Comparative Analysis of the Phase Interaction in Plasma Surfaced NiBSi Overlays with IVB and VIB Transition Metal Carbides
Abstract
:1. Introduction
- injection of refractory particles to the previously initiated liquid weld pool,
- simultaneous introduction of metal−ceramic powder mixtures to the beam or arc,
- melting pre-placed powder mixtures at the surface,
- strengthening of the matrix by in-situ formed particles.
2. Materials and Methods
2.1. Materials
- -
- Specially prepared massive solids for the model wettability and spreadability tests;
- -
- Commercial powders for plasma surfacing.
2.1.1. Composite Matrix Material
2.1.2. Strengthening Carbide Phase
2.1.3. Substrate Material for Plasma Surfacing
2.2. Methods
2.2.1. Wettability and Spreadability Test
2.2.2. Plasma Surfacing
3. Results and Discussion
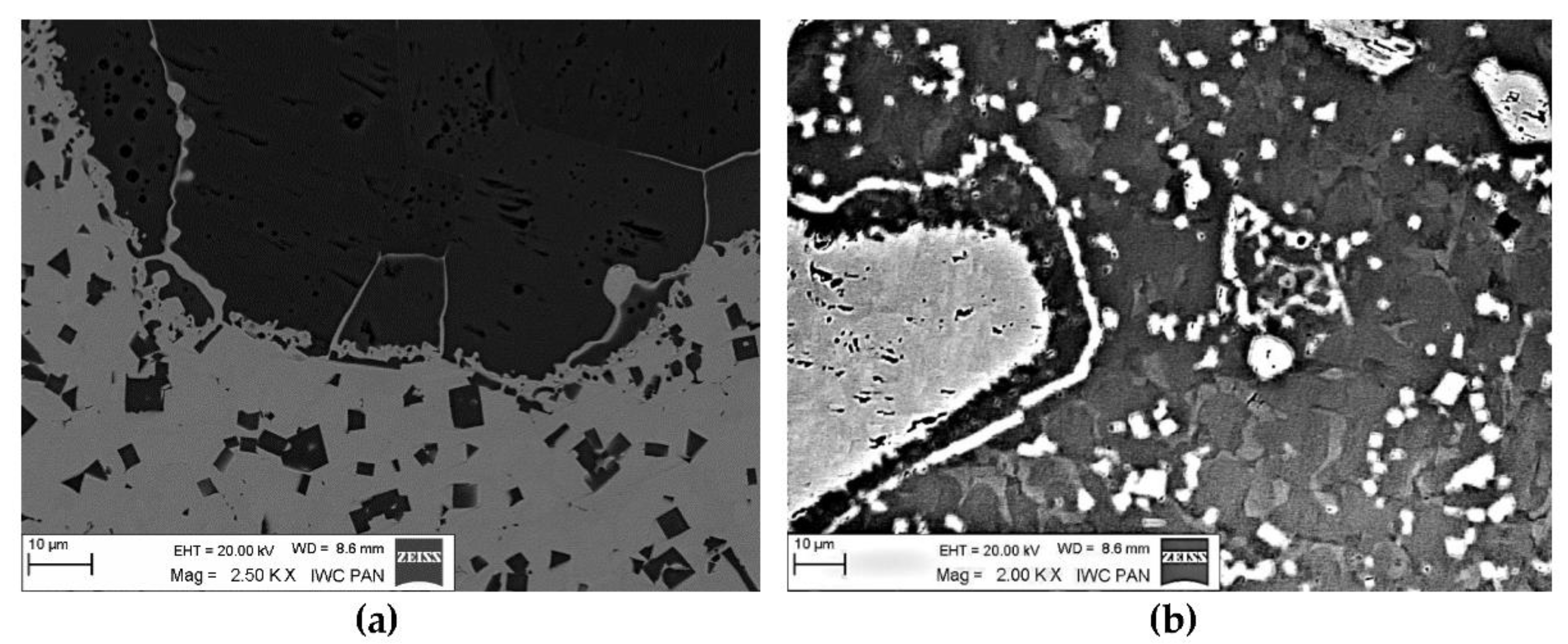
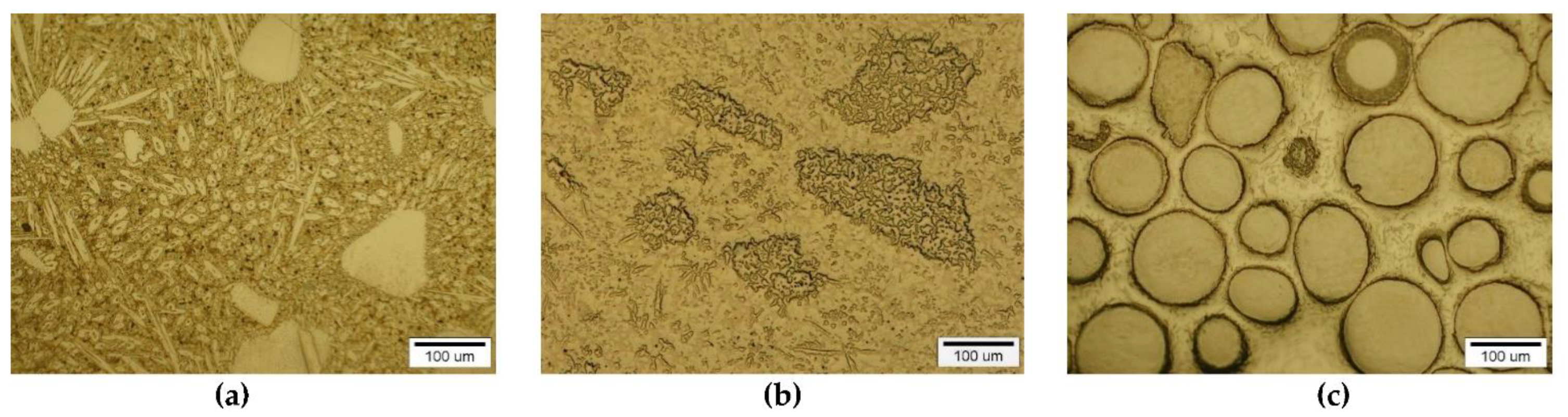
3.1. Interaction in the Model System of Liquid NiBSi Alloy-Solid Carbide Surface
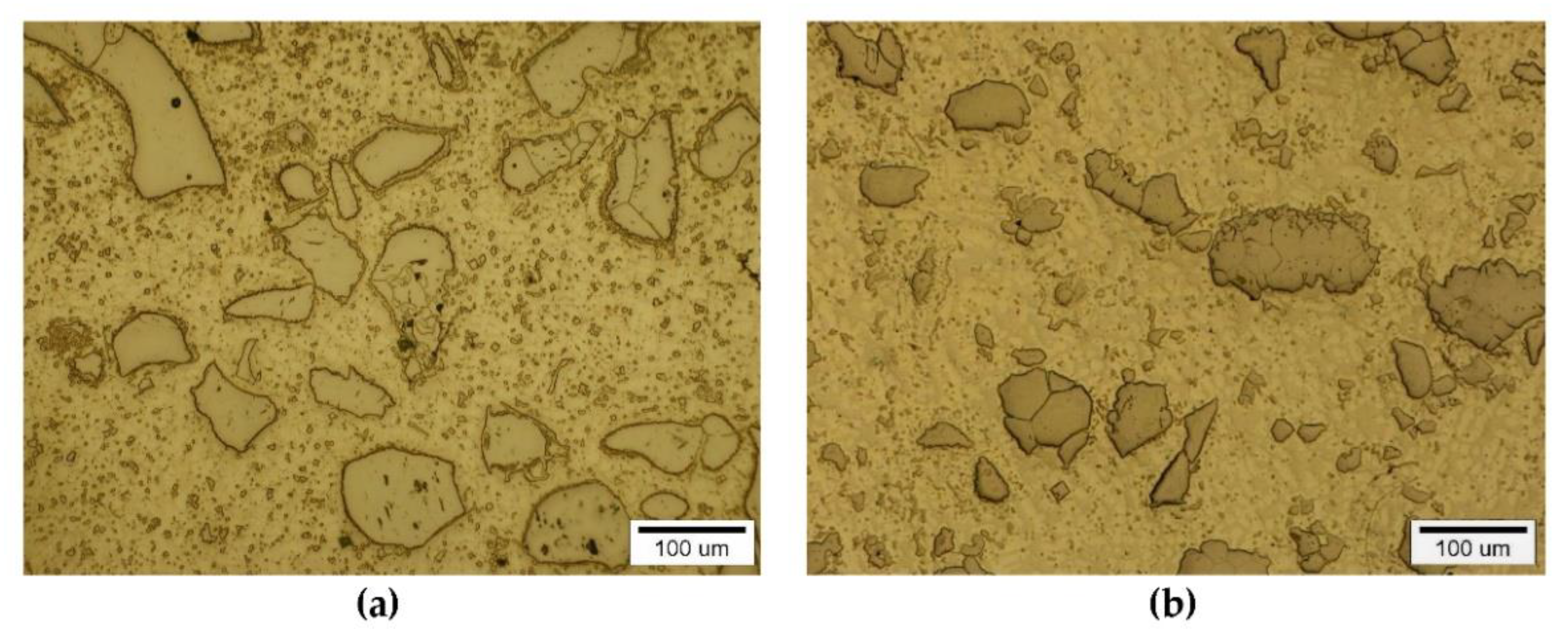
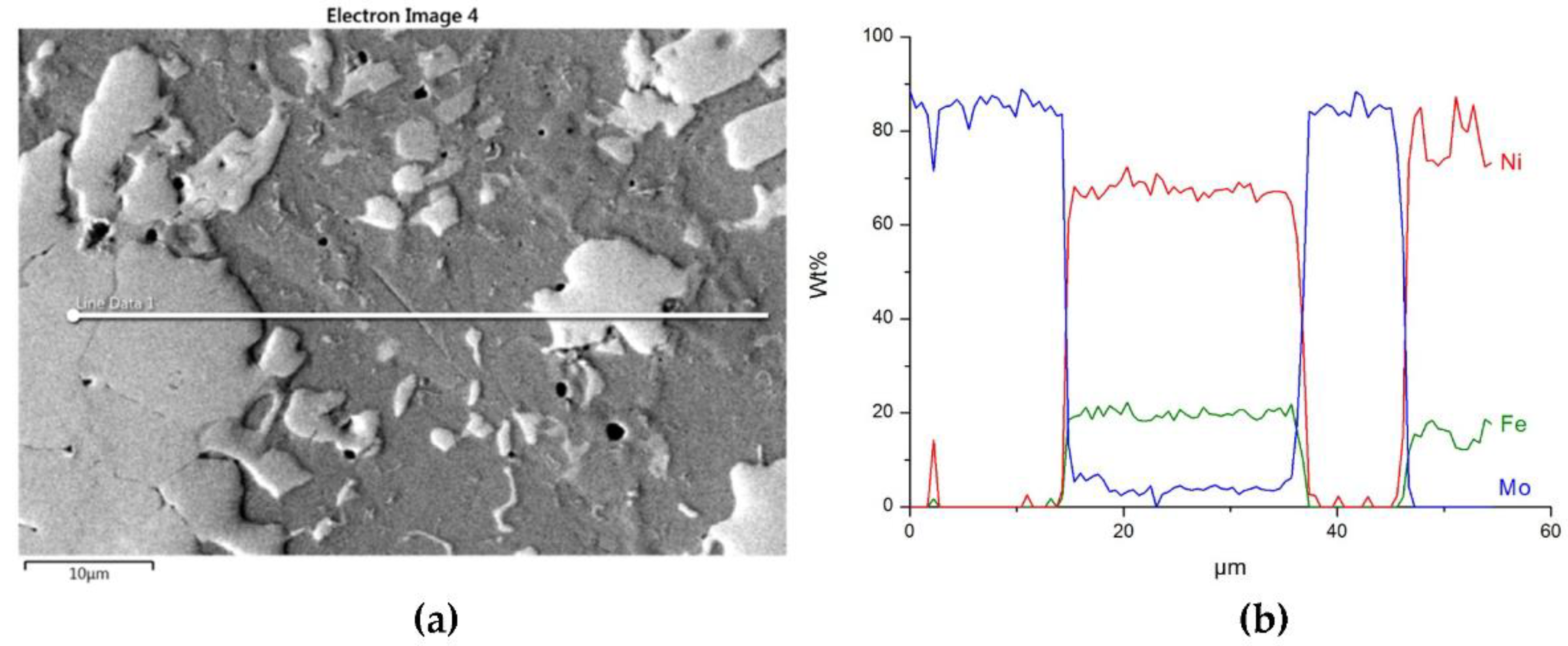
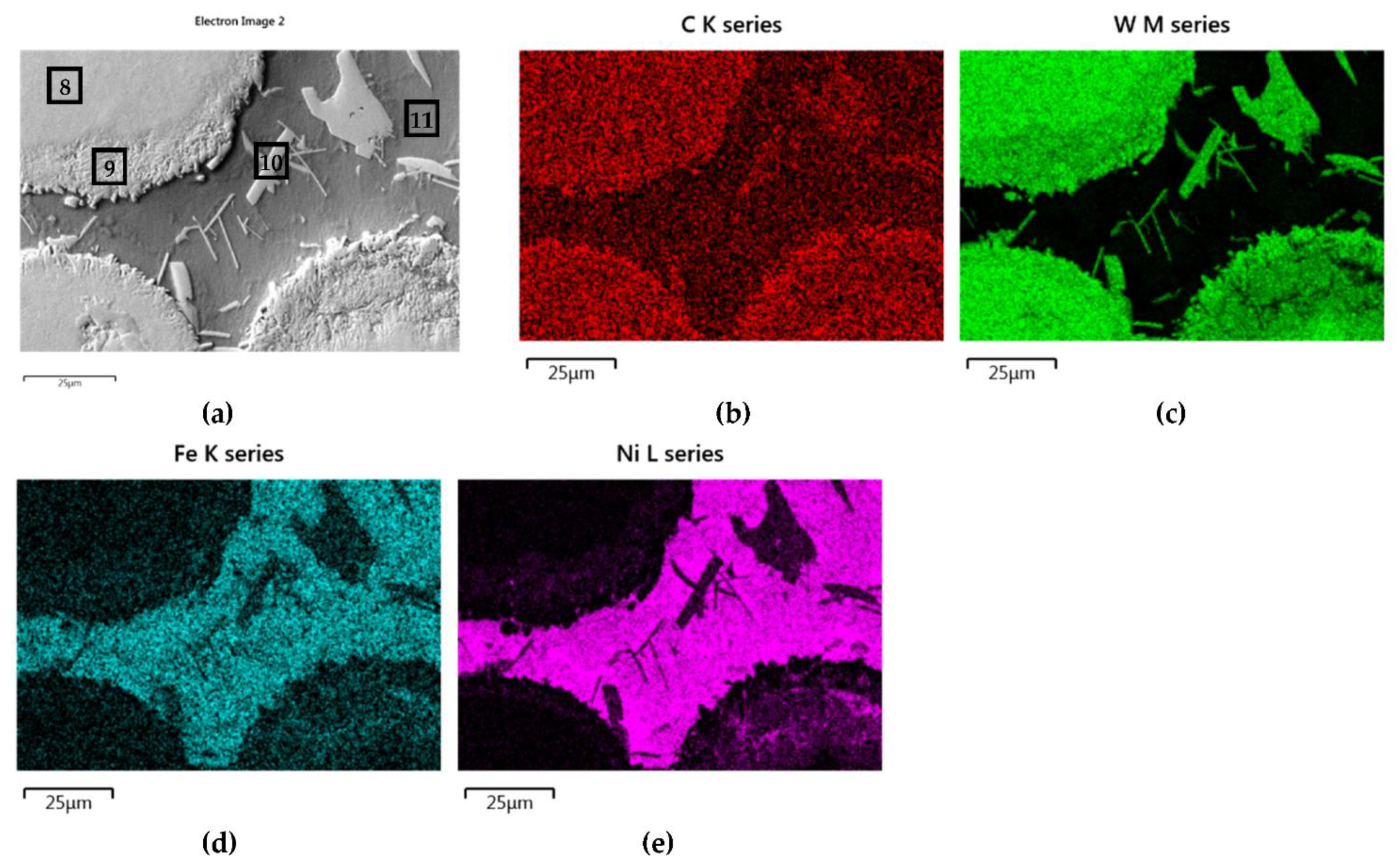
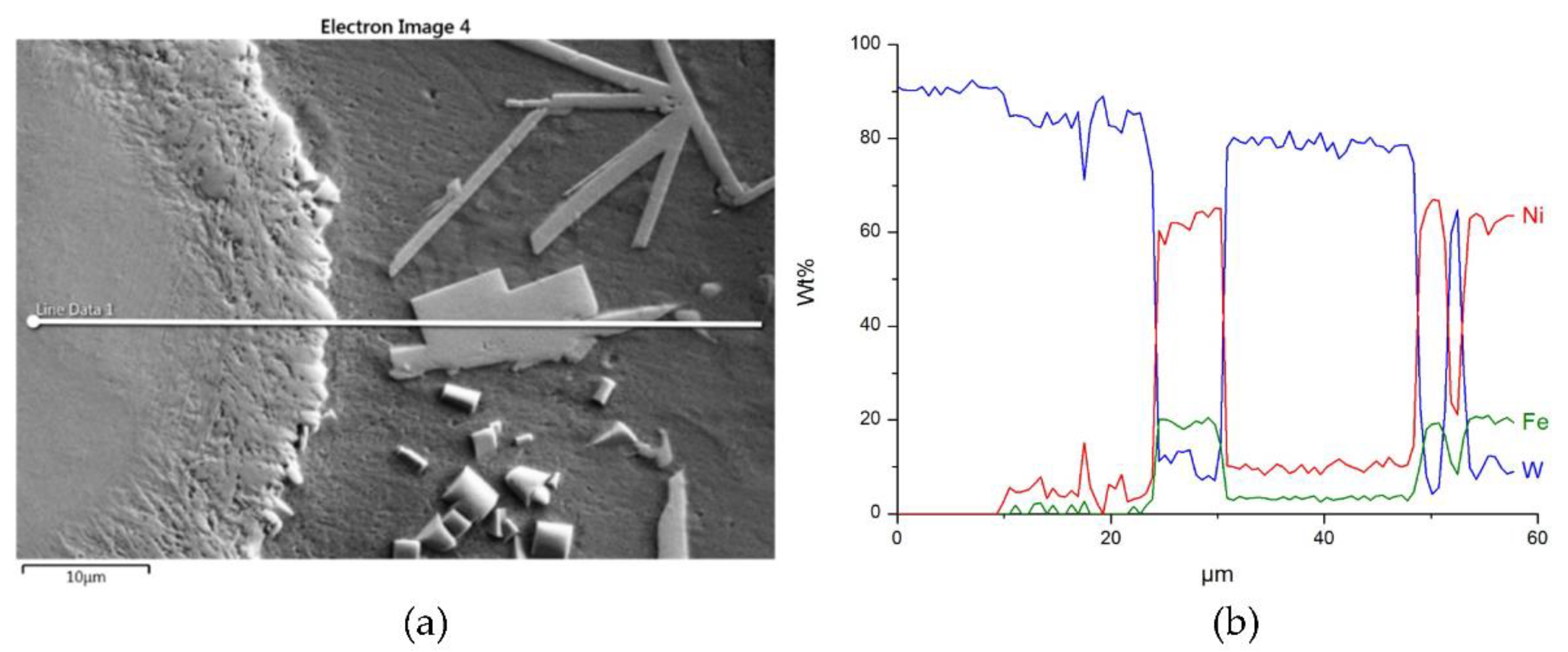
3.2. Interaction of Carbide Powder Particles with Liquid NiBSi during Surfacing
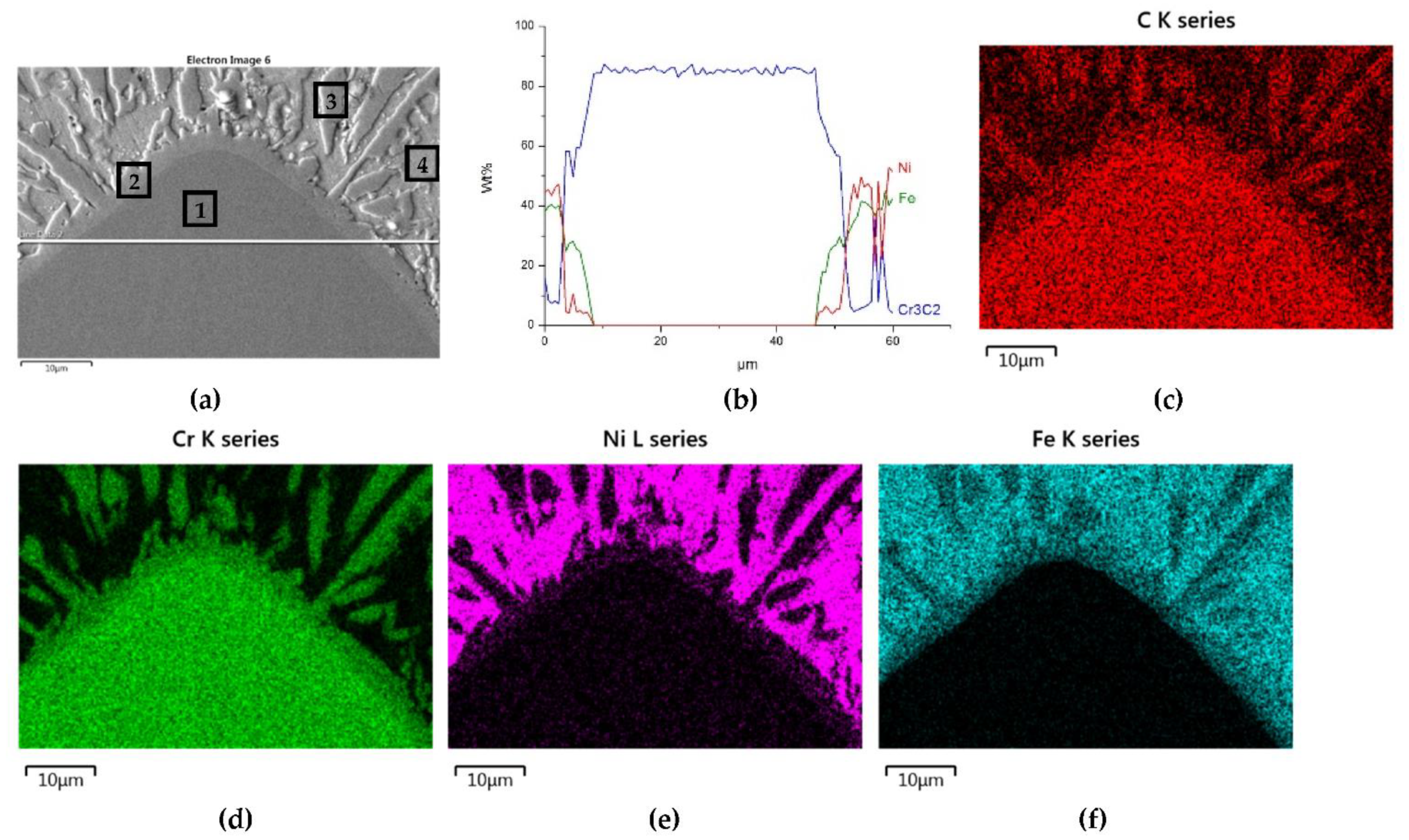
3.2.1. Overlays with Carbides of IVB TM Group
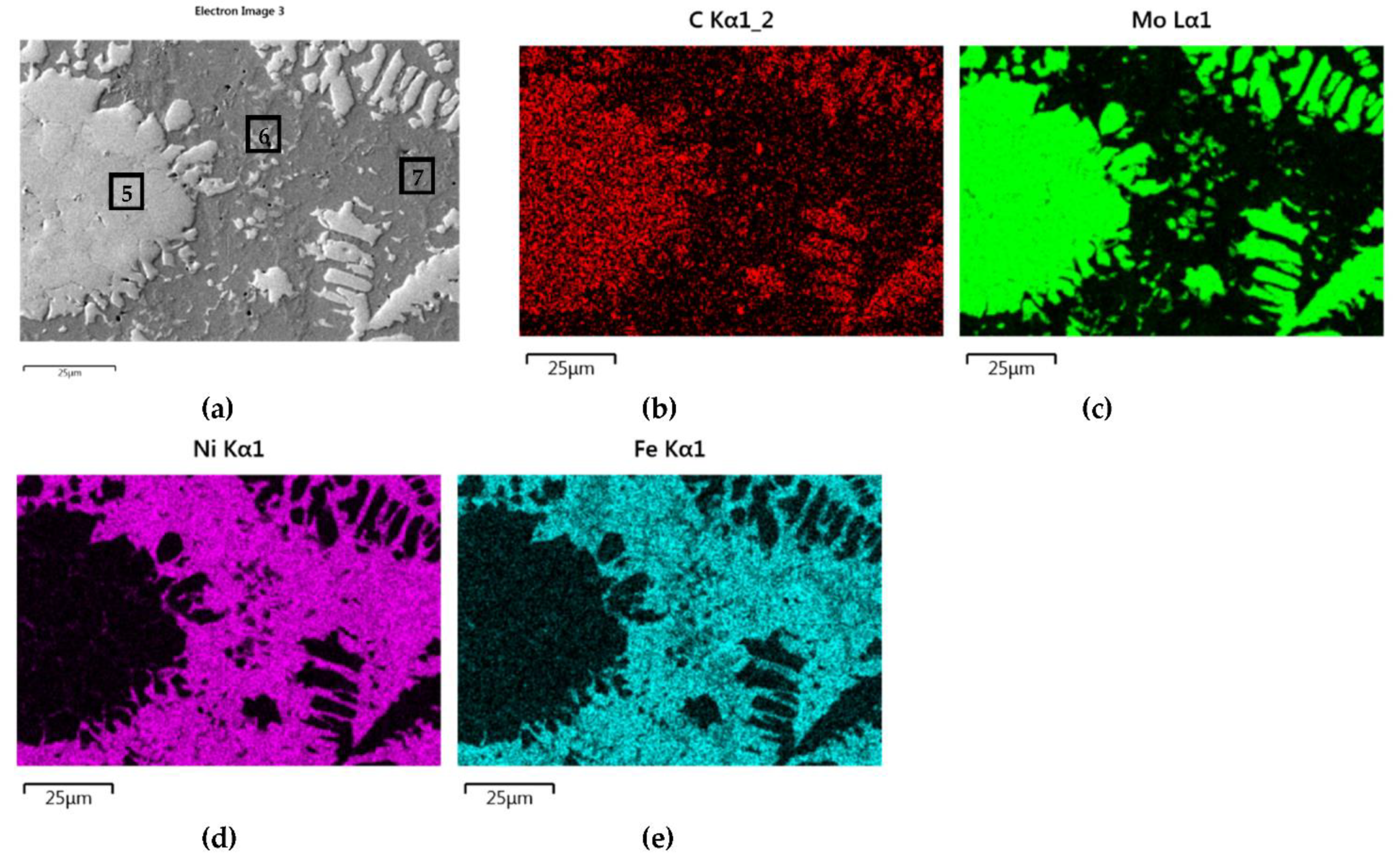
3.2.2. Overlays with Carbides of VIB TM Group
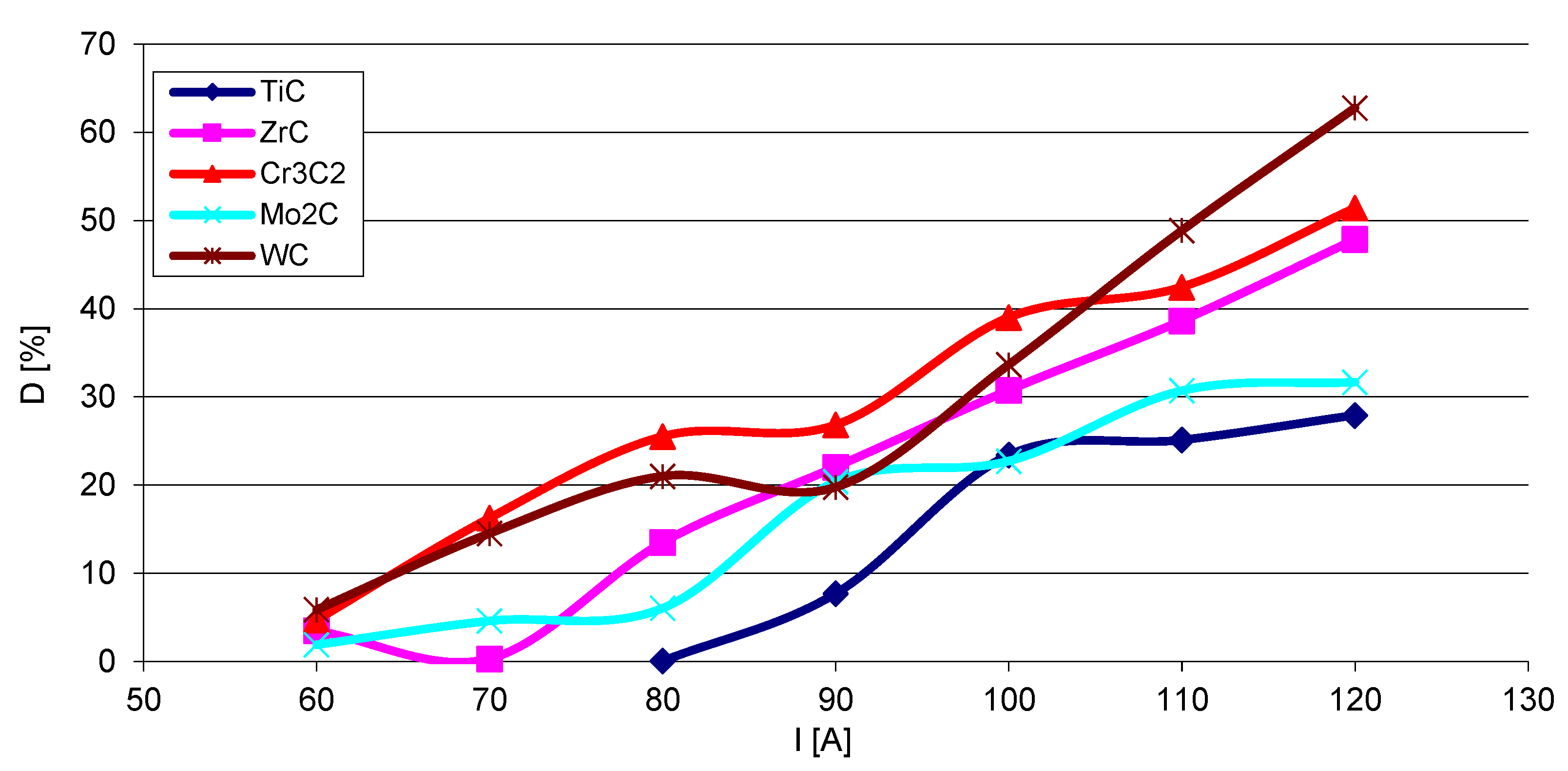
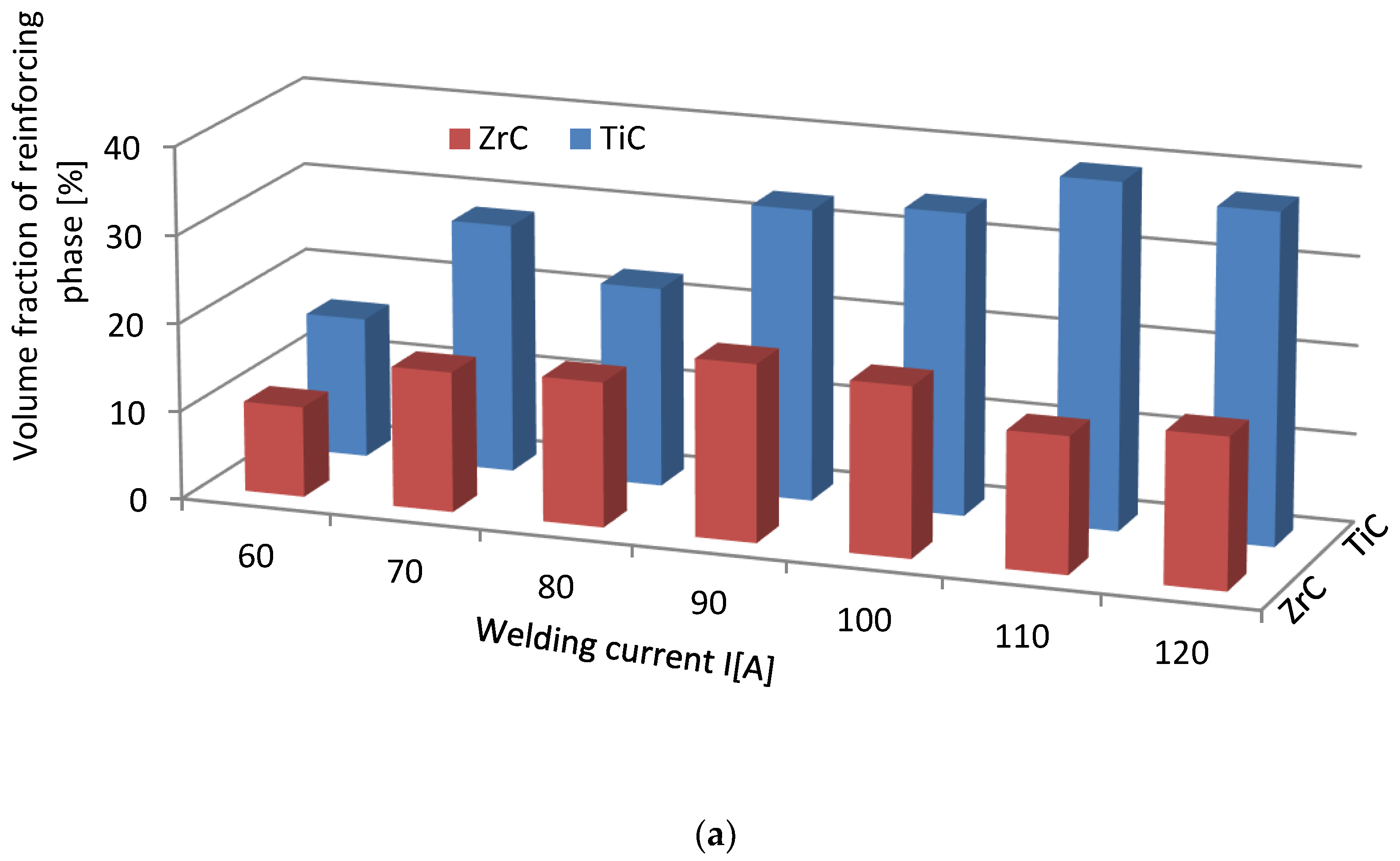
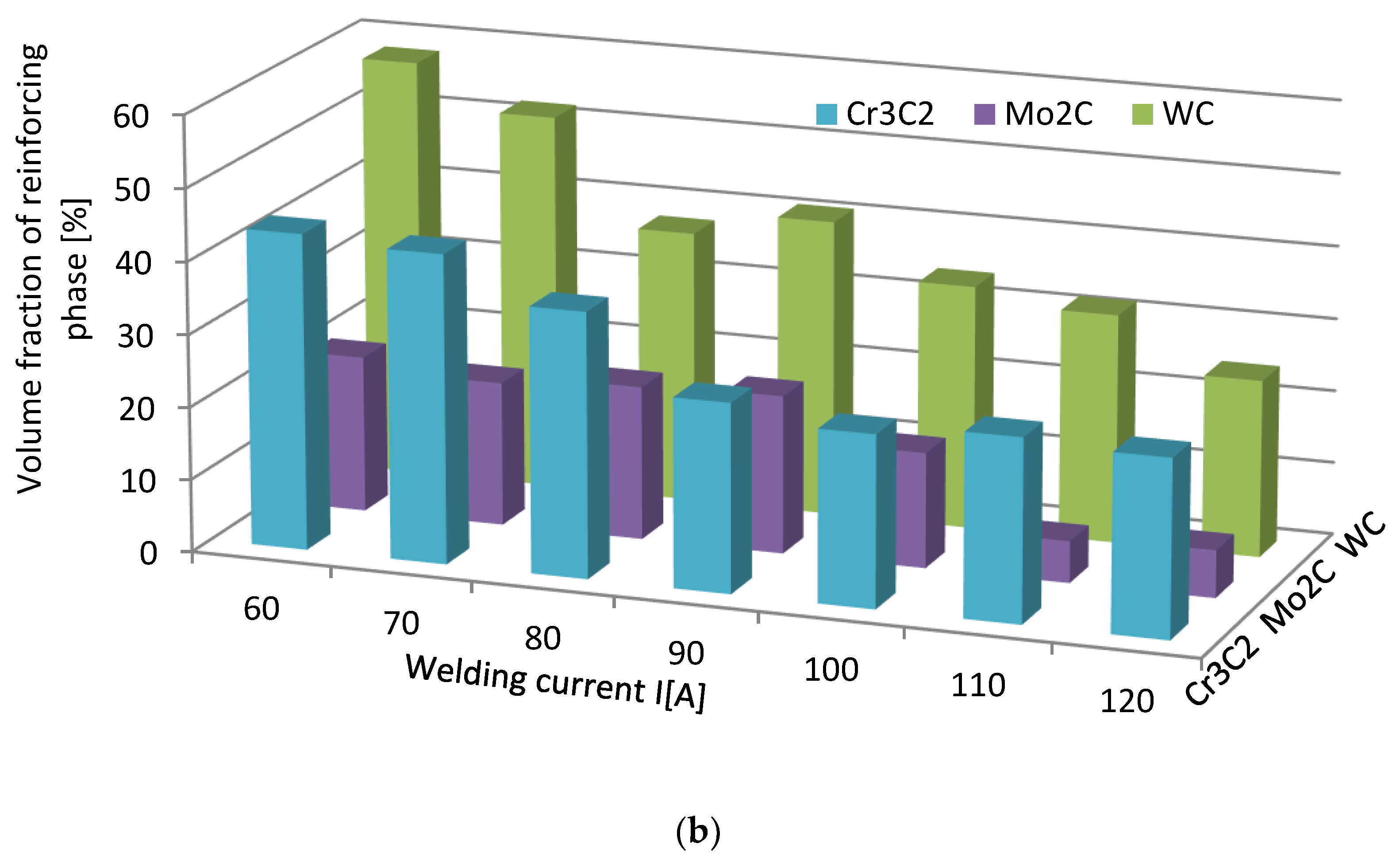
3.3. Strengthening Phase Fraction in Overlays
3.4. Metallurgical Considerations
4. Conclusions
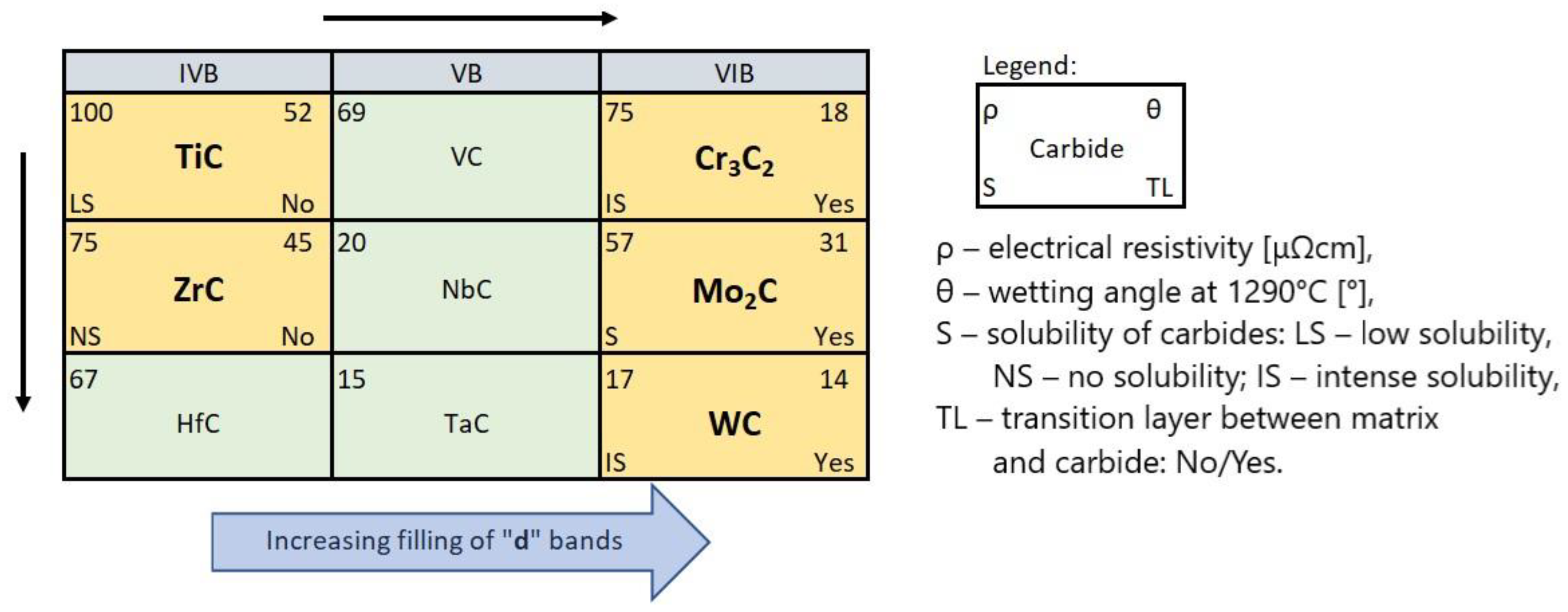
- Carbides of the IVB and VIB TM groups of the periodic table interact with NiBSi alloy, with the increasing intensity related to the group number and the TM location in it. Its measure is the wettability and spreadability, as well as selected aspects of the formation of SCLs: the mechanisms of the disintegration of the strengthening phase in the liquid pool, the solubility of TMCs in the matrix and secondary crystallization, and the formation of transition zones at the interfacial boundaries.
- (a)
- TMCs of the IVB group are good and these of VIB are perfectly wettable with liquid Ni alloy.
- (b)
- The fraction of the strengthening phase particles in SCLs and their distribution are related to the interaction of TMCs with the matrix, which is more intense for the VIB group TMCs.
- (c)
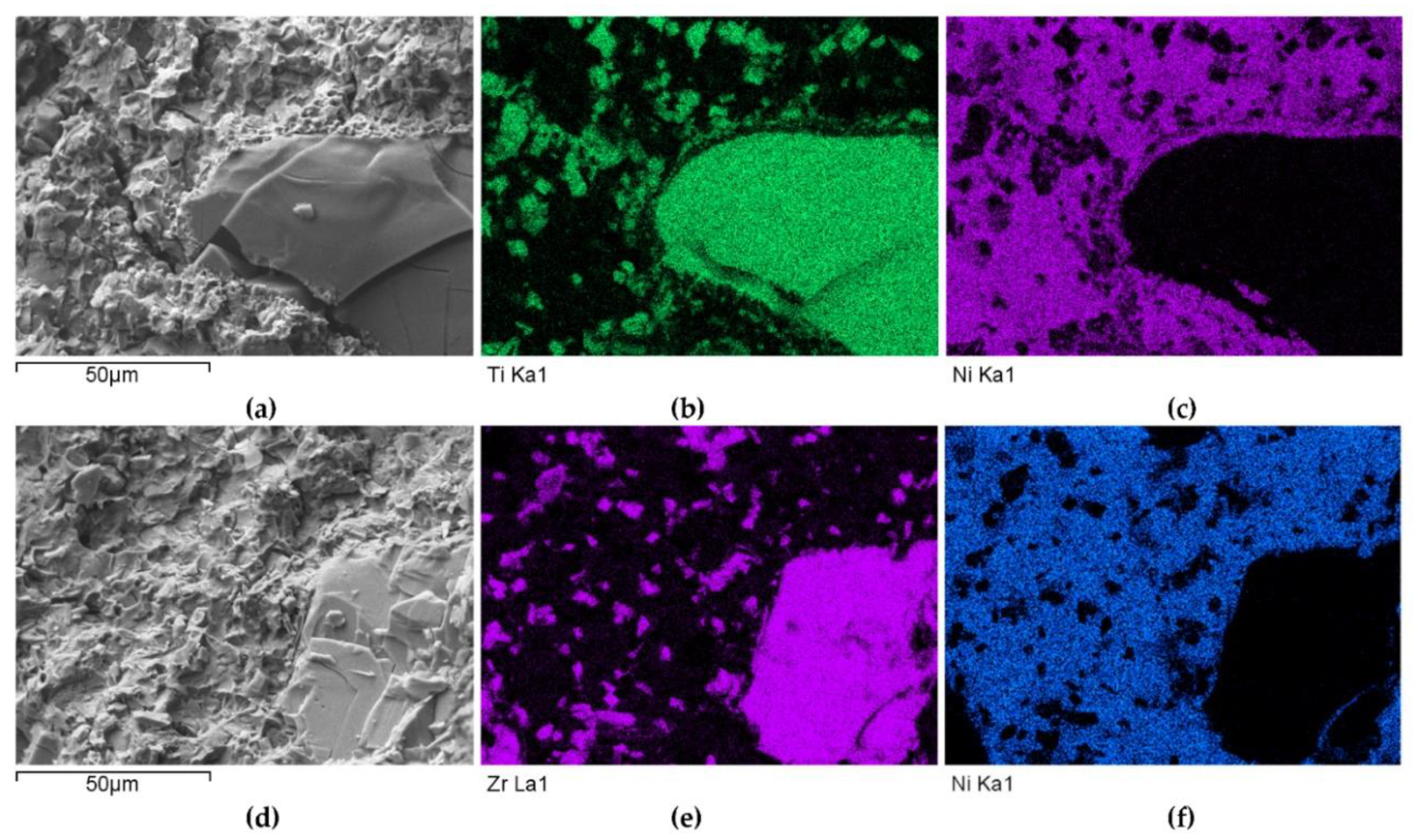
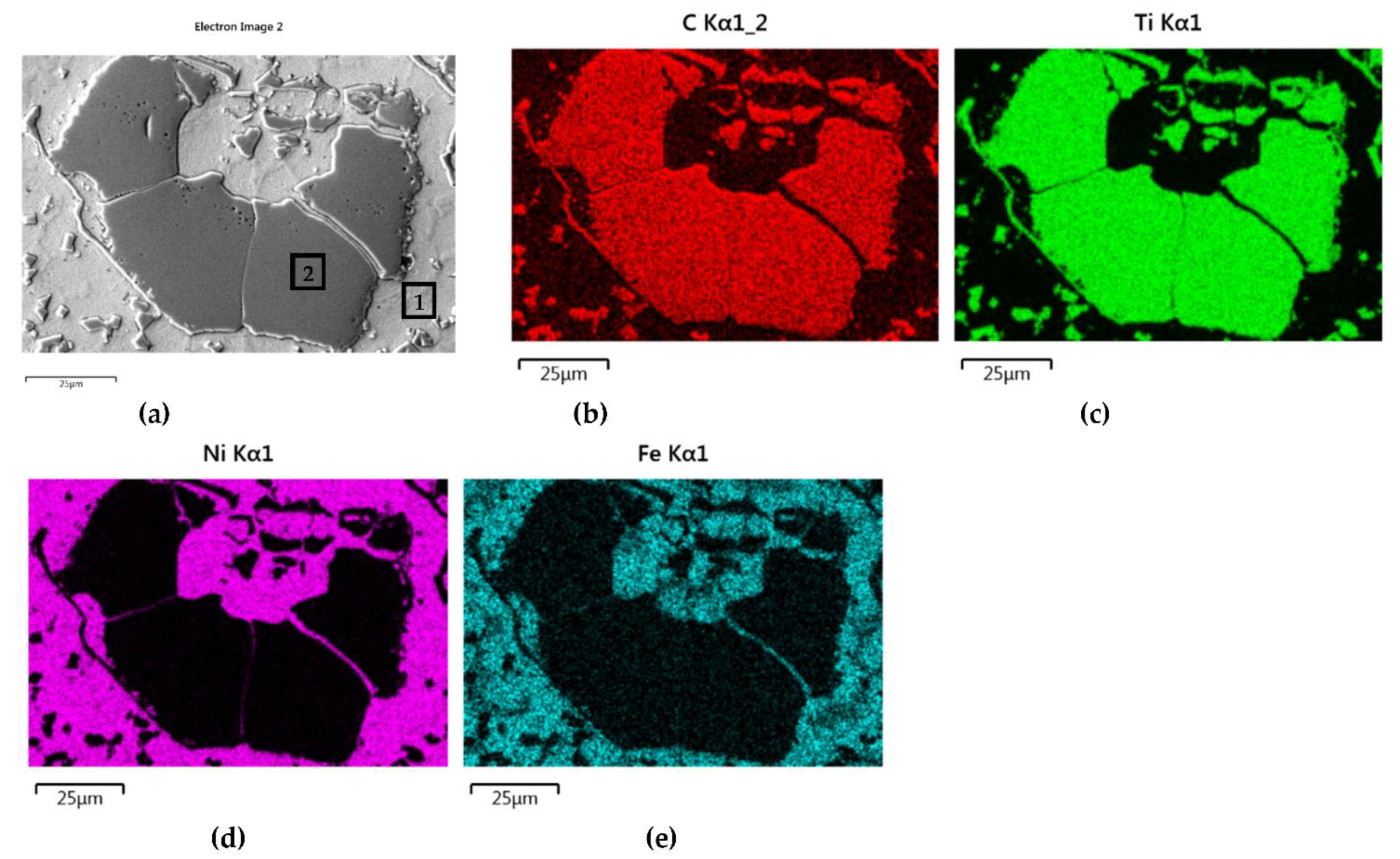
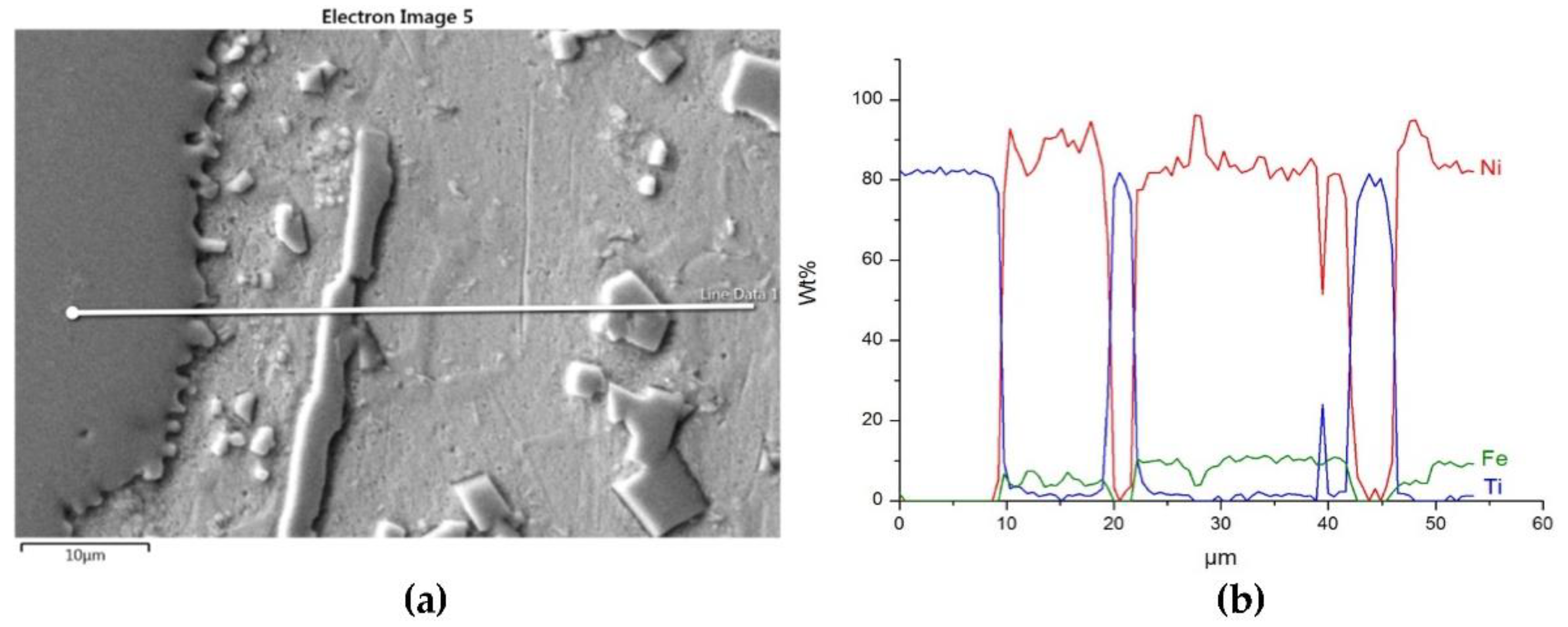
- Particles of TiC and ZrC are decomposed by the penetration of the liquid phase along the grain boundaries. As a result, decomposition of agglomerates on smaller parts occurs and a significant amount of a fine fraction is formed. In contrast with this, Cr3C2, Mo2C, and WC particles are dissolved partially or completely, enriching the matrix in Cr, Mo, and W, respectively. New phases are crystallized from the supersaturated solution when cooling.
- (d)
- No transition zones are formed at the interfacial boundaries of the IVB group TMCs, unlike in the case of the VIB group TMCs.
- (e)
- There is a tendency for both of the studied groups of TMCs to intensify the interaction with the Ni alloy matrix, with an increase in the atomic number of the parent metal forming the given carbide.
- Fe coming from the partially PTAW melted substrate plays an active role in the system, along with temperature depressants B and Si present in the matrix of the composite.
- The obtained experimental results can be successfully interpreted in light of the electronic structure of TMCs and their physicochemical properties.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, L.M. Application of Hardmetals as Thermal Spray Coatings. Int. J. Refract. Met. Hard Mater. 2015, 49, 350–364. [Google Scholar] [CrossRef]
- Pawlowski, L. Thick Laser Coatings: A Review. J. Therm. Spray Technol. 1999, 8, 279–295. [Google Scholar] [CrossRef]
- Zhong, M.; Liu, W. Laser Surface Cladding: The State of the Art and Challenges. Proc. Inst. Mech. Eng. Part C J. Mech. Eng. Sci. 2010, 224, 1041–1060. [Google Scholar] [CrossRef]
- Praedeep, G.R.C.; Ramesh, A.; Prasad, B.D. A Review Paper on Hardfacing Processes and Materials. Int. J. Eng. Sci. Technol. 2010, 11, 6507–6510. [Google Scholar]
- Czupryński, A. Microstructure and Abrasive Wear Resistance of Metal Matrix Composite Coatings Deposited on Steel Grade AISI 4715 by Powder Plasma Transferred Arc Welding Part 2. Mechanical and Structural Properties of a Nickel-Based Alloy Surface Layer Reinforced with Particles of Tungsten Carbide and Synthetic Metal–Diamond Composite. Materials 2021, 14, 2805. [Google Scholar] [CrossRef]
- Meng, Q.W.; Geng, L.; Zhang, B.Y. Laser cladding on Ni-base Composite Coatings onto Ti6Al4V Substrates with Pre-Placed B4C+NiCrBSi Powders. Surf. Coat. Technol. 2006, 200, 4923–4928. [Google Scholar] [CrossRef]
- Li, C.-W.; Chang, K.-C.; Yeh, A.-C.; Yeh, J.-W.; Lin, S.-J. Microstructure Characterization of Cemented Carbide Fabricated by Selective Laser Melting Process. Int. J. Refract. Met. Hard Mater. 2018, 75, 225–233. [Google Scholar] [CrossRef]
- Chao, M.-J.; Niu, X.; Yuan, B.; Liang, E.-J.; Wang, D.-S. Preparation and Characterization of in Situ Synthesized B4C Particulate Reinforced Nickel Composite Coatings by Laser Cladding. Surf. Coatings Technol. 2006, 201, 1102–1108. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H. A Novel Load-Insensitive Co3Mo2Si Reinforced In-Situ Metal-Matrix Composite Coating for Wear Resistance Application. Mater. Lett. 2010, 64, 2494–2497. [Google Scholar] [CrossRef]
- Betts, J.C. The Direct Laser Deposition of AISI316 Stainless Steel and Cr3C2 Powder. J. Mater. Process. Technol. 2009, 209, 5229–5238. [Google Scholar] [CrossRef]
- Asthana, R.; Sobczak, N. Wettability, Spreading, and Interfacial Phenomena in High-Temperature Coatings. JOM-E 2000, 52, 1–19. [Google Scholar]
- Oukach, S.; Pateyron, B.; Pawłowski, L. Physical and chemical Phenomena Occurring Between Solid Ceramics and Liquid Metals and Alloys at Laser and Plasma Composite Coatings Formation: A review. Surf. Sci. Rep. 2019, 74, 213–241. [Google Scholar] [CrossRef]
- Huebner, J.; Kata, D.; Rutkowski, P.; Petrzak, P.; Kusiński, J. Grain-Boundary Interaction between Inconel 625 and WC during Laser Metal Deposition. Materials 2018, 11, 1797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eustathopoulos, N.; Nicholas, M.G.; Drevet, B. Wettability at High Temperatures, 1st ed.; Pergamon Press: Amsterdam, The Netherlands; Lausanne, NY, USA; Oxford, UK, 1999; Volume 3. [Google Scholar]
- Bourell, D.; Kruth, J.P.; Leu, M.; Levy, G.; Rosen, D.; Beese, A.; Clare, A. Materials for Additive Manufacturing. CIRP Ann. 2017, 66, 659–681. [Google Scholar] [CrossRef]
- Rojasa, J.G.M.; Wolfeb, T.; Flecka, B.A.; Qureshi, A.J. Plasma Transferred Arc Additive Manufacturing of Nickel Metal Matrix Composites. Manuf. Lett. 2018, 18, 31–34. [Google Scholar] [CrossRef]
- Zhong, Y.; Xia, X.; Shi, F.; Zhan, J.; Tu, J.; Fan, H.J. Transition Metal Carbides and Nitrides in Energy Storage and Conversion. Adv. Sci. 2016, 3, 1500286. [Google Scholar] [CrossRef] [PubMed]
- Dinh, K.N.; Liang, Q.; Du, C.-F.; Zhao, J.; Tok, A.; Mao, H.; Yan, Q. Nanostructured Metallic Transition Metal Carbides, Nitrides, Phosphides, and Borides for Energy Storage and Conversion. Nano Today 2019, 25, 99–121. [Google Scholar] [CrossRef]
- Xiao, P.; Ge, X.; Wang, H.; Liu, Z.; Fisher, A.C.; Wang, X. Novel Molybdenum Carbide-Tungsten Carbide Composite Nanowires and Their Electrochemical Activation for Efficient and Stable Hydrogen Evolution. Adv. Funct. Mater. 2015, 25, 1520–1526. [Google Scholar] [CrossRef]
- Sebakhy, K.O.; Vitale, G.; Hassan, A.; Pereira-Almao, P. New Insights into the Kinetics of Structural Transformation and Hydrogenation Activity of Nano-crystalline Molybdenum Carbide. Catal. Lett. 2018, 148, 904–923. [Google Scholar] [CrossRef]
- Li, T.; Liu, B.; Liu, Y.; Guo, W.; Fu, A.; Li, L.; Yan, N.; Fang, Q. Microstructure and Mechanical Properties of Particulate Reinforced NbMoCrTiAl High Entropy Based Composite. Entropy 2018, 20, 517. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Wu, H.; Zhou, R.; Zhang, N.; Yin, Y.; Liang, L.; Liu, Y.; Li, J.; Shan, Q.; Li, Q.; et al. Microstructures and Tribological Properties of TiC Reinforced FeCoNiCuAl High-Entropy Alloy at Normal and Elevated Temperature. Metals 2020, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Akhtar, F. Processing and Characterization of Refractory Quaternary and Quinary High-Entropy Carbide Composite. Entropy 2019, 21, 474. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, R. New Advances in High-Entropy Alloys. Entropy 2020, 22, 1158. [Google Scholar] [CrossRef]
- Wang, Z.H.; Wang, H.; He, D.Y.; Cui, L.; Jiang, J.M.; Zhou, Z.; Zhao, Q.Y. Microstructure Characterization of In Situ NbC/high Entropy Alloys by Plasma Cladding. Rare Met. Mater. Eng. 2015, 44, 3156–3160. [Google Scholar]
- Hardfacing Alloys. Available online: www.deloro.com (accessed on 8 September 2021).
- Tokunaga, T.; Nishio, K.; Ohtani, H.; Hasebe, M. Phase Equilibria in the Ni-Si-B System. Mater. Trans. 2003, 44, 1651–1654. [Google Scholar] [CrossRef] [Green Version]
- Rumble, J.R. CRC Handbook of Chemistry and Physics, 99th ed.; Taylor&Francis: Boca Raton, FL, USA; London, UK, 2018. [Google Scholar]
- Bober, M.; Senkara, J.; Wendler, B. Persistence of the Thin Layers of Transition Metal Carbides in Contact with Liquid NiBSi alloy. Weld. Technol. Rev. 2021, 93, 5–12. [Google Scholar] [CrossRef]
- Ramqvist, L. Wetting of Metallic Carbides by Liquid Copper, Nickel, Cobalt and Iron. Int. J. Powder Met. 1965, 1, 2–21. [Google Scholar]
- Naidich, Y.V. The Wettability of Solids by Liquid Metals. Progress in Surface and Membrane Sciences 1981, 14, 353–484. [Google Scholar]
- Bober, M.; Senkara, J. Comparative Tests of Plasma-Surfaced Nickel Layers with Chromium and Titanium Carbides. Weld. Int. 2016, 30, 107–111. [Google Scholar] [CrossRef]
- Bober, M. Formation and Structure of the Composite Coatings Ni-NbC deposited by plasma transferred arc. Weld. Technol. Rev. 2018, 90, 65–69. [Google Scholar]
- Okamoto, H.; Massalski, T.B. Binary Alloy Phase Diagrams Requiring Further Studies. J. Phase Equilibria Diffus. 1994, 15, 500–521. [Google Scholar] [CrossRef]
- Lengauer, W. Transition Metal Carbides, Nitrides and Carbonitrides. In Handbook of Ceramic Hard Materials; Riedel, R., Ed.; Wiley-VCH Verlag GmbH&Co: Weinheim, Germany, 2000. [Google Scholar]
- Pankratz, L.B. Thermodynamic Properties of Carbides, Nitrides, and Other Selected Substances; United States Bureau of Mines Reports: Washington, DC, USA, 1994; Available online: https://digital.library.unt.edu/ark:/67531/metadc12836/ (accessed on 31 August 2021).
- Wang, Q.; German, K.E.; Oganov, A.R.; Dong, H.; Feya, O.D.; Zubavichus, Y.V.; Murzin, V.Y. Explaining Stability of Transition Metal Carbides—and Why TcC Does Not Exist. RSC Adv. 2016, 6, 16197–16202. [Google Scholar] [CrossRef]
- Sevy, A.; Matthew, D.J.; Morse, M.D. Bond Dissociation Energies of TiC, ZrC, HfC, ThC, NbC, and TaC. J. Chem. Phys. 2018, 149, 044306. [Google Scholar] [CrossRef]
- Niessen, A.; de Boer, F.; Boom, R.; de Châtel, P.; Mattens, W.; Miedema, A. Model Predictions for the Enthalpy of Formation of Transition Metal Alloys II. Calphad 1983, 7, 51–70. [Google Scholar] [CrossRef]
- Ushakov, S.V.; Navrotsky, A.; Hong, Q.-J.; Van De Walle, A. Carbides and Nitrides of Zirconium and Hafnium. Materials 2019, 12, 2728. [Google Scholar] [CrossRef] [Green Version]
- Gubanov, V.A.; Ivanovsky, A.L.; Zhukov, V.P. Electronic Structure of Refractory Carbides and Nitrides; Cambridge University Press: Cambridge, UK, 1994. [Google Scholar]
- Johnson, E.L.; Davis, Q.C.; Morse, M.D. Predissociation Measurements of Bond Dissociation Energies: VC, VN, and VS. J. Chem. Phys. 2016, 144, 234306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sevy, A.; Huffaker, R.F.; Morse, M.D. Bond Dissociation Energies of Tungsten Molecules: WC, WSi, WS, WSe, and WCl. J. Phys. Chem. A 2017, 121, 9446–9457. [Google Scholar] [CrossRef]
- Häglund, J.; Guillermet, A.F.; Grimvall, G.; Körling, M. Theory of Bonding in Transition-Metal Carbides and Nitrides. Phys. Rev. B 1993, 48, 11685–11691. [Google Scholar] [CrossRef] [PubMed]
- Greczynski, G.; Primetzhofer, D.; Hultman, L. Reference Binding Energies of Transition Metal Carbides by Core-Level X-Ray Photoelectron Spectroscopy Free from Ar+ Etching Artefacts. Appl. Surf. Sci. 2018, 436, 102–110. [Google Scholar] [CrossRef]
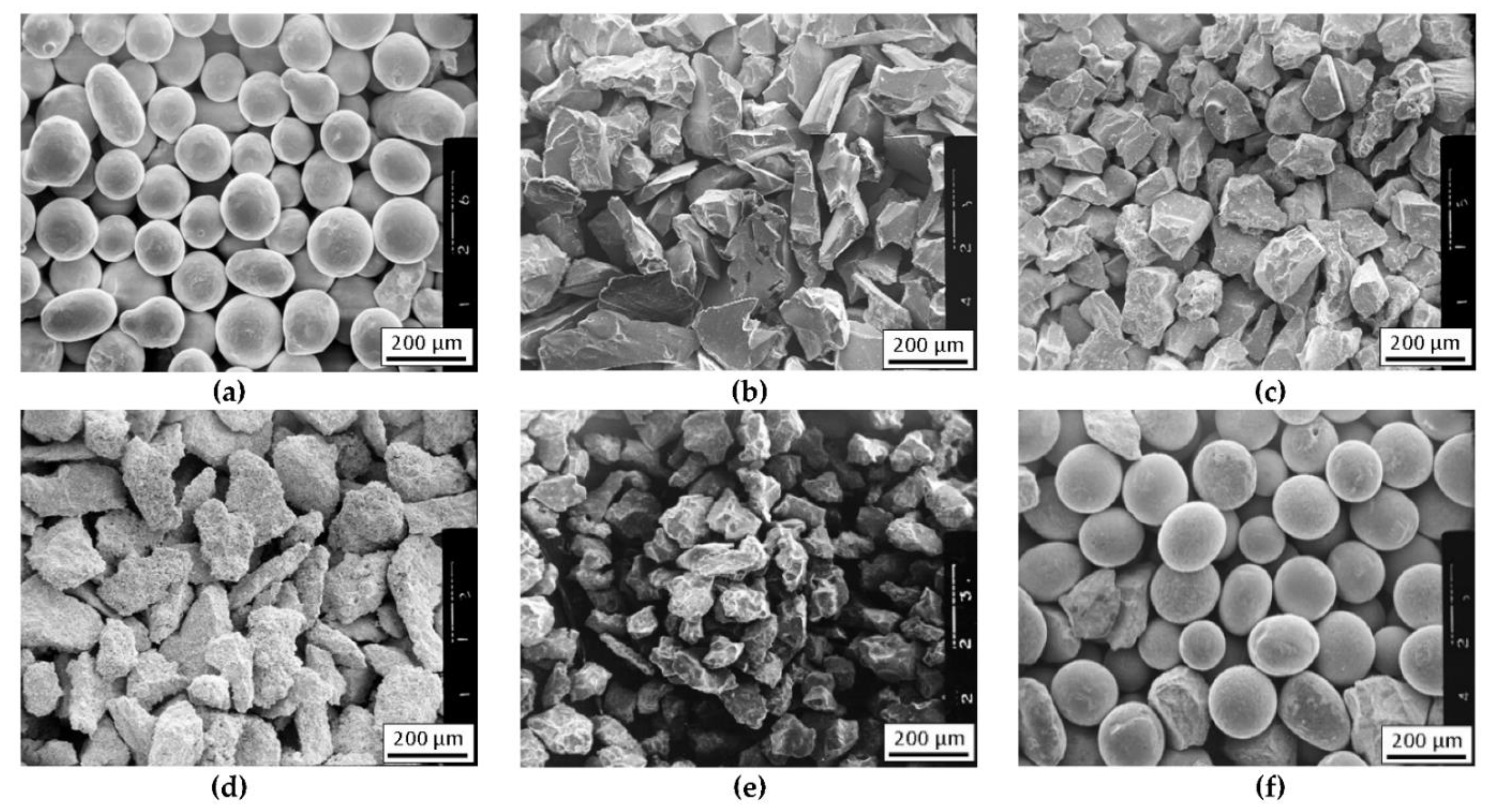
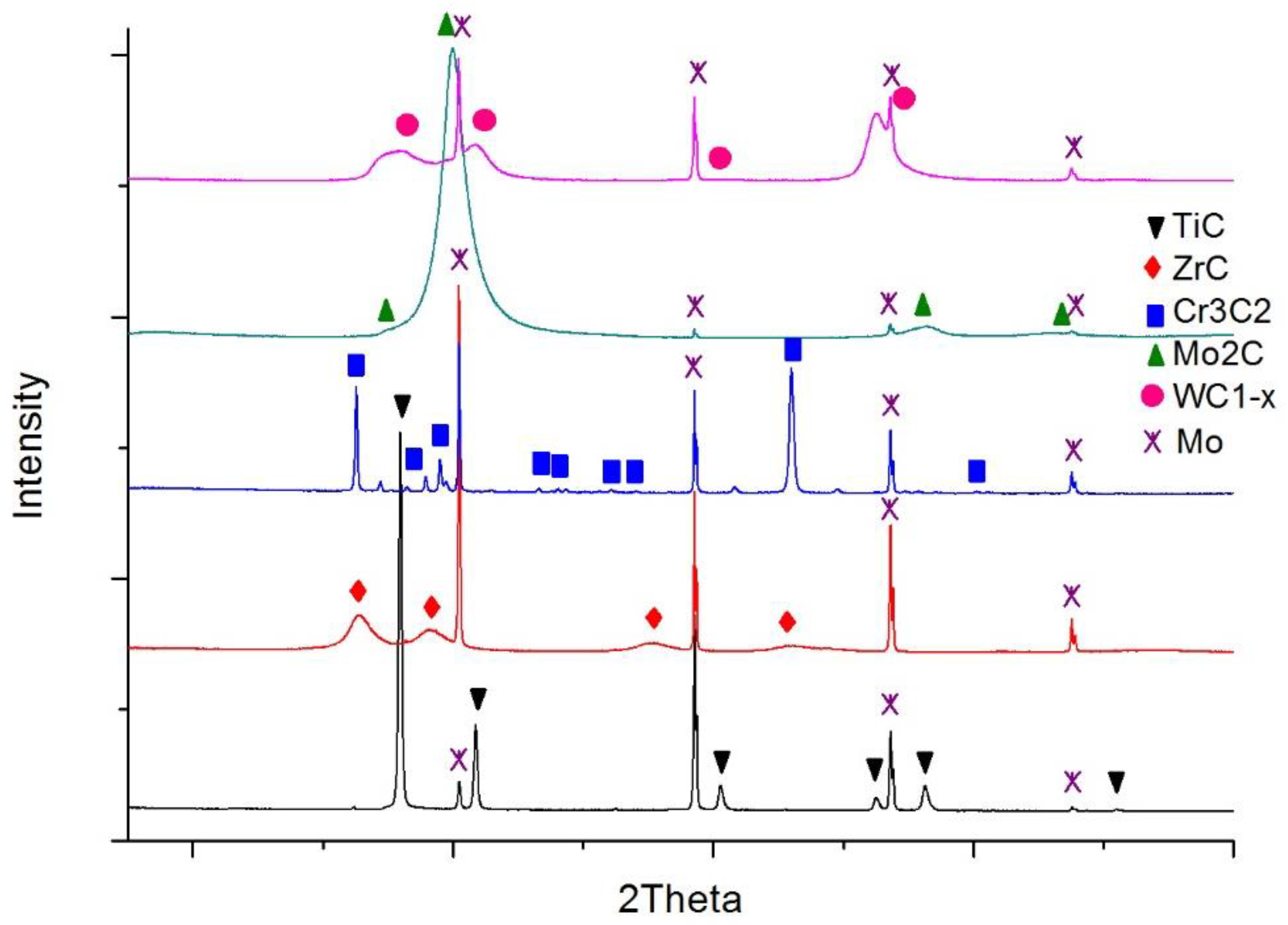
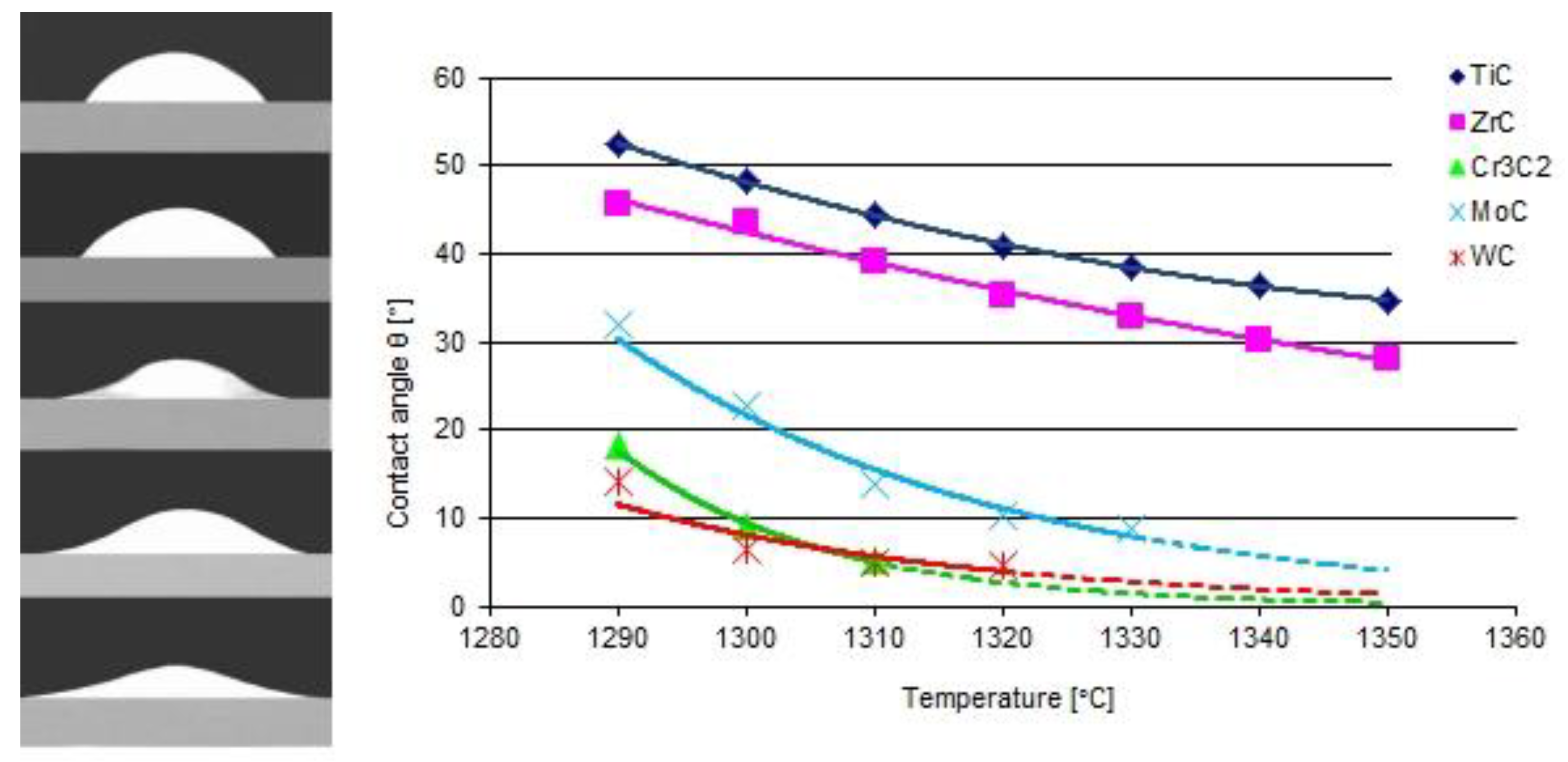
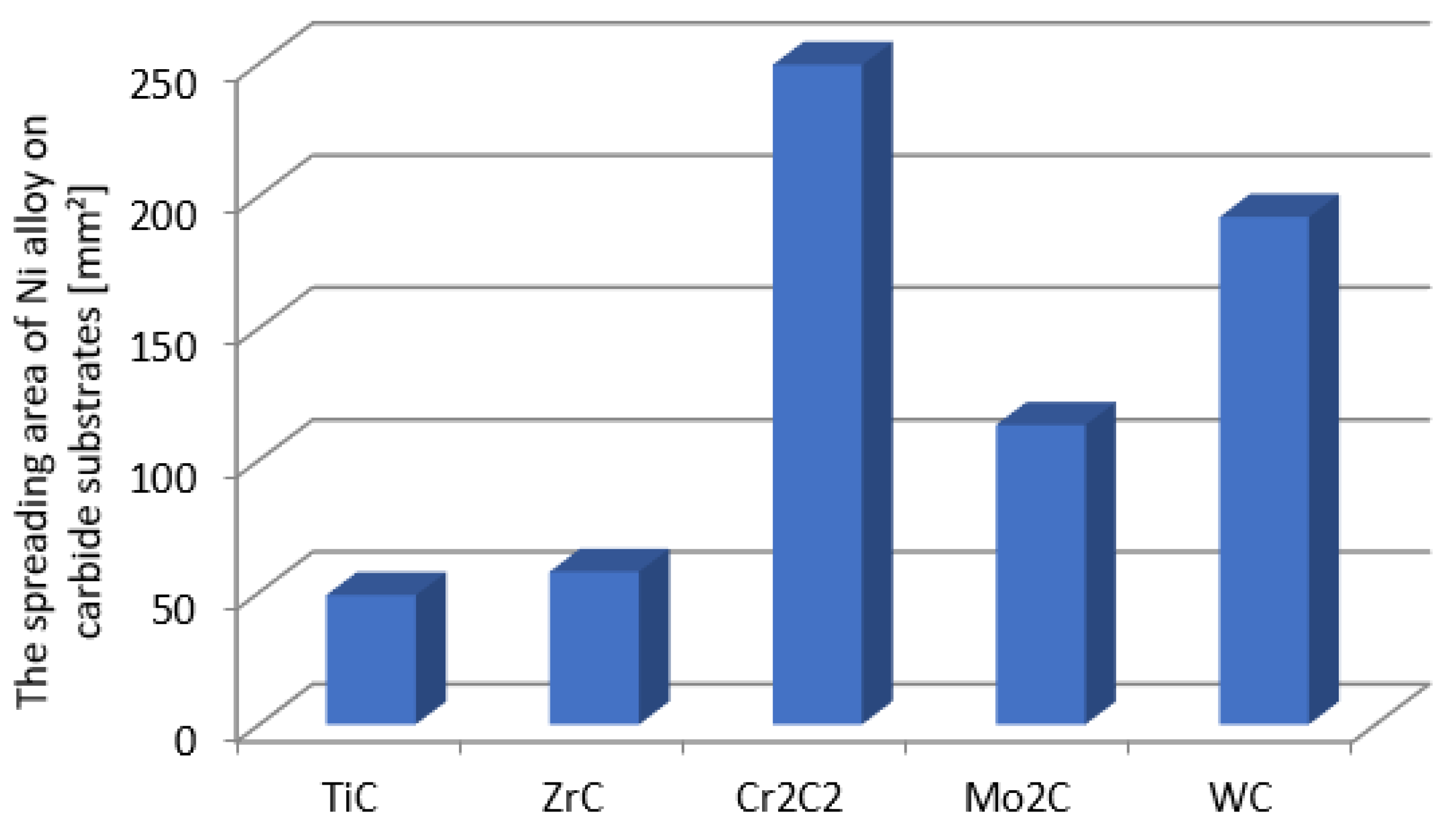


| Material | Elements, Wt% | |||||
|---|---|---|---|---|---|---|
| C | Si | B | Fe | Ni | ||
| Manufacturer’s certificate | Commercial powder | 0.03 | 2.40 | 1.40 | 0.40 | Bal. |
| AAS measured | Commercial powder | - | 2.18 | 1.21 | 0.09 | Bal. |
| Cast alloy ϕ 3 mm | - | 2.45 | 1.17 | 0.20 | Bal. | |
| Type of Coating | TiC | ZrC | Cr3C2 | Mo2C | WC |
|---|---|---|---|---|---|
| Coating thickness [μm] | 4.7 | 3.0 | 2.4 | 7.8 | 1.3 |
| Parameters | Value | |
|---|---|---|
| Main arc current (welding current) | 60, 70, 80, 90, 100, 110, and 120 A | |
| Internal arc current | 40 A | |
| Plasma arc voltage | 25 V | |
| Powder output | 6 g/min | |
| Surfacing rate | 50 mm/min | |
| Gas flow (argon): | ● Plasma generating (orifice) gas | 1.5 L/min |
| ● Shielding gas | 8 L/min | |
| ● Powder transporting gas | 5 L/min | |
| Oscillation amplitude | 8 mm | |
| Oscillating speed | 450 mm/min | |
| Plasmatron-welded substrate distance | 15 mm | |
| Nozzle diameter | 4 mm | |
| Transition Metals Group | Carbide | Welding Current [A] |
|---|---|---|
| IVB | TiC | 90 |
| ZrC | 90 | |
| VIB | Cr3C2 | 90 |
| Mo2C | 80 | |
| WC | 90 |
| Overlay | Region | Elements, Wt% | |||||
|---|---|---|---|---|---|---|---|
| Ti | Zr | C | Ni | Fe | Si | ||
| NiBSi–TiC | 1 | 80.8 | - | 19.2 | - | - | - |
| 2 | 1.7 | - | 7.8 | 79.6 | 9.2 | 1.8 | |
| NiBSi–ZrC | 3 | - | 82.6 | 17.4 | - | - | - |
| 4 | - | - | 4.3 | 63.8 | 30.6 | 1.4 | |
| Overlay | Region | Elements, Wt% | ||||||
|---|---|---|---|---|---|---|---|---|
| Cr | Mo | W | C | Ni | Fe | Si | ||
| NiBSi–Cr3C2 | 1 | 85.3 | - | - | 14.7 | - | - | - |
| 2 | 60.2 | - | - | 10.6 | 4.3 | 24.9 | ||
| 3 | 33.1 | - | - | 9.2 | 22 | 35.5 | 0.3 | |
| 4 | 5 | - | - | 4.3 | 50 | 39.2 | 1.6 | |
| NiBSi–Mo2C | 5 | - | 84 | - | 16 | - | - | - |
| 6 | - | 4.8 | - | 7.2 | 68.7 | 17.6 | 1.6 | |
| 7 | - | 1.2 | - | 7.5 | 80.7 | 10.5 | ||
| NiBSi–WC | 8 | - | - | 90.6 | 9.4 | - | - | - |
| 9 | - | - | 83 | 10.9 | 4.5 | 1.5 | - | |
| 10 | - | - | 79.7 | 8.2 | 8.9 | 3.2 | - | |
| 11 | - | - | 10.3 | 7.1 | 61.1 | 20.2 | 1.3 | |
| TM Group | Carbide | Tf [°C] | ρ [μΩcm] | ΔGF [kJ/mole C] | ETMC [eV] | EBD [eV] | ΔHNi mix [kJ/mole] |
|---|---|---|---|---|---|---|---|
| IVB | TiC | 3067 [40] | 100 | −164.73 | −1.62 | 3.857 | −154 |
| ZrC | 3572 [40] | 75 | −186.11 | −1.63 | 4.892 | −236 | |
| HfC | 3982 [40] | 67 | −214.16 | −1.88 | 4.426 | −250 [41] | |
| VB | VC0.88 | 2650 | 69 | −91.40 | −0.83 | 4.109 [42] | −75 |
| NbC | 3610 | 20 | −133.27 | −1.06 | 5.620 | n.a. | |
| TaC | 3985 | 15 | −140.70 | −1.17 | 4.975 | −133 | |
| VIB | Cr3C2 | 1810 | 75 | −114.32 | n.a. | n.a. | −27 |
| Mo2C | 2520 | 57 | −62.13 | −0.18 * | n.a. | −32 | |
| WC | 2776 | 17 | −34.01 | −0.24 | 4.289 [43] | −14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bober, M.; Senkara, J.; Li, H. Comparative Analysis of the Phase Interaction in Plasma Surfaced NiBSi Overlays with IVB and VIB Transition Metal Carbides. Materials 2021, 14, 6617. https://doi.org/10.3390/ma14216617
Bober M, Senkara J, Li H. Comparative Analysis of the Phase Interaction in Plasma Surfaced NiBSi Overlays with IVB and VIB Transition Metal Carbides. Materials. 2021; 14(21):6617. https://doi.org/10.3390/ma14216617
Chicago/Turabian StyleBober, Mariusz, Jacek Senkara, and Hong Li. 2021. "Comparative Analysis of the Phase Interaction in Plasma Surfaced NiBSi Overlays with IVB and VIB Transition Metal Carbides" Materials 14, no. 21: 6617. https://doi.org/10.3390/ma14216617
APA StyleBober, M., Senkara, J., & Li, H. (2021). Comparative Analysis of the Phase Interaction in Plasma Surfaced NiBSi Overlays with IVB and VIB Transition Metal Carbides. Materials, 14(21), 6617. https://doi.org/10.3390/ma14216617






