Efficient Use of Graphene Oxide and Silica Fume in Cement-Based Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
2.3. Specimen Testing
3. Results
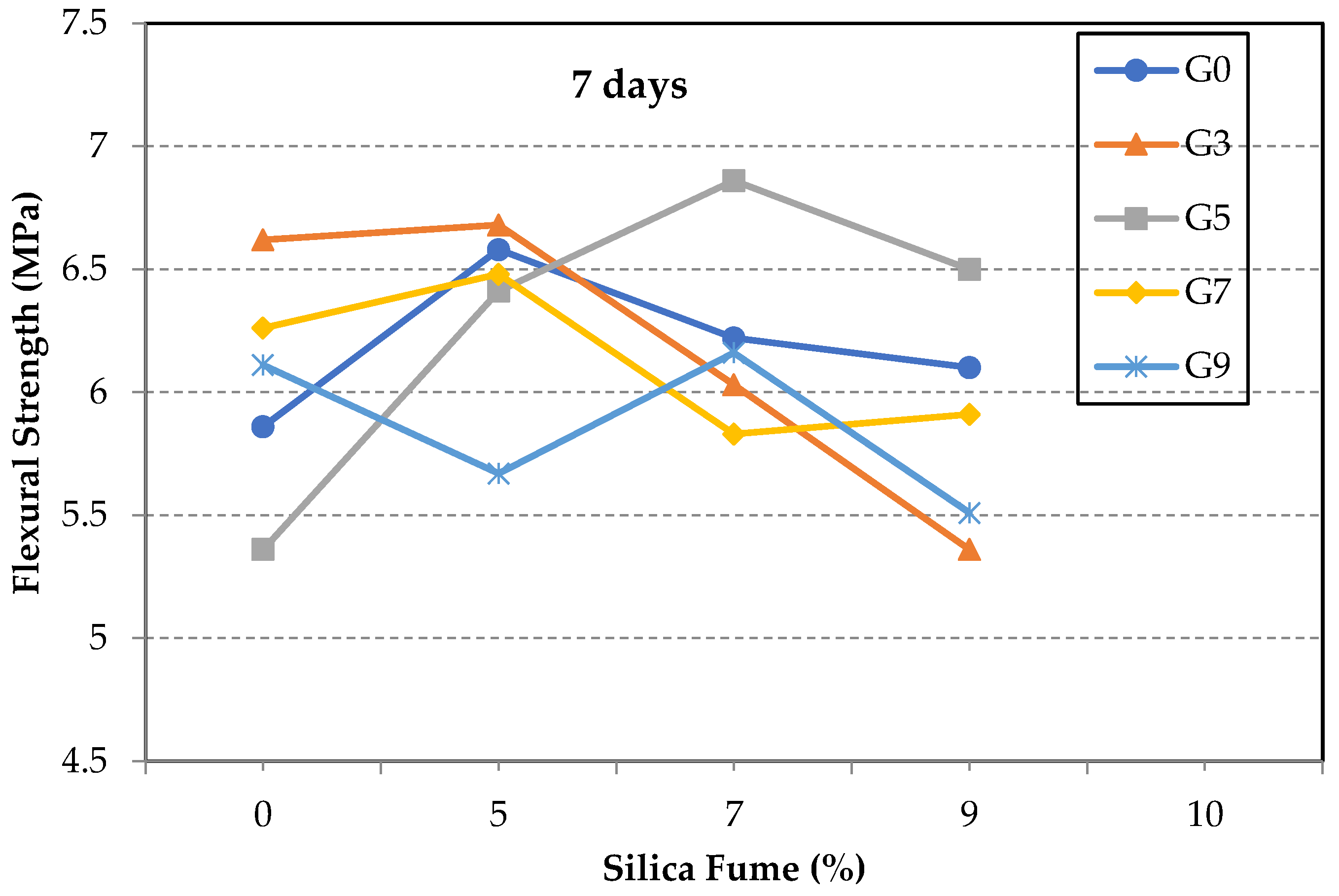
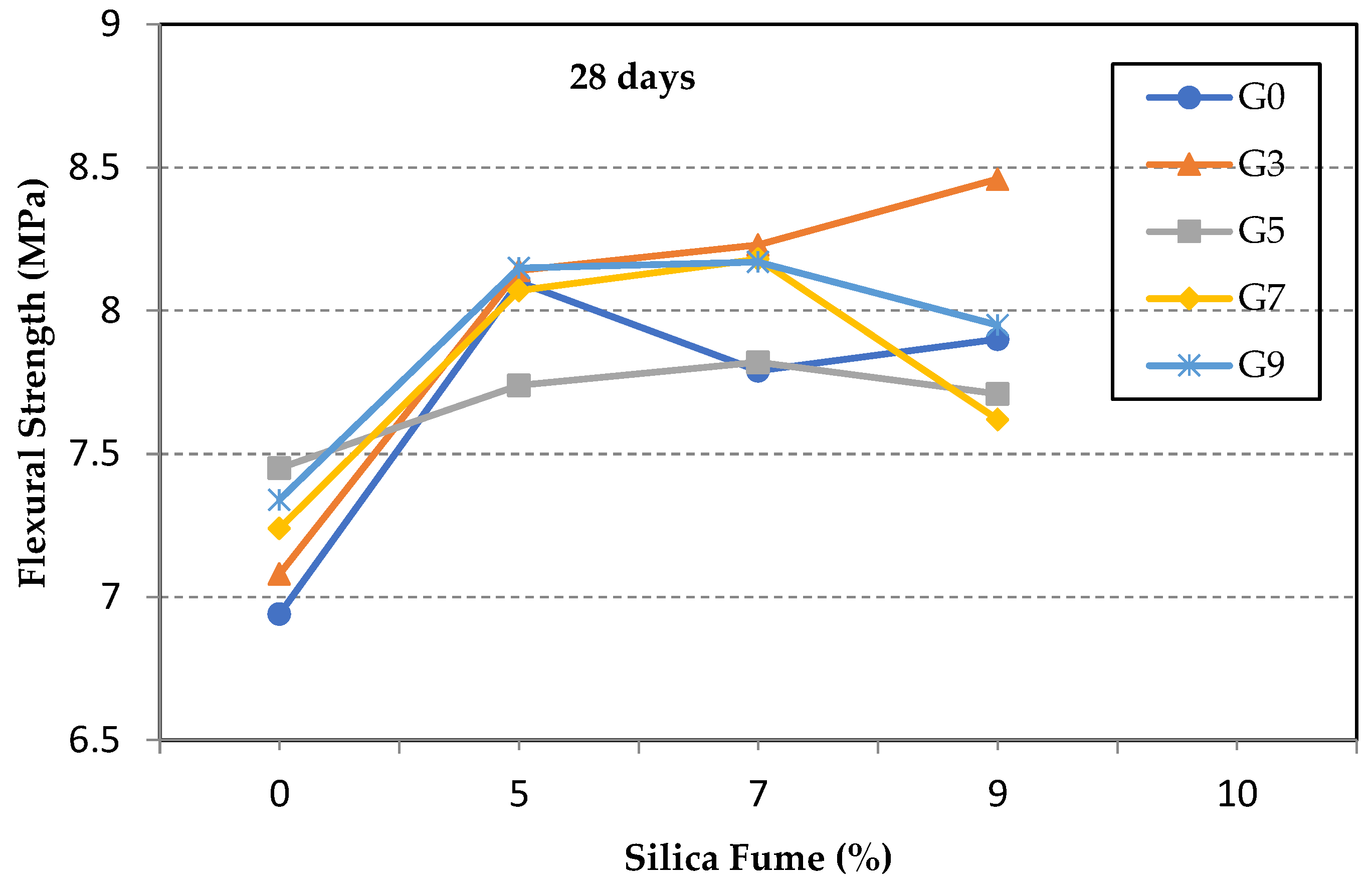
3.1. Flexural Strength of GO/SF Cement Composite
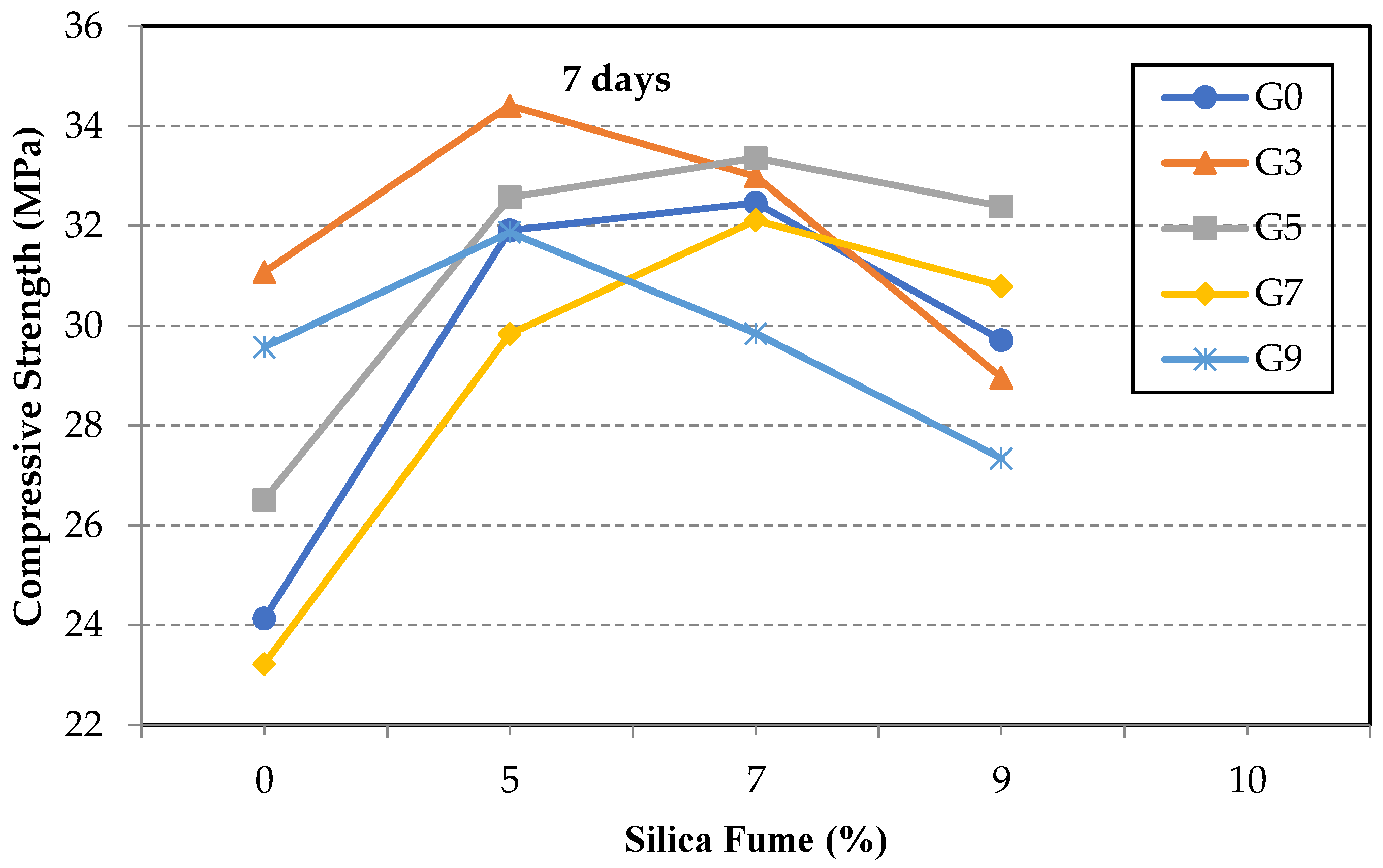
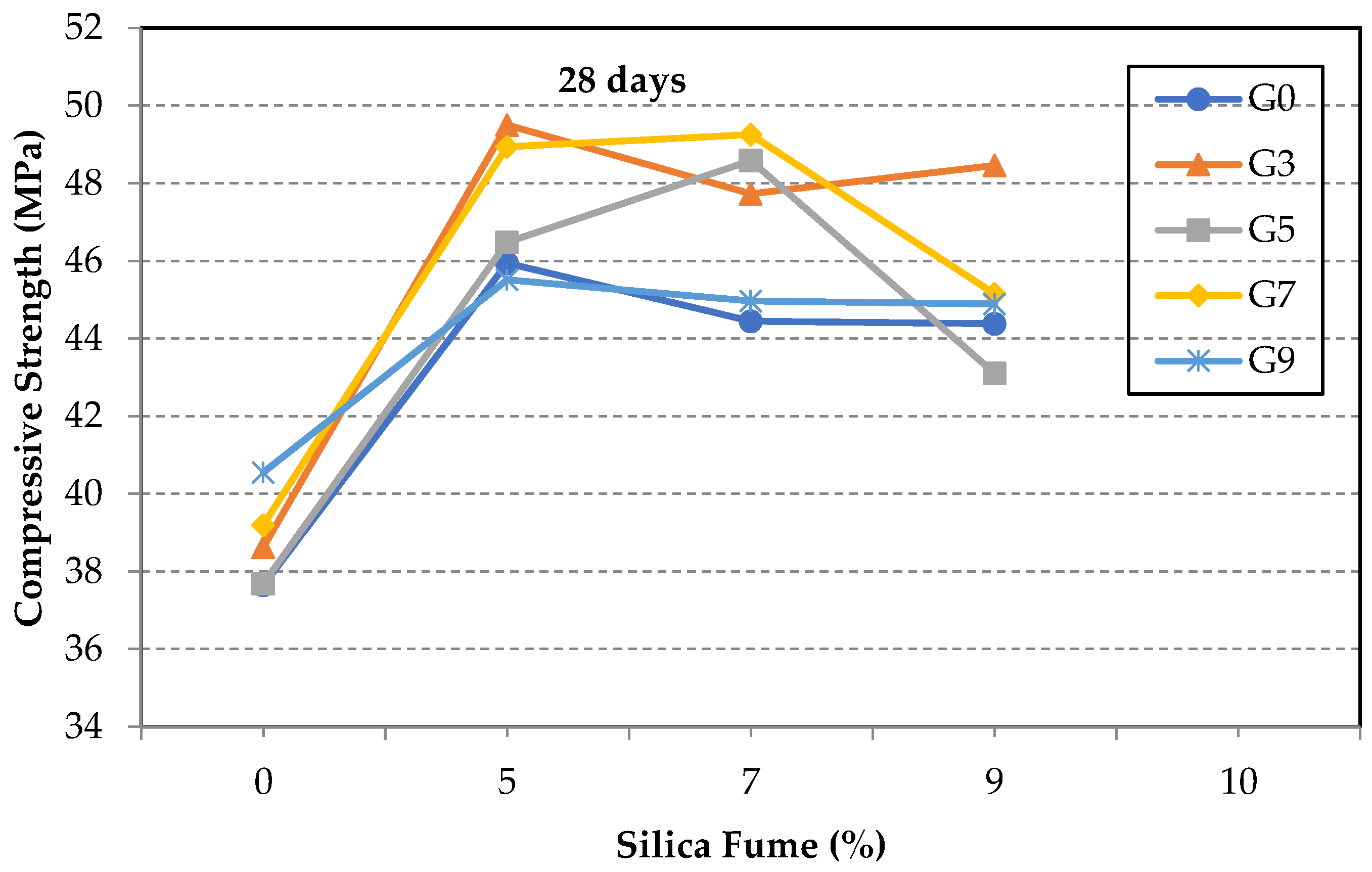
3.2. Compressive Strength of GO/SF Cement Composite
3.3. Microstructure of GO/SF Cement Composite
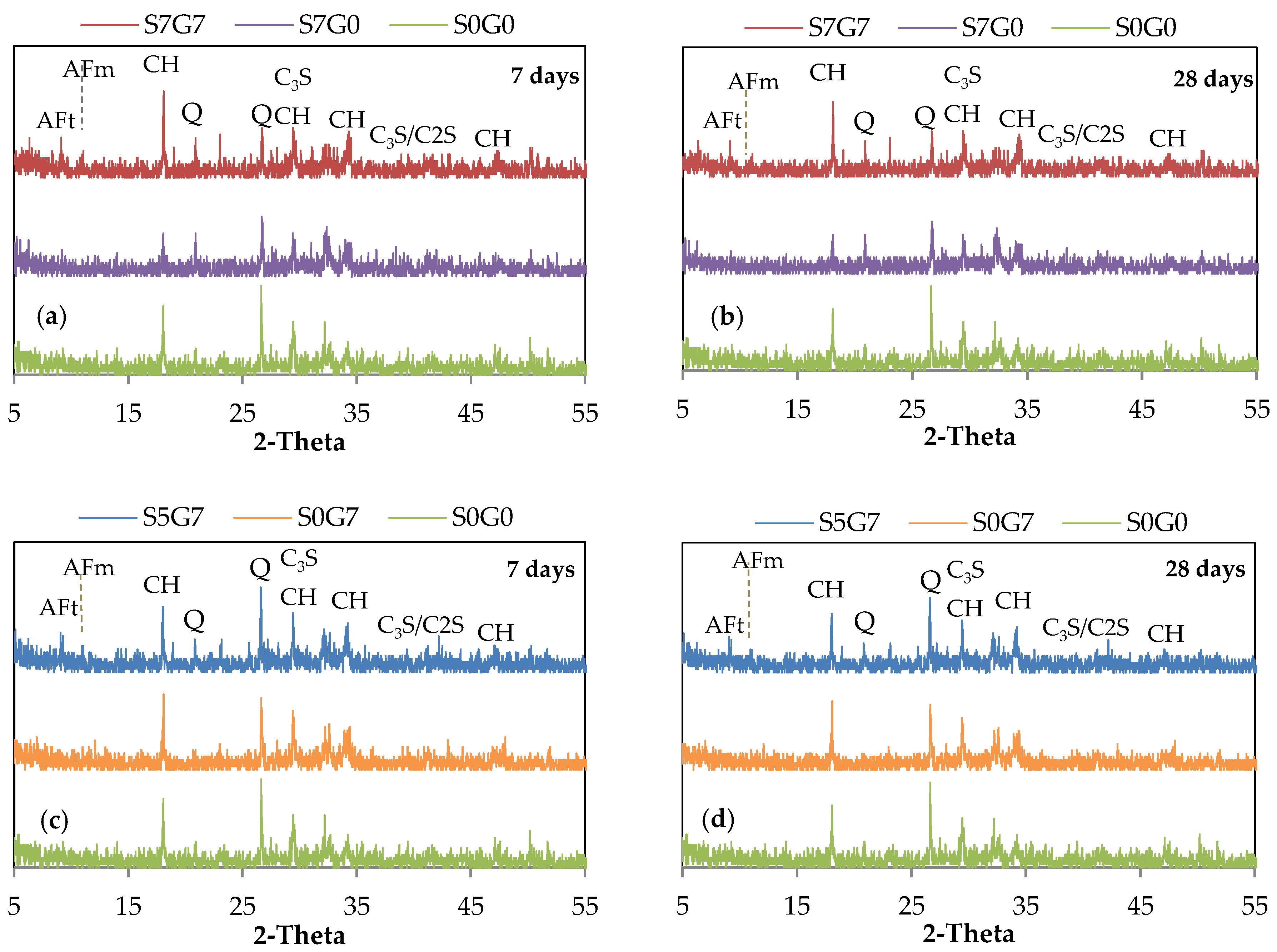
3.4. XRD Analysis of GO/SF Cement Composite
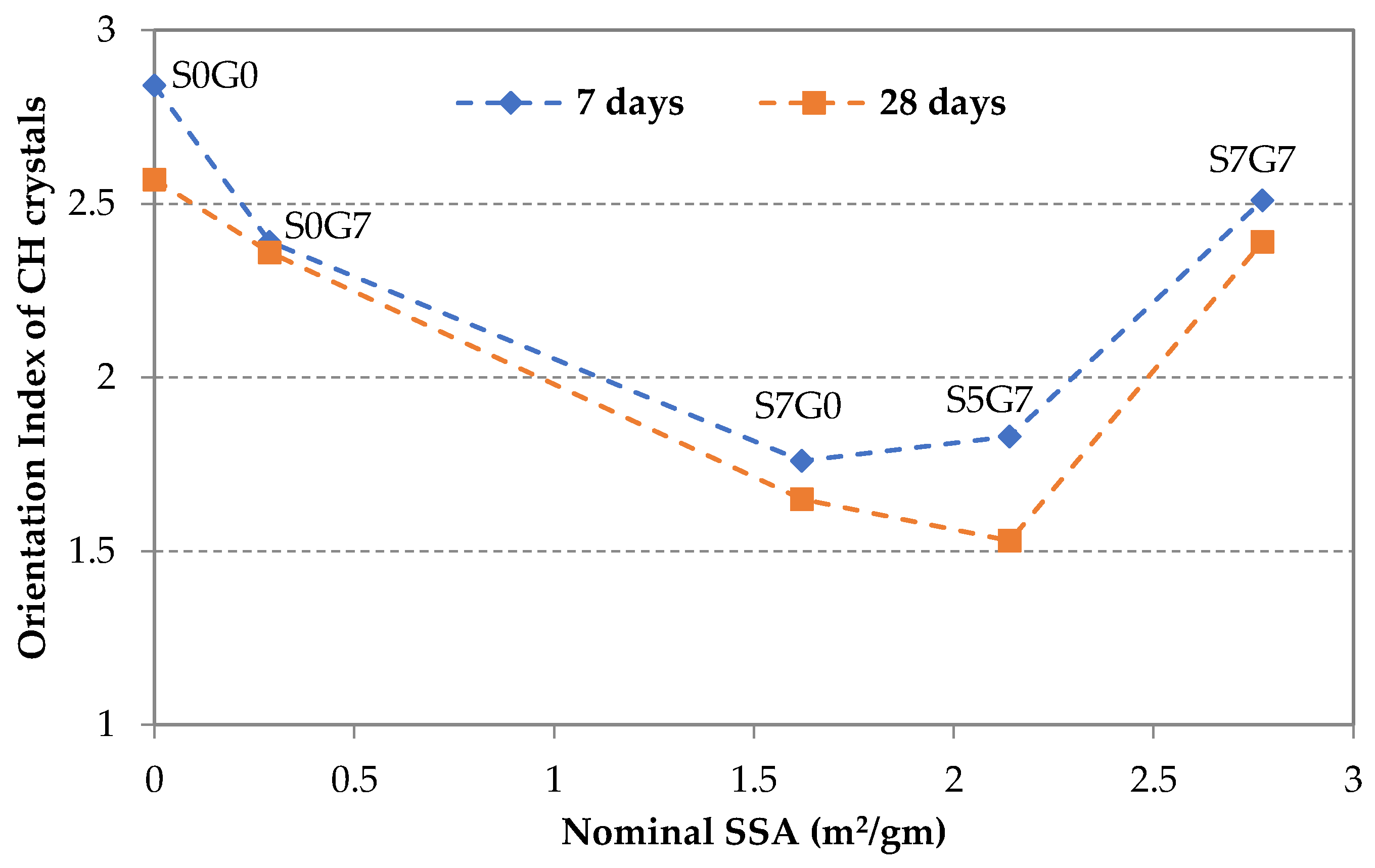
3.5. CH Orientation at the Fracture Surface of GO/SF Cement Composite
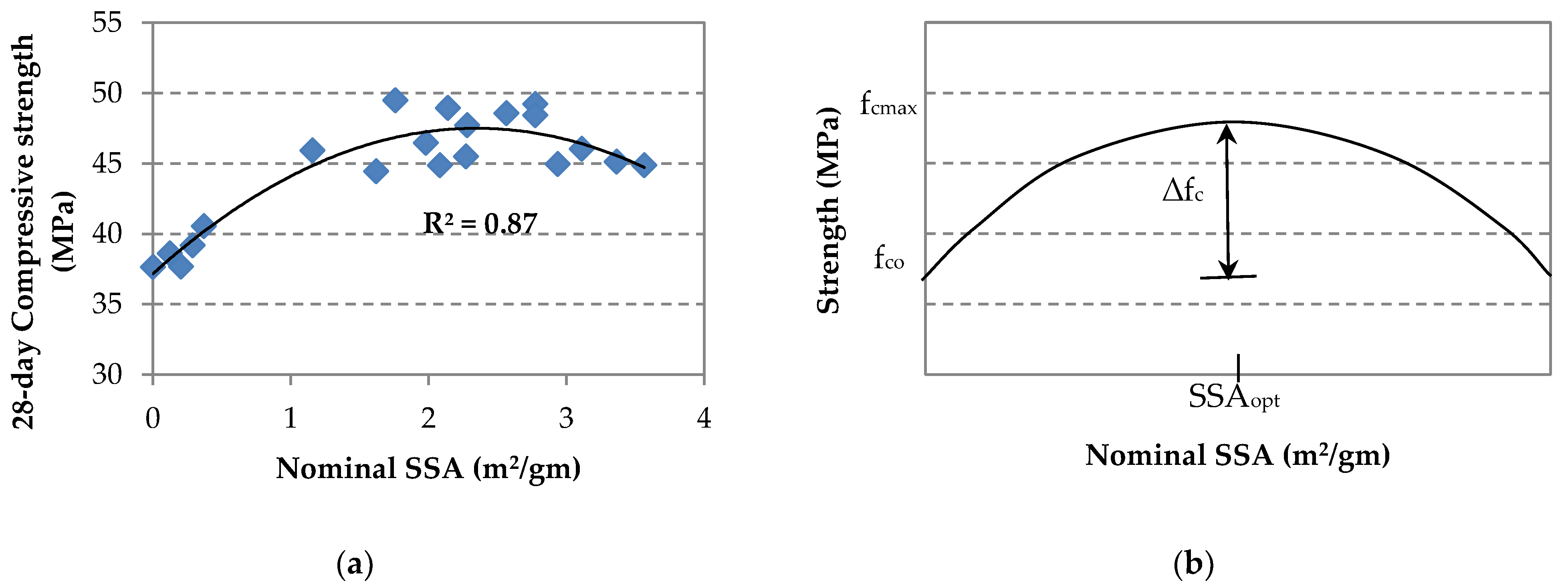
4. Estimation of Strength Enhancement in GO/SF Cement Composite
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lv, S.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J.; Zhou, Q. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, J.; Ouyang, D. Effect of Graphene Oxide on Mechanical Properties of Cement Mortar and its Strengthening. Materials 2019, 12, 3753. [Google Scholar] [CrossRef] [Green Version]
- Long, W.-J.; Wei, J.-J.; Xing, F.; Khayat, K.H. Enhanced dynamic mechanical properties of cement paste modified with graphene oxide nanosheets and its reinforcing mechanism. Cem. Concr. Compos. 2018, 93, 127–139. [Google Scholar] [CrossRef]
- Wang, J.; Dong, S.; Yu, X.; Han, B. Mechanical properties of graphene-reinforced reactive powder concrete at different strain rates. J. Mater. Sci. 2020, 55, 3369–3387. [Google Scholar] [CrossRef]
- Lu, Z.; Hou, D.; Meng, L.; Sun, G.; Lu, C.; Li, Z. Mechanism of cement paste reinforced by graphene oxide/carbon nanotubes composites with enhanced mechanical properties. RSC Adv. 2015, 5, 100598–100605. [Google Scholar] [CrossRef]
- Mohammed, A.; Al-Saadi, N.T.K.; Al-Mahaidi, R. Utilization of Graphene Oxide to Synthesize High-Strength Cement-Based Adhesive. J. Mater. Civ. Eng. 2017, 29, 04016258. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano reinforced cement and concrete composites and new perspective from graphene oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.M.; Li, W.G.; Li, C.Y.; Sanjayan, J.G.; Duan, W.H.; Li, Z. Effects of graphene oxide agglomerates on workability, hydration, microstructure and compressive strength of cement paste. Constr. Build. Mater. 2017, 145, 402–410. [Google Scholar] [CrossRef]
- Mohammed, A.; Sanjayan, J.G.; Duan, W.H.; Nazari, A. Graphene Oxide Impact on Hardened Cement Expressed in Enhanced Freeze-Thaw Resistance. J. Mater. Civ. Eng. 2016, 28, 04016072. [Google Scholar] [CrossRef]
- Mohammed, A.; Sanjayan, J.G.; Duan, W.H.; Nazari, A. Incorporating graphene oxide in cement composites: A study of transport properties. Constr. Build. Mater. 2015, 84, 341–347. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Lu, C.-X.; Liu, B.-W.; Zhang, K.; Li, C.-Z. Influence of graphene oxide additions on the microstructure and mechanical strength of cement. New Carbon Mater. 2015, 30, 349–356. [Google Scholar] [CrossRef]
- Kim, H.K.; Nam, I.W.; Lee, H.-K. Enhanced effect of carbon nanotube on mechanical and electrical properties of cement composites by incorporation of silica fume. Compos. Struct. 2014, 107, 60–69. [Google Scholar] [CrossRef]
- Bai, S.; Jiang, L.; Xu, N.; Jin, M.; Jiang, S. Enhancement of mechanical and electrical properties of graphene/cement composite due to improved dispersion of graphene by addition of silica fume. Constr. Build. Mater. 2018, 164, 433–441. [Google Scholar] [CrossRef]
- Ovid’Ko, I.A. Mechanical properties of graphene. Rev. Adv. Mater. Sci. 2013, 34, 1–11. [Google Scholar]
- Gao, W. The Chemistry of Graphene Oxide. In Graphene Oxide: Reduction Recipes, Spectroscopy, and Applications; Gao, W., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 61–95. [Google Scholar] [CrossRef]
- Du, H.; Pang, S.D. Dispersion and stability of graphene nanoplatelet in water and its influence on cement composites. Construction and Building Materials 2018, 167, 403–413. [Google Scholar] [CrossRef]
- Shamsaei, E.; de Souza, F.B.; Yao, X.; Benhelal, E.; Akbari, A.; Duan, W. Graphene-based nanosheets for stronger and more durable concrete: A review. Constr. Build. Mater. 2018, 183, 642–660. [Google Scholar] [CrossRef]
- Suk, J.W.; Piner, R.D.; An, J.; Ruoff, R.S. Mechanical Properties of Monolayer Graphene Oxide. ACS Nano 2010, 4, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W.; Nguyen, S.T.; Ruoff, R.S. Graphene oxide papers modified by divalent ions—Enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide-cement composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Gong, K.; Pan, Z.; Korayem, A.H.; Qiu, L.; Li, D.; Collins, F.; Wang, C.M.; Duan, W.H. Reinforcing Effects of Graphene Oxide on Portland Cement Paste. J. Mater. Civ. Eng. 2015, 27, A4014010. [Google Scholar] [CrossRef]
- Birenboim, M.; Nadiv, R.; Alatawna, A.; Buzaglo, M.; Schahar, G.; Lee, J.; Kim, G.; Peled, A.; Regev, O. Reinforcement and workability aspects of graphene-oxide-reinforced cement nanocomposites. Compos. Part B Eng. 2019, 161, 68–76. [Google Scholar] [CrossRef]
- Zhu, X.; Kang, X. Effect of graphene oxide (GO) on the hydration and dissolution of alite in a synthetic cement system. J. Mater. Sci. 2020, 55, 3419–3433. [Google Scholar] [CrossRef]
- Lu, Z.; Li, X.; Hanif, A.; Chen, B.; Parthasarathy, P.; Yu, J.; Li, Z. Early-age interaction mechanism between the graphene oxide and cement hydrates. Constr. Build. Mater. 2017, 152, 232–239. [Google Scholar] [CrossRef]
- Chuah, S.; Li, W.; Chen, S.J.; Sanjayan, J.G.; Duan, W.H. Investigation on dispersion of graphene oxide in cement composite using different surfactant treatments. Constr. Build. Mater. 2018, 161, 519–527. [Google Scholar] [CrossRef]
- Liu, J.; Li, Q.; Xu, S. Reinforcing Mechanism of Graphene and Graphene Oxide Sheets on Cement-Based Materials. J. Mater. Civ. Eng. 2019, 31, 04019014. [Google Scholar] [CrossRef]
- Tyson, B.M.; Al-Rub, R.K.A.; Yazdanbakhsh, A.; Grasley, Z. Carbon Nanotubes and Carbon Nanofibers for Enhancing the Mechanical Properties of Nanocomposite Cementitious Materials. J. Mater. Civ. Eng. 2011, 23, 1028–1035. [Google Scholar] [CrossRef]
- Baomin, W.; Shuang, D. Effect and mechanism of graphene nanoplatelets on hydration reaction, mechanical properties and microstructure of cement composites. Constr. Build. Mater. 2019, 228, 116720. [Google Scholar] [CrossRef]
- Reales, O.A.M.; Duda, P.; Filho, R.D.T. Effect of a Carbon Nanotube/Surfactant Aqueous Dispersion on the Rheological and Mechanical Properties of Portland Cement Pastes. J. Mater. Civ. Eng. 2018, 30, 04018259. [Google Scholar] [CrossRef]
- Liu, J.; Fu, J.; Yang, Y.; Gu, C. Study on dispersion, mechanical and microstructure properties of cement paste incorporating graphene sheets. Constr. Build. Mater. 2019, 199, 1–11. [Google Scholar] [CrossRef]
- Snellings, R.; Mertens, G.; Cizer, Ö.; Elsen, J. Early age hydration and pozzolanic reaction in natural zeolite blended cements: Reaction kinetics and products by in situ synchrotron X-ray powder diffraction. Cem. Concr. Res. 2010, 40, 1704–1713. [Google Scholar] [CrossRef]
- Zelić, J.; Rušić, D.; Veža, D.; Krstulović, R. The role of silica fume in the kinetics and mechanisms during the early stage of cement hydration. Cem. Concr. Res. 2000, 30, 1655–1662. [Google Scholar] [CrossRef]
- Roy, R.; Mitra, A.; Ganesh, A.T.; Sairam, V. Effect of Graphene Oxide Nanosheets dispersion in cement mortar composites incorporating Metakaolin and Silica Fume. Constr. Build. Mater. 2018, 186, 514–524. [Google Scholar] [CrossRef]
- Li, X.; Korayem, A.H.; Li, C.; Liu, Y.; He, H.; Sanjayan, J.G.; Duan, W.H. Incorporation of graphene oxide and silica fume into cement paste: A study of dispersion and compressive strength. Constr. Build. Mater. 2016, 123, 327–335. [Google Scholar] [CrossRef]
- Lu, Z.; Hou, D.; Hanif, A.; Hao, W.; Li, Z.; Sun, G. Comparative evaluation on the dispersion and stability of graphene oxide in water and cement pore solution by incorporating silica fume. Cem. Concr. Compos. 2018, 94, 33–42. [Google Scholar] [CrossRef]
- Sanchez, F.; Ince, C. Microstructure and macroscopic properties of hybrid carbon nanofiber/silica fume cement composites. Compos. Sci. Technol. 2009, 69, 1310–1318. [Google Scholar] [CrossRef]
- BS EN 197-1. Cement—Part 1: Composition, Specifications and Conformity Criteria for Common Cements; BSI: London, UK, 2011. [Google Scholar]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Montes-Navajas, P.; Asenjo, N.G.; Santamaría, R.; Menéndez, R.; Corma, A.; García, H. Surface Area Measurement of Graphene Oxide in Aqueous Solutions. Langmuir 2013, 29, 13443–13448. [Google Scholar] [CrossRef]
- Esmaeili, A.; Entezari, M. Facile and fast synthesis of graphene oxide nanosheets via bath ultrasonic irradiation. J. Colloid Interface Sci. 2014, 432C, 19–25. [Google Scholar] [CrossRef]
- Alazmi, A.; El Tall, O.; Rasul, S.; Hedhili, M.N.; Patole, S.P.; Costa, P.M.F.J. A process to enhance the specific surface area and capacitance of hydrothermally reduced graphene oxide. Nanoscale 2016, 8, 17782–17787. [Google Scholar] [CrossRef] [PubMed]
- BS EN 196-1. Methods of Testing Cement. Determination of Strength; BSI: London, UK, 2005. [Google Scholar]
- Zaaba, N.I.; Foo, K.L.; Hashim, U.; Tan, S.J.; Liu, W.-W.; Voon, C.H. Synthesis of Graphene Oxide using Modified Hummers Method: Solvent Influence. Procedia Eng. 2017, 184, 469–477. [Google Scholar] [CrossRef]
- ASTM. ASTM C109/C109M-16a. In Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- ASTM. ASTM C348-14. In Standard Test Method for Flexural Strength of Hydraulic-Cement Mortars; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Ghazizadeh, S.; Du, P.; Skipper, N.T.; Bai, Y. Understanding the behaviour of graphene oxide in Portland cement paste. Cem. Concr. Res. 2018, 111, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Babak, F.; Abolfazl, H.; Alimorad, R.; Parviz, G. Preparation and mechanical properties of graphene oxide: Cement nanocomposites. Sci. World J. 2014, 2014, 1–10. [Google Scholar] [CrossRef]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of nano-SiO2 addition on properties of hardened cement paste as compared with silica fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Kudžma, A.; Škamat, J.; Stonys, R.; Krasnikovs, A.; Kuznetsov, D.; Girskas, G.; Antonovič, V. Study on the Effect of Graphene Oxide with Low Oxygen Content on Portland Cement Based Composites. Materials 2019, 12, 802. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.; Yu, Y.; Qian, X.; Zhan, S.; Qian, K. Effect of nano-TiO2 on the mechanical properties of cement mortar. Constr. Build. Mater. 2012, 29, 241–245. [Google Scholar] [CrossRef]
- Grandet, J.; Ollivier, J.P. Etude de la formation du monocarboaluminate de calcium hydrate au contact d’un granulat calcaire dans une pate de ciment portland. Cem. Concr. Res. 1980, 10, 759–770. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
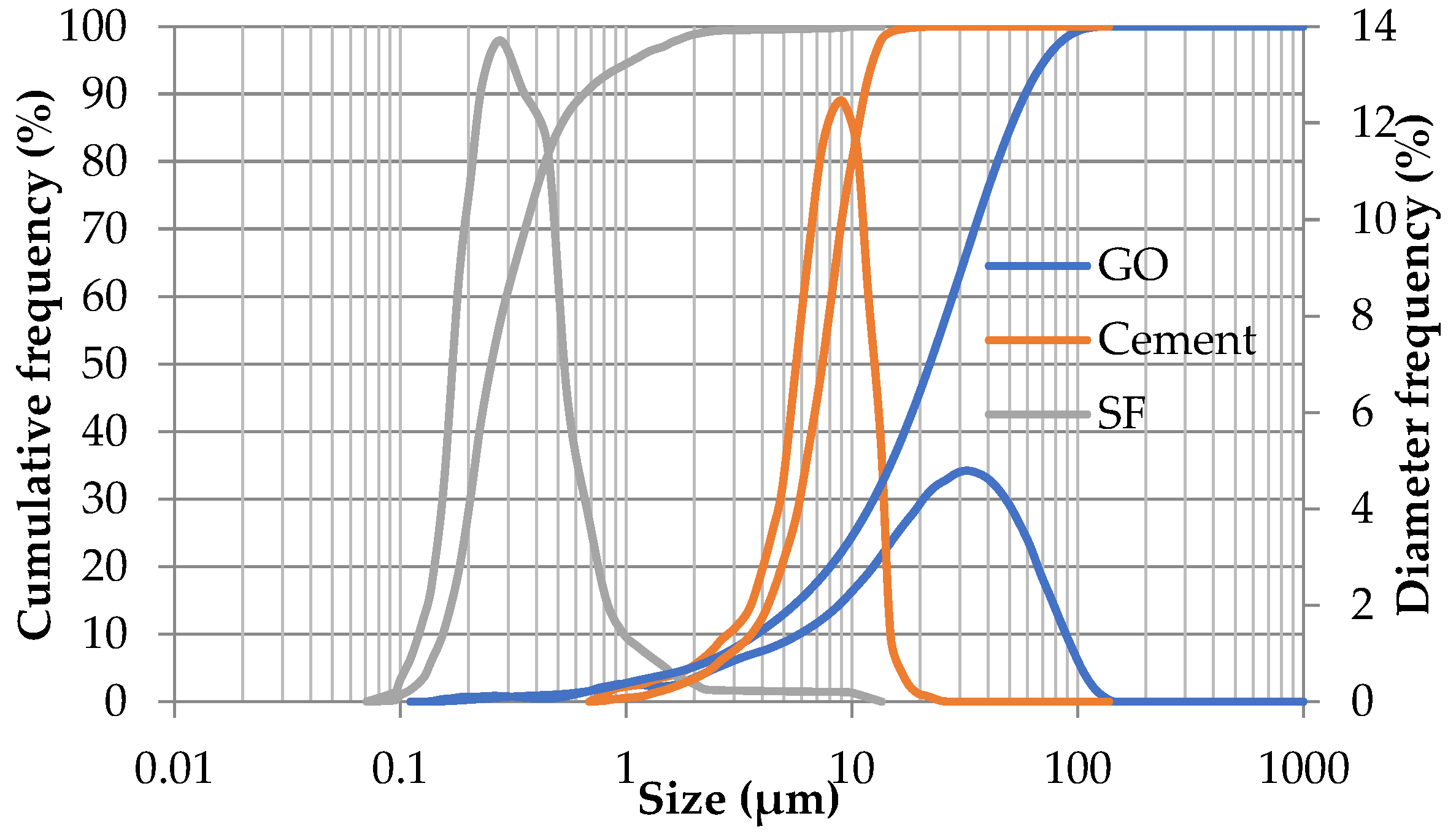
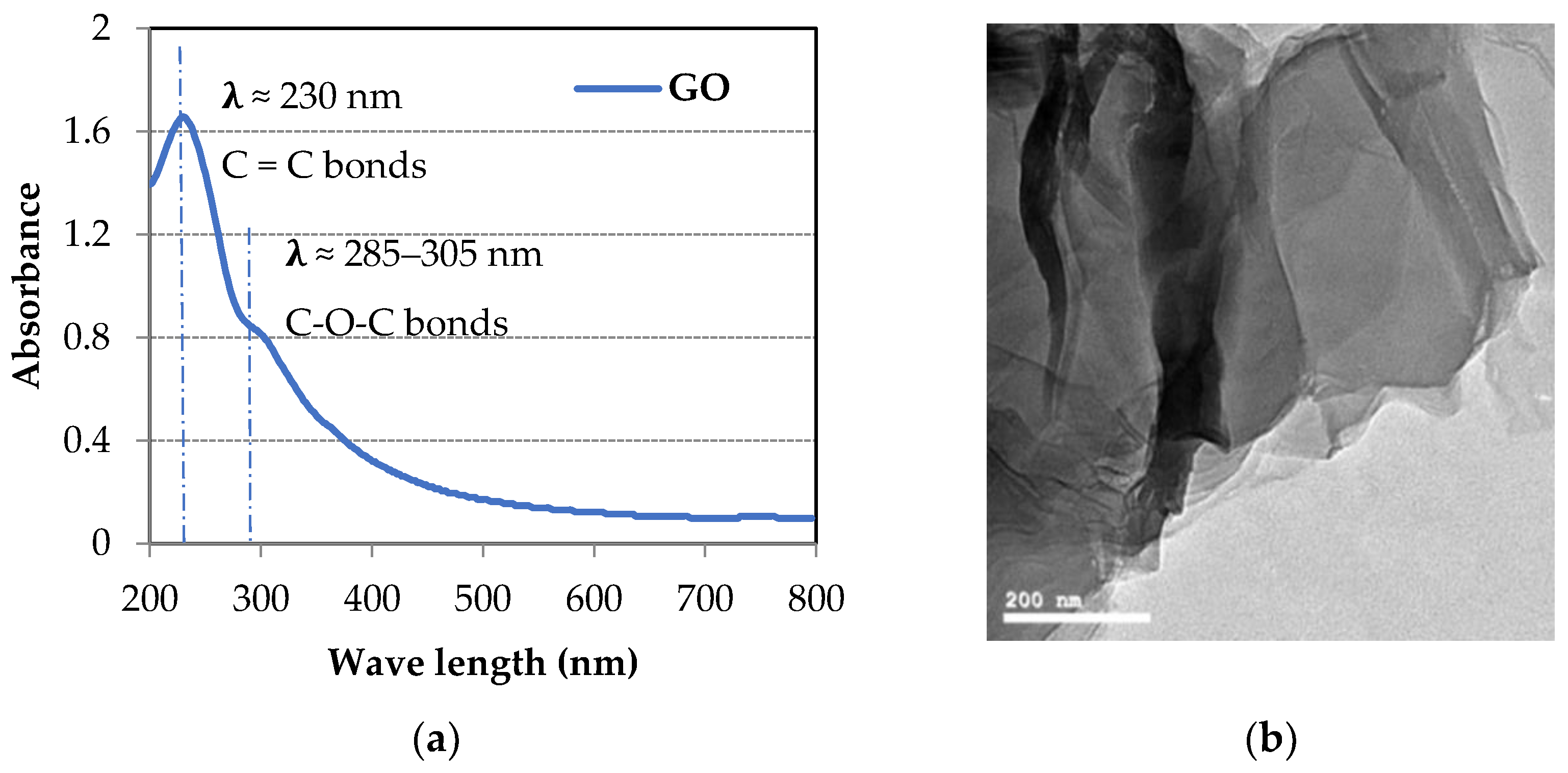


| Material | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | Na2O | K2O |
|---|---|---|---|---|---|---|---|---|
| Cement | 23.77 | 5.41 | 6.2 | 57.14 | 2.28 | 2.25 | 0.64 | 0.25 |
| SF | 93.4 | 0.8 | 1.28 | 0.32 | 0.75 | - | - | 0.35 |
| Test Specimen | Water/Cement Ratio | Binder:Sand | SF/Cement Weight % | GO/Cement Weight % |
|---|---|---|---|---|
| S0G0 | 0.485 | 1:2.75 | 0 | 0 |
| S0G3 | 0.485 | 1:2.75 | 0 | 0.03 |
| S0G5 | 0.485 | 1:2.75 | 0 | 0.05 |
| S0G7 | 0.485 | 1:2.75 | 0 | 0.07 |
| S0G9 | 0.485 | 1:2.75 | 0 | 0.09 |
| S5G0 | 0.485 | 1:2.75 | 5 | 0 |
| S5G3 | 0.485 | 1:2.75 | 5 | 0.03 |
| S5G5 | 0.485 | 1:2.75 | 5 | 0.05 |
| S5G7 | 0.485 | 1:2.75 | 5 | 0.07 |
| S5G9 | 0.485 | 1:2.75 | 5 | 0.09 |
| S7G0 | 0.485 | 1:2.75 | 7 | 0 |
| S7G3 | 0.485 | 1:2.75 | 7 | 0.03 |
| S7G5 | 0.485 | 1:2.75 | 7 | 0.05 |
| S7G7 | 0.485 | 1:2.75 | 7 | 0.07 |
| S7G9 | 0.485 | 1:2.75 | 7 | 0.09 |
| S9G0 | 0.485 | 1:2.75 | 9 | 0 |
| S9G3 | 0.485 | 1:2.75 | 9 | 0.03 |
| S9G5 | 0.485 | 1:2.75 | 9 | 0.05 |
| S9G7 | 0.485 | 1:2.75 | 9 | 0.07 |
| S9G9 | 0.485 | 1:2.75 | 9 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullah, A.; Taha, M.; Rashwan, M.; Fahmy, M. Efficient Use of Graphene Oxide and Silica Fume in Cement-Based Composites. Materials 2021, 14, 6541. https://doi.org/10.3390/ma14216541
Abdullah A, Taha M, Rashwan M, Fahmy M. Efficient Use of Graphene Oxide and Silica Fume in Cement-Based Composites. Materials. 2021; 14(21):6541. https://doi.org/10.3390/ma14216541
Chicago/Turabian StyleAbdullah, Ahmad, Mohamed Taha, Mohamed Rashwan, and Mohamed Fahmy. 2021. "Efficient Use of Graphene Oxide and Silica Fume in Cement-Based Composites" Materials 14, no. 21: 6541. https://doi.org/10.3390/ma14216541
APA StyleAbdullah, A., Taha, M., Rashwan, M., & Fahmy, M. (2021). Efficient Use of Graphene Oxide and Silica Fume in Cement-Based Composites. Materials, 14(21), 6541. https://doi.org/10.3390/ma14216541







