Original Approach to Synthesize TiO2/ZnO Hybrid Nanosponges Used as Photoanodes for Photoelectrochemical Applications
Abstract
:1. Introduction
2. Experimental Procedure
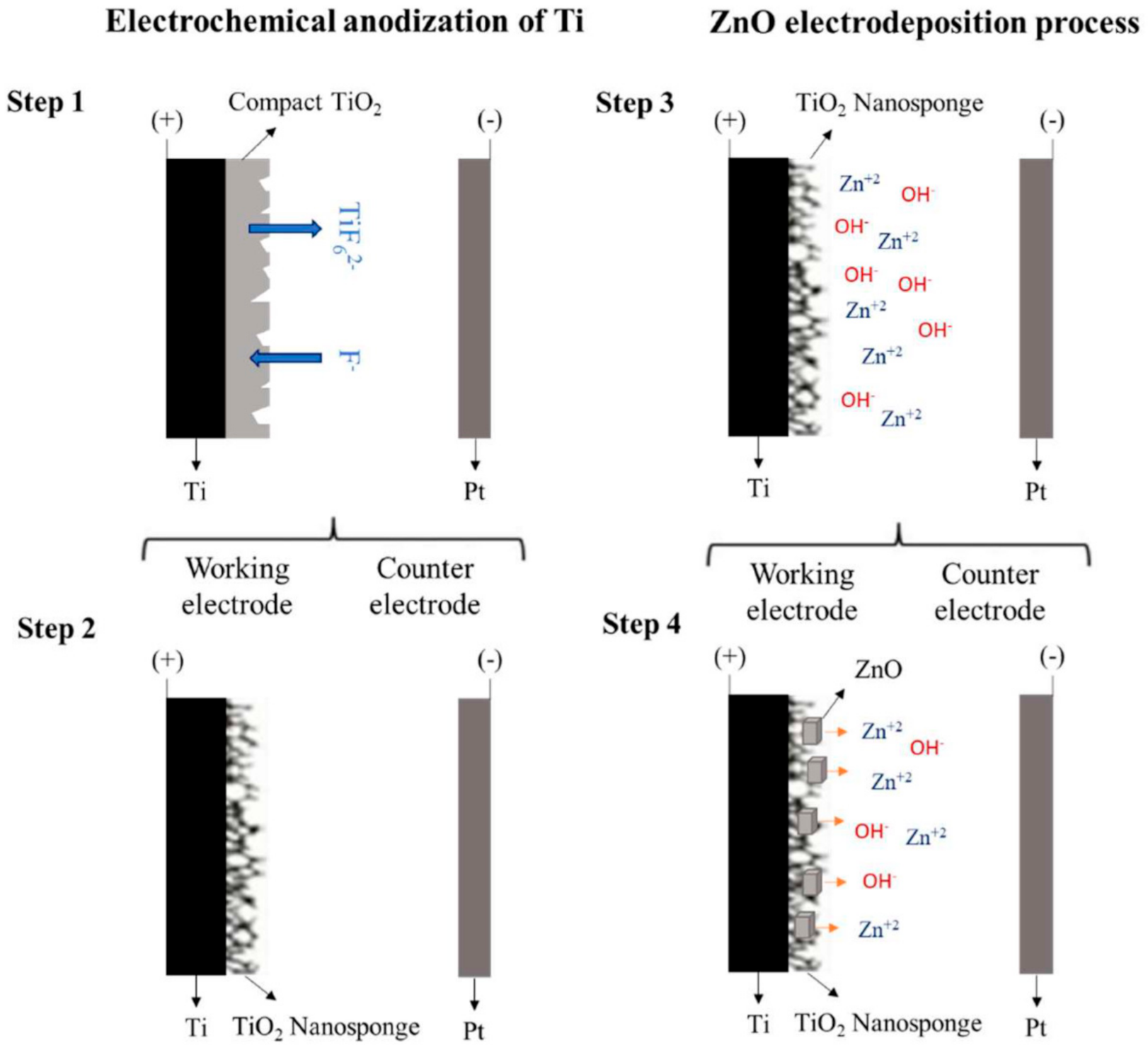
2.1. Preparation of the Photocatalysts
2.2. Morphological, Chemical, and Structural Characterization
2.3. Photoelectrochemical Water Splitting Tests
3. Results and Discussion
3.1. Synthesis of TiO2 Nanosponges
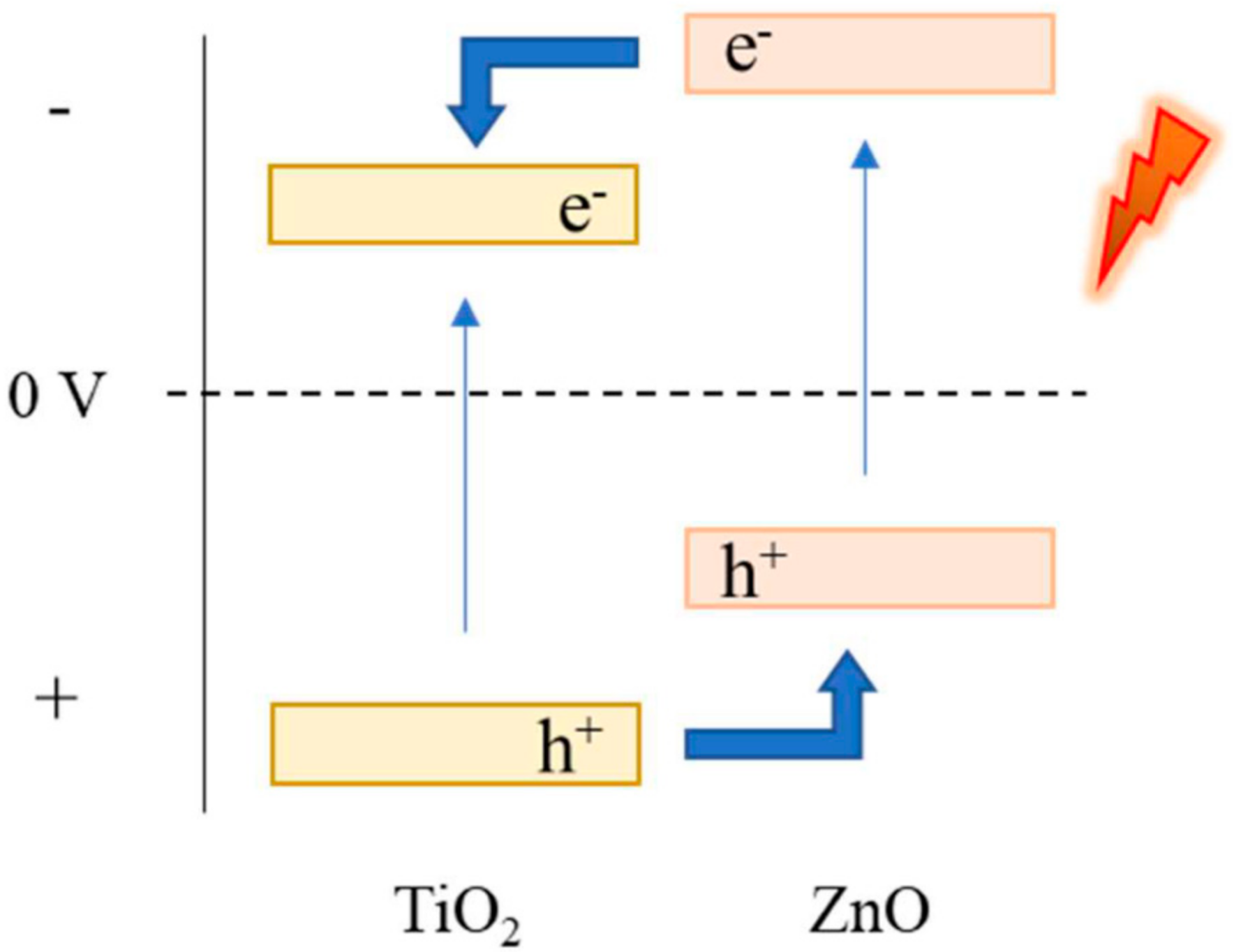
3.2. Synthesis of TiO2/ZnO Hybrid Nanosponges Used as Photoanodes
3.2.1. Influence of Electrodeposition Time
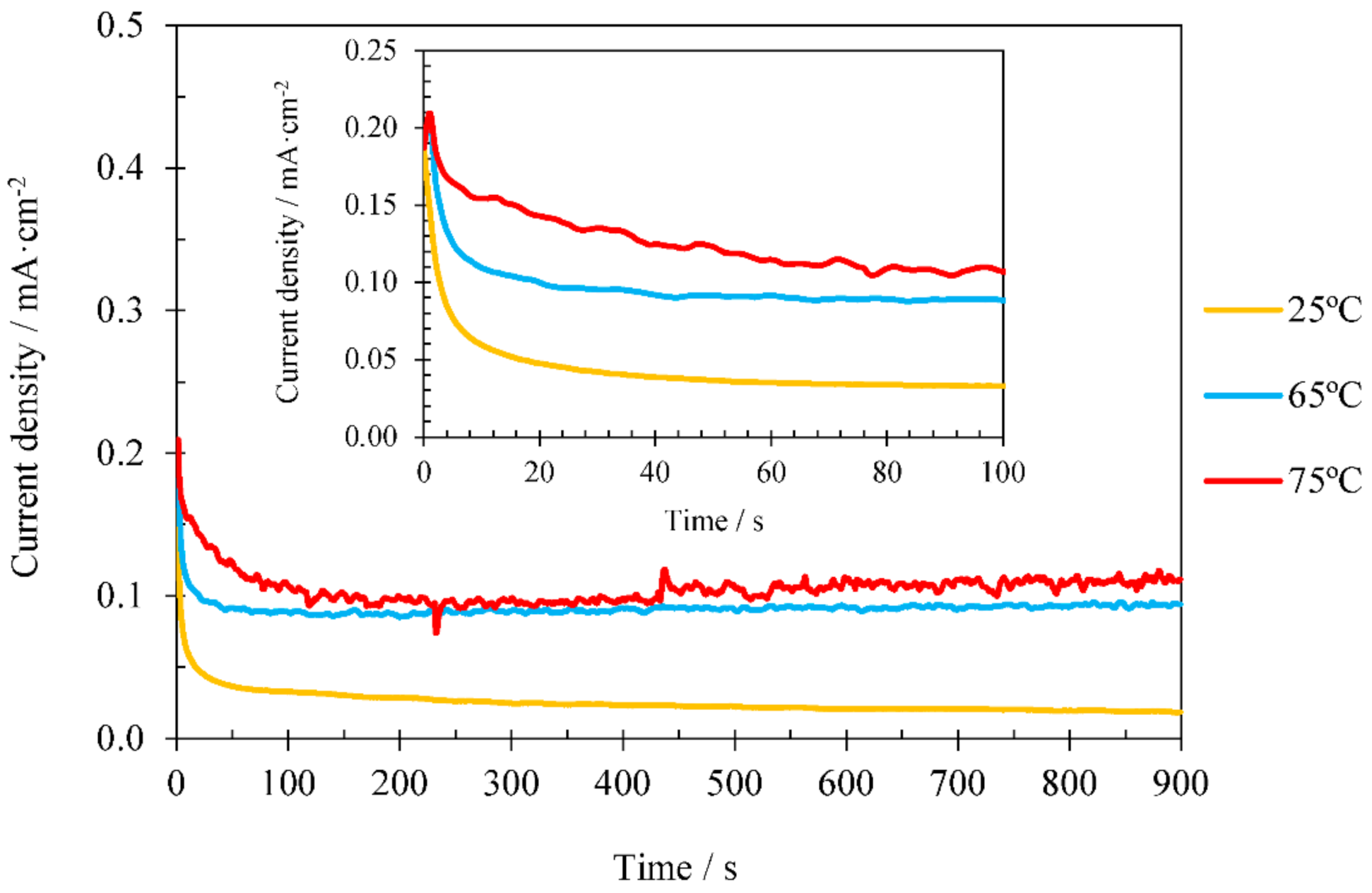
3.2.2. Influence of Electrodeposition Temperature
3.2.3. Influence of Zn(NO3)2 Concentration
3.3. Statistical Analysis
3.4. Chemical Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fonseca, J.D.; Camargo, M.; Commenge, J.M.; Falk, L.; Gil, I.D. Trends in design of distributed energy systems using hydrogen as energy vector: A systematic literature review. Int. J. Hydrog. Energy 2019, 4, 9486–9504. [Google Scholar] [CrossRef]
- Cabezas, M.D.; Frak, A.E.; Sanguinetti, A.; Franco, J.I.; Fasoli, H.J. Hydrogen energy vector: Demonstration pilot plant with minimal peripheral equipment. Int. J. Hydrog. Energy 2014, 39, 18165–18172. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- He, X.; Cai, Y.; Zhang, H.; Liang, C. Photocatalytic degradation of organic pollutants with Ag decorated free-standing TiO2 nanotube arrays and interface electrochemical response. J. Mater. Chem. 2011, 21, 475–480. [Google Scholar] [CrossRef]
- Liu, Z.; Pesic, B.; Raja, K.S.; Rangaraju, R.R.; Misra, M. Hydrogen generation under sunlight by self ordered TiO2 nanotube arrays. Int. J. Hydrog. Energy 2009, 34, 3250–3257. [Google Scholar] [CrossRef]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Wang, M.; Ioccozia, J.; Sun, L.; Lin, C.; Lin, Z. Inorganic-modified semiconductor TiO2 nanotube arrays for photocatalysis. Energy Environ. Sci. 2014, 7, 2182–2202. [Google Scholar] [CrossRef]
- Regonini, D.; Bowen, C.R.; Jaroenworaluck, A.; Stevens, R. A review of growth mechanism, structure and crystallinity of anodized TiO2 nanotubes. Mater. Sci. Eng. R Rep. 2013, 74, 377–406. [Google Scholar] [CrossRef] [Green Version]
- Roduner, E. Size matters: Why nanomaterials are different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Aijo John, K.; Naduvath, J.; Remillard, S.K.; Shaji, S.; DeYoung, P.A.; Kellner, Z.T.; Mallick, S.; Thankamoniamma, M.; Okram, G.S.; Philip, R.R. A simple method to fabricate metal doped TiO2 nanotubes. Chem. Phys. 2019, 523, 198–204. [Google Scholar] [CrossRef]
- Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-García, D.M.; García-Antón, J. Enhancement of photoelectrochemical activity for water splitting by controlling hydrodynamic conditions on titanium anodization. J. Power Sources 2015, 286, 224–231. [Google Scholar] [CrossRef]
- Guo, L.; Gao, G.; Liu, X.; Liu, F. Preparation and characterization of TiO2 nanosponge. Mater. Chem. Phys. 2008, 111, 322–325. [Google Scholar] [CrossRef]
- Blasco-Tamarit, E.; Muñoz-Portero, M.J.; Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-Antón, J. The effect of Reynolds number on TiO2 nanosponges doped with Li+ cations. New J. Chem. 2018, 42, 11054–11063. [Google Scholar] [CrossRef]
- Fernández-Domene, R.M.; Sánchez-Tovar, R.; Sánchez-González, S.; García-Antón, J. Photoelectrochemical characterization of anatase-rutile mixed TiO2 nanosponges. Int. J. Hydrog. Energy 2016, 41, 18380–18388. [Google Scholar] [CrossRef] [Green Version]
- Rosseler, O.; Shankar, M.V.; Karkmaz-Le Du, M.; Schmidlin, L.; Keller, N.; Keller, V. Solar light photocatalytic hydrogen production from water over Pt and Au/TiO2(anatase/rutile) photocatalysts: Influence of noble metal and porogen promotion. J. Catal. 2010, 269, 179–190. [Google Scholar] [CrossRef]
- Sánchez-Tovar, R.; Blasco-Tamarit, E.; Fernández-Domene, R.M.; Lucas-Granados, B.; García-Antón, J. Should TiO2 nanostructures doped with Li+ be used as photoanodes for photoelectrochemical water splitting applications? J. Catal. 2017, 349, 41–52. [Google Scholar] [CrossRef]
- Chen, L.-C.; Tsai, S.-F.; Chen, J.-H.; Wang, G.-W. Preparation of Vertically Aligned ZnO/TiO2 Core-Shell Composites for Dye-Sensitized Solar Cells. Int. J. Photoenergy 2013, 2013, 417964. [Google Scholar] [CrossRef]
- Liao, Y.; Zhang, K.; Wang, X.; Zhang, D.; Li, Y.; Su, H.; Zhang, H.; Zhong, Z. Preparation of ZnO@TiO2 nanotubes heterostructured film by thermal decomposition and their photocatalytic performances. RSC Adv. 2018, 8, 8064–8070. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Yu, J.; Liu, S.; Zhai, P.; Jiang, L. Effects of calcination temperatures on photocatalytic activity of SnO2/TiO2 composite films prepared by an EPD method. J. Hazard. Mater. 2008, 154, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.H.; Chiu, Y.H.; Shao, P.W.; Hsu, Y.J. Metal-Particle-Decorated ZnO Nanocrystals: Photocatalysis and Charge Dynamics. ACS Appl. Mater. Interfaces 2016, 8, 32754–32763. [Google Scholar] [CrossRef]
- Lu, J.; Jin, H.; Dai, Y.; Yang, K.; Huang, B. Effect of electronegativity and charge balance on the visible-light- responsive photocatalytic activity of nonmetal doped anatase TiO2. Int. J. Photoenergy 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.C.; Chou, C.Y.; Yi, S.R.; Chen, H.D. Deposition of heterojunction of ZnO on hydrogenated TiO2 nanotube arrays by atomic layer deposition for enhanced photoelectrochemical water splitting. Int. J. Hydrog. Energy 2019, 44, 28685–28697. [Google Scholar] [CrossRef]
- Lin, L.; Yang, Y.; Men, L.; Wang, X.; He, D.; Chai, Y.; Zhao, B.; Ghoshroy, S.; Tang, Q. A highly efficient TiO2@ZnO n-p-n heterojunction nanorod photocatalyst. Nanoscale 2013, 5, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yuan, Y.; Liang, L.; Cheng, Y.; Shi, G.; Jin, L. Preparation and photoelectrocatalytic activity of ZnO nanorods embedded in highly ordered TiO2 nanotube arrays electrode for azo dye degradation. J. Hazard. Mater. 2008, 158, 517–522. [Google Scholar] [CrossRef]
- Liu, W.; Su, P.; Chen, S.; Wang, N.; Ma, Y.; Liu, Y.; Wang, J.; Zhang, Z.; Li, H.; Webster, T.J. Synthesis of TiO2 nanotubes with ZnO nanoparticles to achieve antibacterial properties and stem cell compatibility. Nanoscale 2014, 6, 9050–9062. [Google Scholar] [CrossRef]
- Fan, J.; Zamani, R.; Fábrega, C.; Shavel, A.; Flox, C.; Ibáñez, M.; Andreu, T.; López, A.M.; Arbiol, J.; Morante, J.R.; et al. Solution-growth and optoelectronic performance of ZnO:Cl/TiO2 and ZnO:Cl/ZnxTiOy/TiO2 core-shell nanowires with tunable shell thickness. J. Phys. D Appl. Phys. 2012, 45, 415301. [Google Scholar] [CrossRef]
- Peleyeju, M.G.; Arotiba, O.A. Recent trend in visible-light photoelectrocatalytic systems for degradation of organic contaminants in water/wastewater. Environ. Sci. Water Res. Technol. 2018, 4, 1389–1411. [Google Scholar] [CrossRef]
- Subash, B.; Krishnakumar, B.; Swaminathan, M.; Shanthi, M. Highly efficient, solar active, and reusable photocatalyst: Zr-loaded Ag-ZnO for reactive red 120 dye degradation with synergistic effect and dye-sensitized mechanism. Langmuir 2013, 29, 939–949. [Google Scholar] [CrossRef]
- Movsesyan, L.; Maijenburg, A.W.; Goethals, N.; Sigle, W.; Spende, A.; Yang, F.; Kaiser, B.; Jaegermann, W.; Park, S.; Mul, G.; et al. ZnO Nanowire Networks as Photoanode Model Systems for Photoelectrochemical Applications. Nanomaterials 2018, 8, 693. [Google Scholar] [CrossRef] [Green Version]
- Nundy, S.; Eom, T.y.; Song, K.Y.; Park, J.S.; Lee, H.J. Hydrothermal synthesis of mesoporous ZnO microspheres as NOX gas sensor materials—Calcination effects on microstructure and sensing performance. Ceram. Int. 2020, 46, 19354–19364. [Google Scholar] [CrossRef]
- Pugazhendhi, K.; D’Almeida, S.; Kumar, P.N.; Mary, J.S.S.; Tenkyong, T.; Sharmila, D.J.; Madhavan, J.; Shyla, J.M. Hybrid TiO2 /ZnO and TiO2 /Al plasmon impregnated ZnO nanocomposite photoanodes for DSSCs: Synthesis and characterisation. Mater. Res. Express 2018, 5, 045053. [Google Scholar] [CrossRef]
- Perkgoz, N.K.; Toru, R.S.; Unal, E.; Sefunc, M.A.; Tek, S.; Mutlugun, E.; Soganci, I.M.; Celiker, H.; Celiker, G.; Demir, H.V. Photocatalytic hybrid nanocomposites of metal oxide nanoparticles enhanced towards the visible spectral range. Appl. Catal. B Environ. 2011, 105, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Tovar, R.; Blasco-Tamarit, E.; Fernández-Domene, R.M.; Villanueva-Pascual, M.; García-Antón, J. Electrochemical formation of novel TiO2-ZnO hybrid nanostructures for photoelectrochemical water splitting applications. Surf. Coat. Technol. 2020, 388, 125605. [Google Scholar] [CrossRef]
- Schmidt, S.; Greczynski, G.; Goyenola, C.; Gueorguiev, G.K.; Czigány, Z.; Jensen, J.; Ivanov, I.G.; Hultman, L. Surface & Coatings Technology CF x thin solid fi lms deposited by high power impulse magnetron sputtering: Synthesis and characterization. Surf. Coat. Technol. 2011, 206, 646–653. [Google Scholar]
- Gueorguiev, G.K.; Czigány, Z.; Furlan, A.; Stafström, S.; Hultman, L. Intercalation of P atoms in Fullerene-like CPx. Chem. Phys. Lett. 2011, 501, 400–403. [Google Scholar] [CrossRef] [Green Version]
- Gueorguiev, G.K.; Goyenola, C.; Schmidt, S.; Hultman, L. CF x: A first-principles study of structural patterns arising during synthetic growth. Chem. Phys. Lett. 2011, 516, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Dai, S.; Li, Y.; Du, Z.; Carter, K.R. Electrochemical Deposition of ZnO Hierarchical Nanostructures from Hydrogel Coated Electrodes. J. Electrochem. Soc. 2013, 160, D156–D162. [Google Scholar] [CrossRef] [Green Version]
- Therese, G.H.A.; Kamath, P.V. Electrochemical synthesis of metal oxides and hydroxides. Chem. Mater. 2000, 12, 1195–1204. [Google Scholar] [CrossRef]
- Jiang, S.; Wu, M.; Zhou, Y.; Wen, Y.; Yang, C.; Zhang, S. Effects of electrodeposition conditions on the microstructures of ZnO thin films. Integr. Ferroelectr. 2007, 88, 33–43. [Google Scholar] [CrossRef]
- Cho, H.W.; Liao, K.L.; Yang, J.S.; Wu, J.J. Revelation of rutile phase by Raman scattering for enhanced photoelectrochemical performance of hydrothermally-grown anatase TiO2 film. Appl. Surf. Sci. 2018, 440, 125–132. [Google Scholar] [CrossRef]
- Mozaffari, S.A.; Ranjbar, M.; Kouhestanian, E.; Amoli, H.S.; Armanmehr, M.H. An investigation on the effect of electrodeposited nanostructured ZnO on the electron transfer process efficiency of TiO2 based DSSC. Mater. Sci. Semicond. Process. 2015, 40, 285–292. [Google Scholar] [CrossRef]
- Samadipakchin, P.; Mortaheb, H.R.; Zolfaghari, A. ZnO nanotubes: Preparation and photocatalytic performance evaluation. J. Photochem. Photobiol. A Chem. 2017, 337, 91–99. [Google Scholar] [CrossRef]
- Skompska, M.; Zarebska, K. Electrodeposition of ZnO Nanorod Arrays on Transparent Conducting Substrates—A Review. Electrochim. Acta 2014, 127, 467–488. [Google Scholar] [CrossRef]
- Goux, A.; Pauporté, T.; Chivot, J.; Lincot, D. Temperature effects on ZnO electrodeposition. Electrochim. Acta 2005, 50, 2239–2248. [Google Scholar] [CrossRef]
- Otani, S.; Katayama, J.; Umemoto, H.; Matsuoka, M. Effect of Bath Temperature on the Electrodeposition Mechanism of Zinc Oxide Film from Zinc Nitrate Solution. J. Electrochem. Soc. 2006, 153, C551. [Google Scholar] [CrossRef]
- Yilmaz, C.; Unal, U. Effect of Zn(NO3)2 concentration in hydrothermal-electrochemical deposition on morphology and photoelectrochemical properties of ZnO nanorods. Appl. Surf. Sci. 2016, 368, 456–463. [Google Scholar] [CrossRef]
- Chen, H.; Zhu, L.; Liu, H.; Li, W. Effects of preparing conditions on the nanostructures electrodeposited from the Zn(NO3)2 electrolyte containing KCl. Thin Solid Films 2013, 534, 205–213. [Google Scholar] [CrossRef]
- Asib, N.A.M.; Juhaizat, K.A.; Mamat, M.H.; Rusop, M.; Khusaimi, Z. Influence of different stabilizers to the growth of ZnO nanostructures on TiO2 seed layer Influence of Different Stabilizers to the Growth of ZnO Nanostructures on TiO2 Seed Layer. AIP Conf. Proc. 2019, 2151, 020016. [Google Scholar]
- Liu, R.; Ye, H.; Xiong, X.; Liu, H. Fabrication of TiO2/ZnO composite nanofibers by electrospinning and their photocatalytic property. Mater. Chem. Phys. 2010, 121, 432–439. [Google Scholar] [CrossRef]
- Pérez-larios, A.; Lopez, R.; Hernández-gordillo, A.; Tzompantzi, F.; Gómez, R.; Torres-guerra, L.M. Improved hydrogen production from water splitting using TiO2—ZnO mixed oxides photocatalysts. Fuel 2012, 100, 139–143. [Google Scholar] [CrossRef]
- Konan, F.K.; Hartiti, B.; Batan, A.; Aka, B. X-ray diffraction, XPS, and Raman spectroscopy of coated ZnO:Al (1−7 at%) nanoparticles. e-J. Surf. Sci. Nanotechnol. 2019, 17, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Pérez-gonzález, M.; Tomás, S.A.; Santoyo-salazar, J.; Morales-luna, M. Enhanced photocatalytic activity of TiO2-ZnO thin fi lms deposited by dc reactive magnetron sputtering. Ceram. Int. 2017, 43, 8831–8838. [Google Scholar] [CrossRef]
- Moulder, C.; Jill., J.F. Handbook of X-ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics Division; Perkin-Elmer Corp: Eden Prairie, MN, USA, 1992; ISBN 0962702625. [Google Scholar]
- Liu, W.; Chen, S.; Zhang, Z.; Webster, T.J. Antibacterial properties of TiO2 nanotubes incorporated with ZnO. In Proceedings of the 2014 40th Annual Northeast Bioengineering Conference (NEBEC), Boston, MA, USA, 25–27 April 2014; pp. 14–15. [Google Scholar] [CrossRef]
- Fern, R.M. Surface & Coatings Technology Novel TiO2-WO3 self-ordered nanotubes used as photoanodes: Influence of Na2WO4 and H2O2 concentration during electrodeposition. Surf. Coat. Technol. 2021, 415, 127124. [Google Scholar]
- Ashraf, S.; Zainizan, M.; Nayan, N.; Embong, Z.; Rohaida, C.; Hak, C.; Adriyanto, F. Neutron beam interaction with rutile TiO2 single crystal (1 1 1): Raman and XPS study on Ti3+-oxygen vacancy formation. Mater. Lett. 2020, 263, 127143. [Google Scholar]
- Blasco-tamarit, E.; Solsona, B.; Sánchez-tovar, R.; García-garcía, D.; Fernández-domene, R.M. In fl uence of annealing atmosphere on photoelectrochemical response of TiO2 nanotubes anodized under controlled hydrodynamic conditions. J. Electroanal. Chem. 2021, 897, 115579. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Nishimura, D.; Doi, H.; Nomura, N.; Hanawa, T. Difference in surface reactions between titanium and zirconium in Hanks ’ solution to elucidate mechanism of calcium phosphate formation on titanium using XPS and cathodic polarization. Mater. Sci. Eng. C 2009, 29, 1702–1708. [Google Scholar] [CrossRef]
- Ghobadi, A.; Ulusoy, T.G.; Garifullin, R.; Guler, M.O.; Okyay, A.K. A Heterojunction Design of Single Layer Hole Tunneling ZnO Passivation Wrapping around TiO2 Nanowires for Superior Photocatalytic Performance. Nat. Publ. Gr. 2016, 6, 1–15. [Google Scholar]
- Kang, Q.; Cao, J.; Zhang, Y.; Liu, L.; Xu, H.; Ye, J. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. A 2013, 1, 5766–5774. [Google Scholar] [CrossRef]
- Muhamad, S.; Mohamed, H.; Naif, K.; Al, M.; Che, H.; Che, A. treatment route and evaluating the impact of various molar concentrations on the structure and optical behaviors. Appl. Phys. A 2020, 126, 1–15. [Google Scholar] [CrossRef]
- Li, G.; Zhao, Q.; Yang, H.; Liu, Z. Fabrication and characterization of ZnO-coated TiO2 nanotube arrays. Compos. Interfaces 2016, 23, 125–132. [Google Scholar] [CrossRef]
- Ewan, B.C.R.; Allen, R.W.K. A figure of merit assessment of the routes to hydrogen. Int. J. Hydrog. Energy 2005, 30, 809–819. [Google Scholar] [CrossRef]
- Dediu, V.; Musat, V.; Cernica, I. Applied Surface Science Nb-TiO2/ZnO nanostructures for chemoresistive alcohol sensing. Appl. Surf. Sci. 2019, 488, 70–76. [Google Scholar] [CrossRef]
- Wang, W.; Wen, H.; Cheng, C.; Hung, C.; Chou, W. Microelectronics Reliability Nanotribological properties of ALD-processed bilayer TiO2/ZnO films. Microelectron. Reliab. 2014, 54, 2754–2759. [Google Scholar] [CrossRef]
- Léonard, G.L.; Pàez, C.A.; Ramírez, A.E.; Mahy, J.G. Interactions between Zn2+ or ZnO with TiO2 to produce an ef fi cient photocatalytic, superhydrophilic and aesthetic glass. J. Photochem. Photobiol. A Chem. 2018, 350, 32–43. [Google Scholar] [CrossRef]

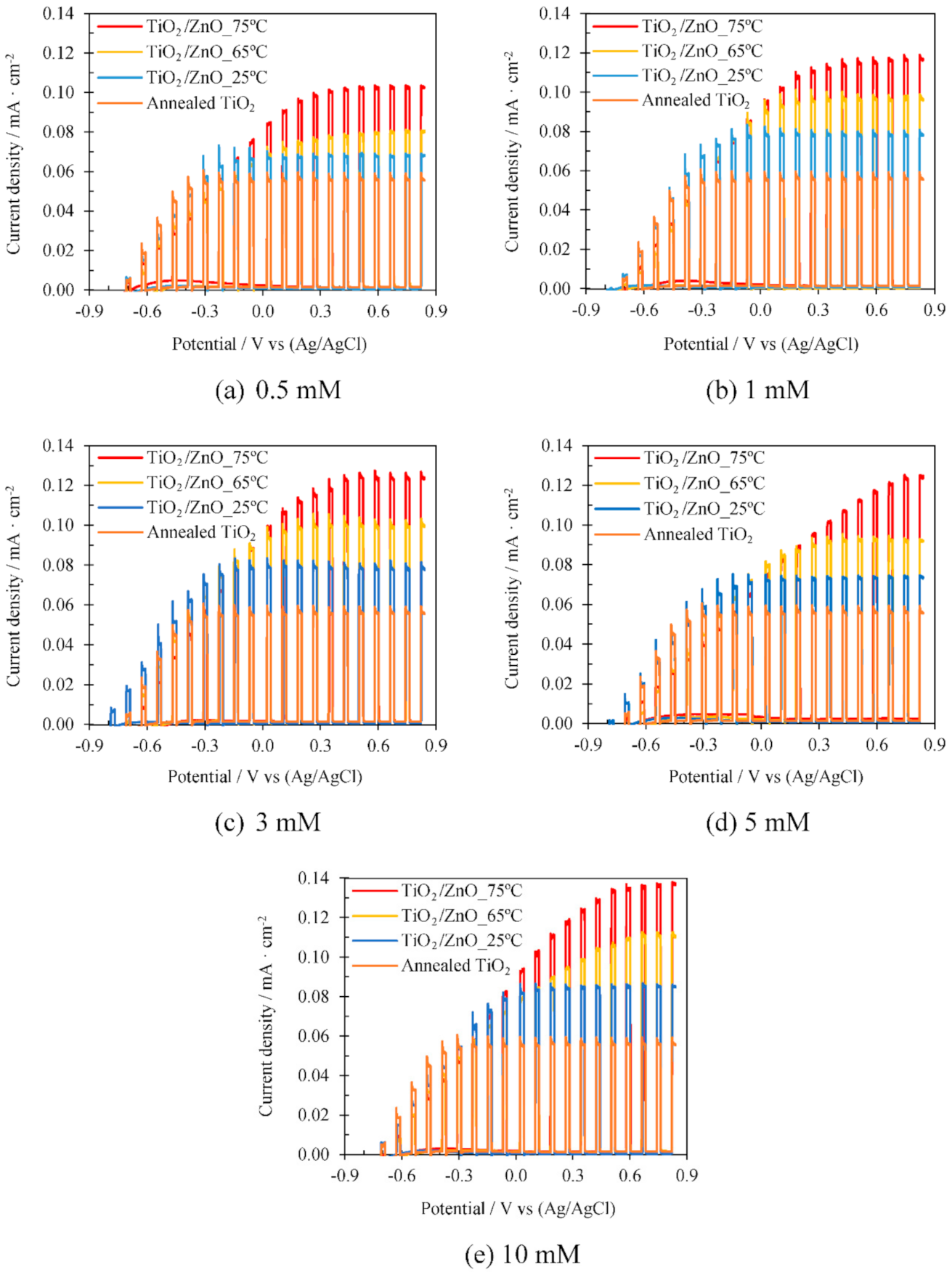
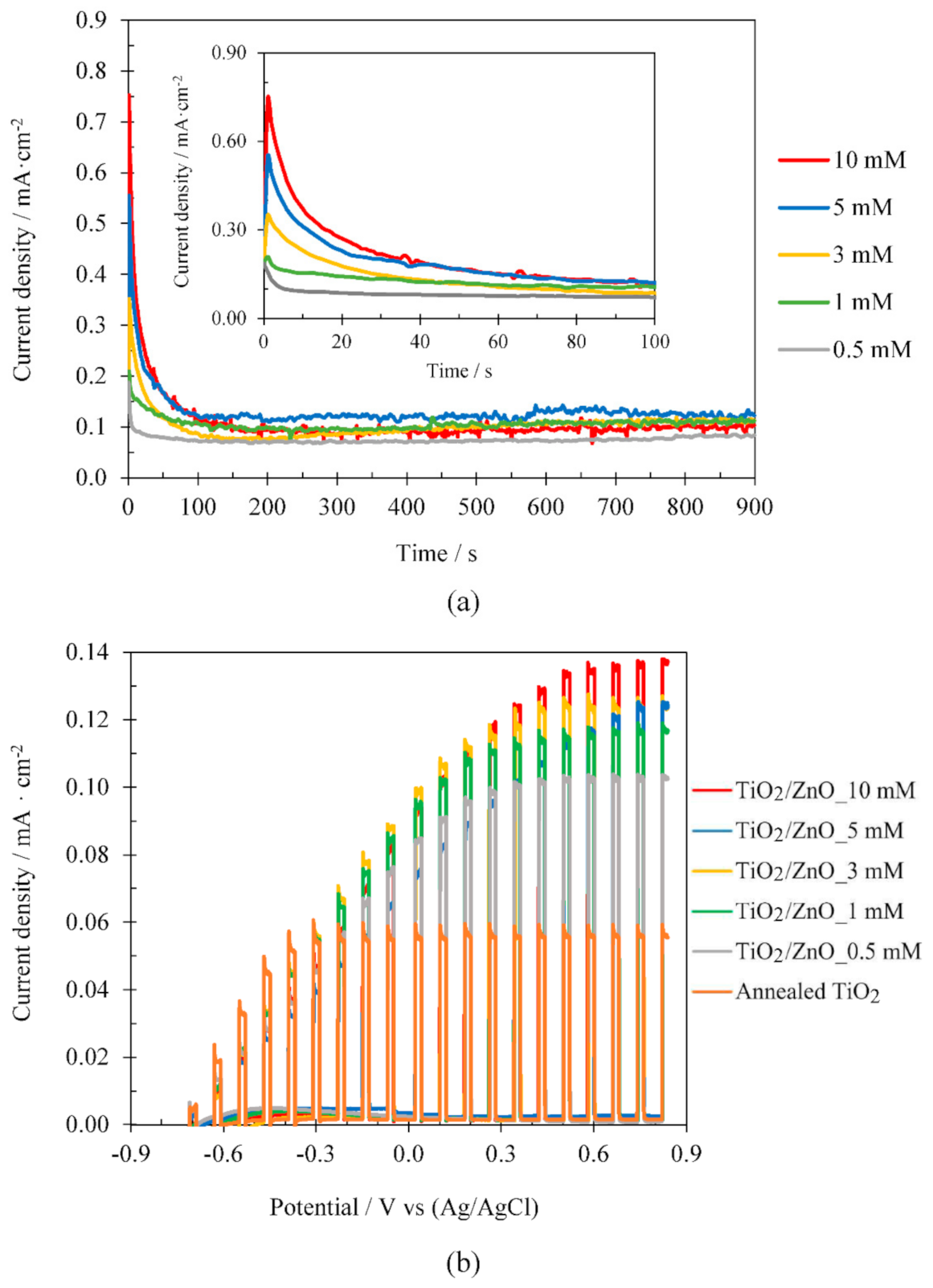
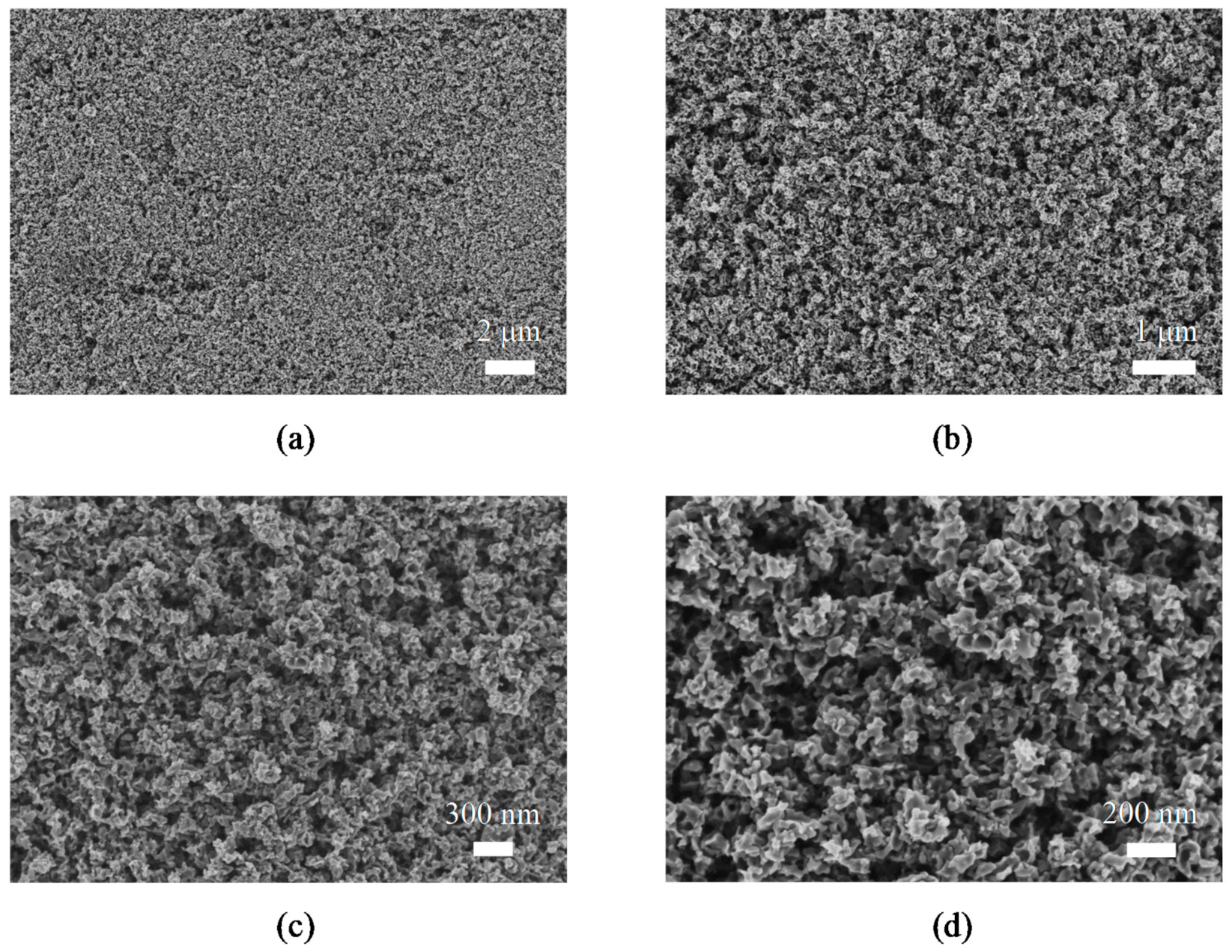
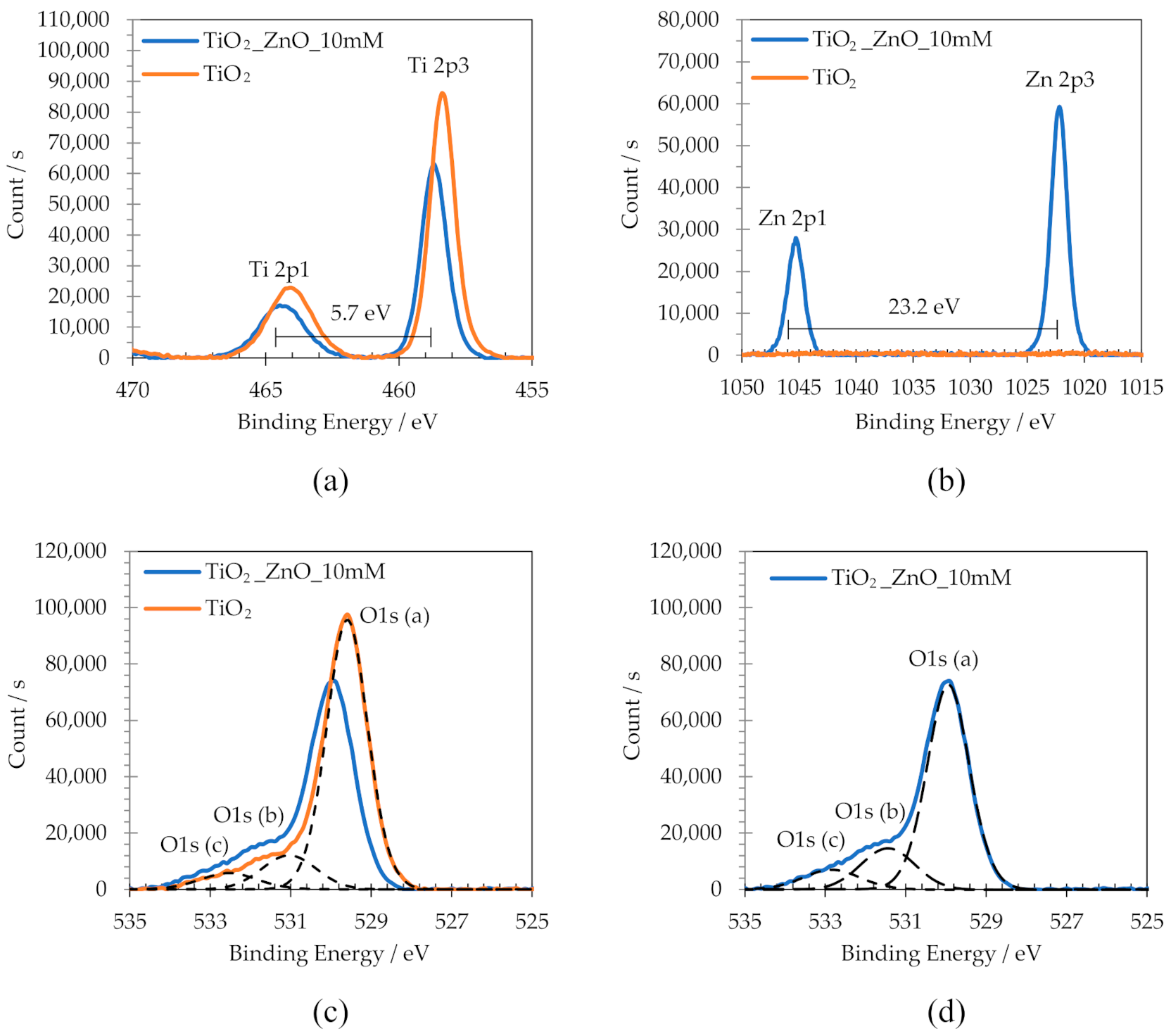
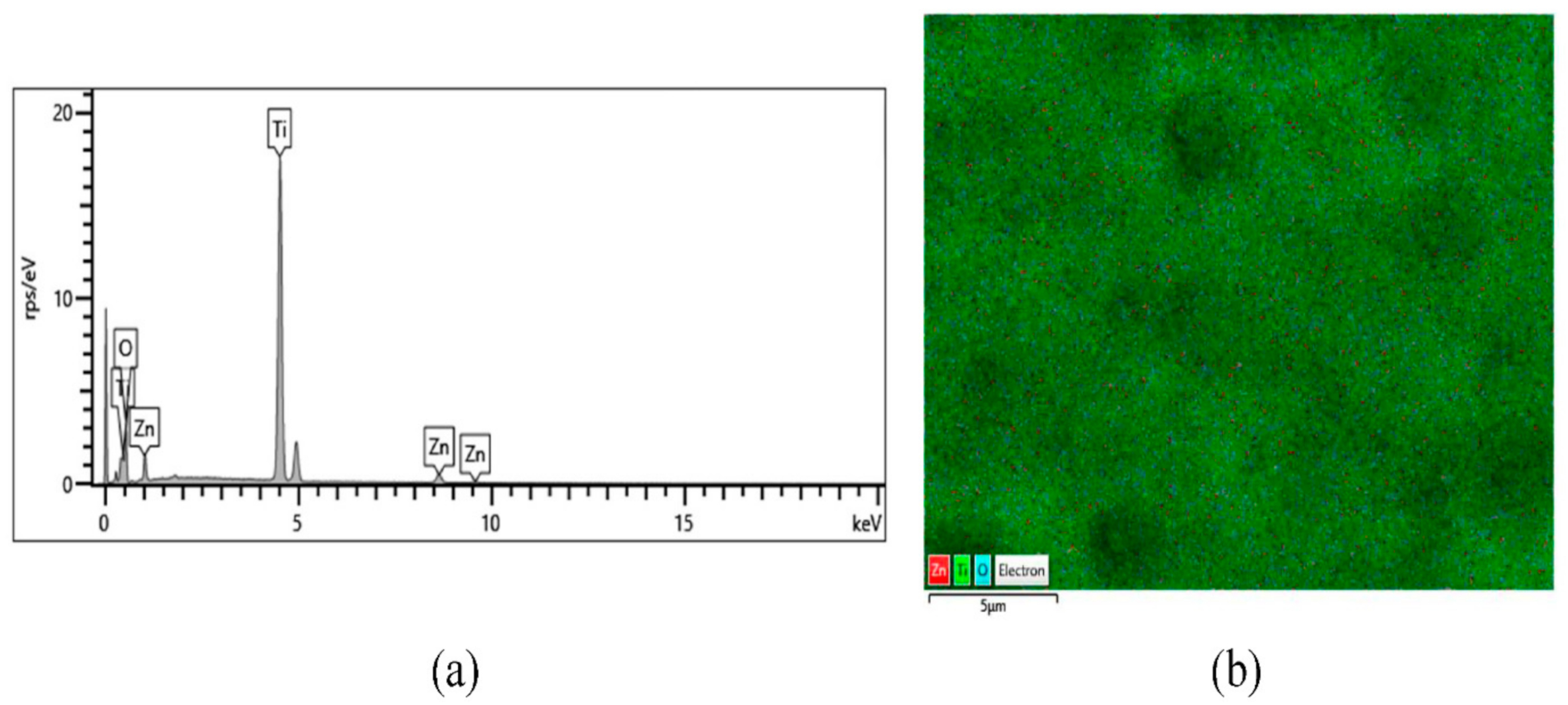

| Temperature (ºC) | Concentration (mM) | i max (mA·cm-2) | Δi (mA·cm-2) | %imp |
|---|---|---|---|---|
| 25 | 0.5 | 0.068 | 0.067 | 21.43 |
| 1 | 0.077 | 0.077 | 37.50 | |
| 3 | 0.078 | 0.077 | 39.29 | |
| 5 | 0.073 | 0.072 | 30.36 | |
| 10 | 0.085 | 0.085 | 51.79 | |
| 65 | 0.5 | 0.093 | 0.093 | 66.07 |
| 1 | 0.096 | 0.096 | 71.43 | |
| 3 | 0.099 | 0.098 | 76.79 | |
| 5 | 0.103 | 0.102 | 83.93 | |
| 10 | 0.109 | 0.105 | 94.64 | |
| 75 | 0.5 | 0.103 | 0.102 | 83.93 |
| 1 | 0.114 | 0.113 | 103.57 | |
| 3 | 0.123 | 0.122 | 119.64 | |
| 5 | 0.117 | 0.112 | 108.93 | |
| 10 | 0.135 | 0.134 | 141.07 |
| TiO2 (%At) | TiO2_1 mM (%At) | TiO2_3 mM (%At) | TiO2_5 mM (%At) | TiO2_10 mM (%At) | |
|---|---|---|---|---|---|
| O1s_a | 58.46 | 49.64 | 47.34 | 46.70 | 41.50 |
| O1s_b | 9.68 | 10.55 | 12.11 | 13.05 | 19.01 |
| O1s_c | 4.94 | 6.35 | 6.58 | 5.86 | 7.42 |
| Ti+4 | 26.91 | 25.25 | 24.72 | 22.07 | 18.32 |
| Zn+2 | 0.00 | 8.20 | 9.25 | 12.32 | 13.77 |
| Sample | Ti | O | Zn | O/Ti | %Imp | |||
|---|---|---|---|---|---|---|---|---|
| Weight Ratio | Atomic Ratio | Weight Ratio | Atomic Ratio | Weight Ratio | Atomic Ratio | Atomic Ratio | ||
| TiO2 | 65.49 | 38.79 | 34.51 | 61.21 | 0.00 | 0.00 | 1.58 | - |
| TiO2/ZnO_25ºC_10mM | 62.23 | 35.85 | 36.98 | 63.81 | 0.79 | 0.34 | 1.78 | 51.79 |
| TiO2/ZnO_65ºC_10mM | 59.03 | 34.04 | 37.30 | 64.40 | 3.67 | 1.55 | 1.89 | 94.64 |
| TiO2/ZnO_75ºC_10mM | 57.81 | 34.43 | 35.04 | 62.45 | 7.15 | 3.12 | 1.81 | 141.07 |
| TiO2/ZnO_75ºC_5mM | 58.08 | 34.10 | 36.07 | 63.39 | 5.85 | 2.51 | 1.86 | 108.93 |
| TiO2/ZnO_75ºC_1mM | 57.56 | 32.92 | 38.11 | 65.26 | 4.33 | 1.82 | 1.98 | 103.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Gázquez, P.J.; Muñoz-Portero, M.J.; Blasco-Tamarit, E.; Sánchez-Tovar, R.; Fernández-Domene, R.M.; García-Antón, J. Original Approach to Synthesize TiO2/ZnO Hybrid Nanosponges Used as Photoanodes for Photoelectrochemical Applications. Materials 2021, 14, 6441. https://doi.org/10.3390/ma14216441
Navarro-Gázquez PJ, Muñoz-Portero MJ, Blasco-Tamarit E, Sánchez-Tovar R, Fernández-Domene RM, García-Antón J. Original Approach to Synthesize TiO2/ZnO Hybrid Nanosponges Used as Photoanodes for Photoelectrochemical Applications. Materials. 2021; 14(21):6441. https://doi.org/10.3390/ma14216441
Chicago/Turabian StyleNavarro-Gázquez, Pedro José, Maria José Muñoz-Portero, Encarna Blasco-Tamarit, Rita Sánchez-Tovar, Ramon Manuel Fernández-Domene, and Jose García-Antón. 2021. "Original Approach to Synthesize TiO2/ZnO Hybrid Nanosponges Used as Photoanodes for Photoelectrochemical Applications" Materials 14, no. 21: 6441. https://doi.org/10.3390/ma14216441
APA StyleNavarro-Gázquez, P. J., Muñoz-Portero, M. J., Blasco-Tamarit, E., Sánchez-Tovar, R., Fernández-Domene, R. M., & García-Antón, J. (2021). Original Approach to Synthesize TiO2/ZnO Hybrid Nanosponges Used as Photoanodes for Photoelectrochemical Applications. Materials, 14(21), 6441. https://doi.org/10.3390/ma14216441






