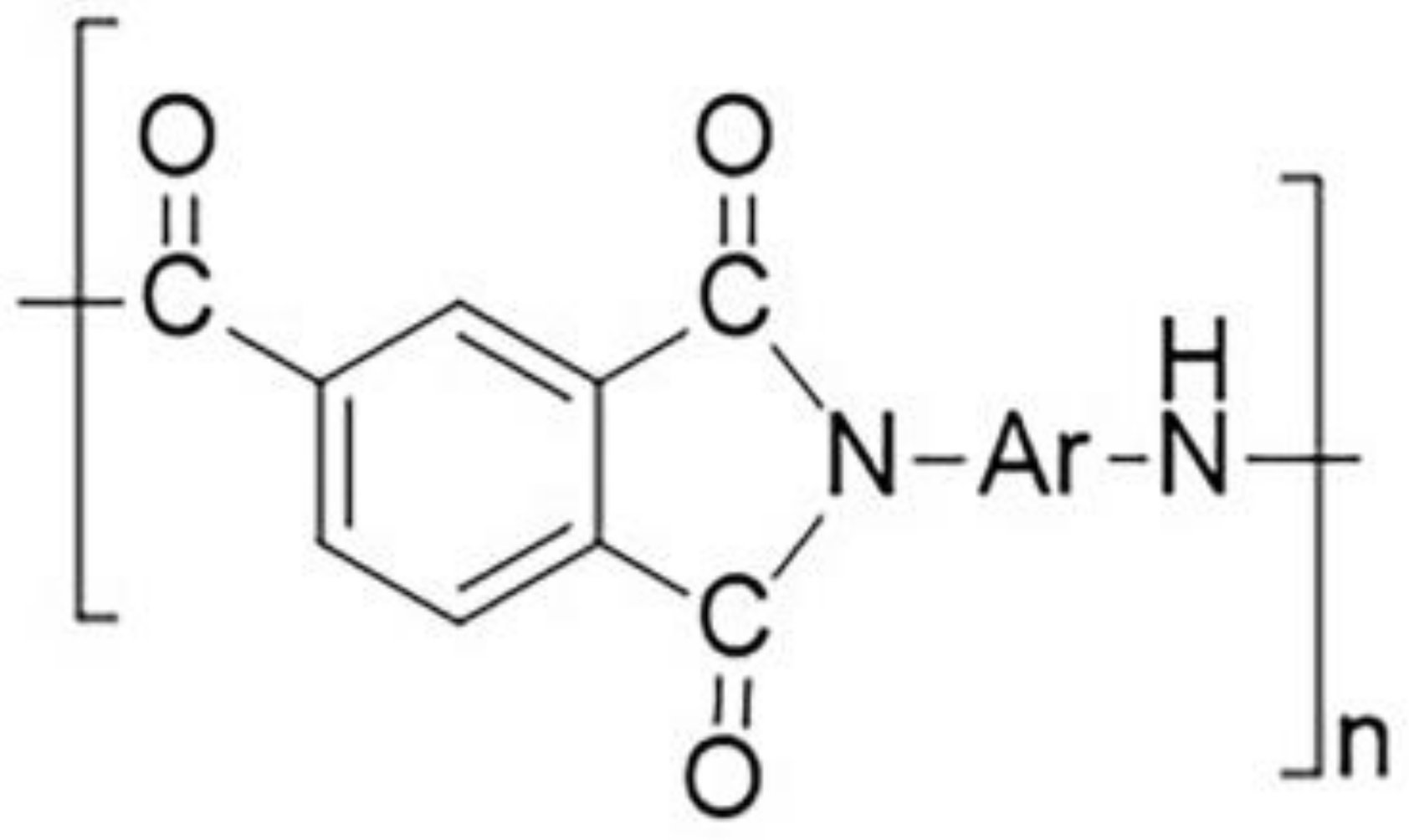
PAI Materials Synthesized by 4,4′-Diaminodiphenyl ether/2,2′-Bis (trifluoromethyl)-4,4′-diaminophenyl ether and Their Properties
Abstract
:1. Introduction
2. Experimental
2.1. Materials
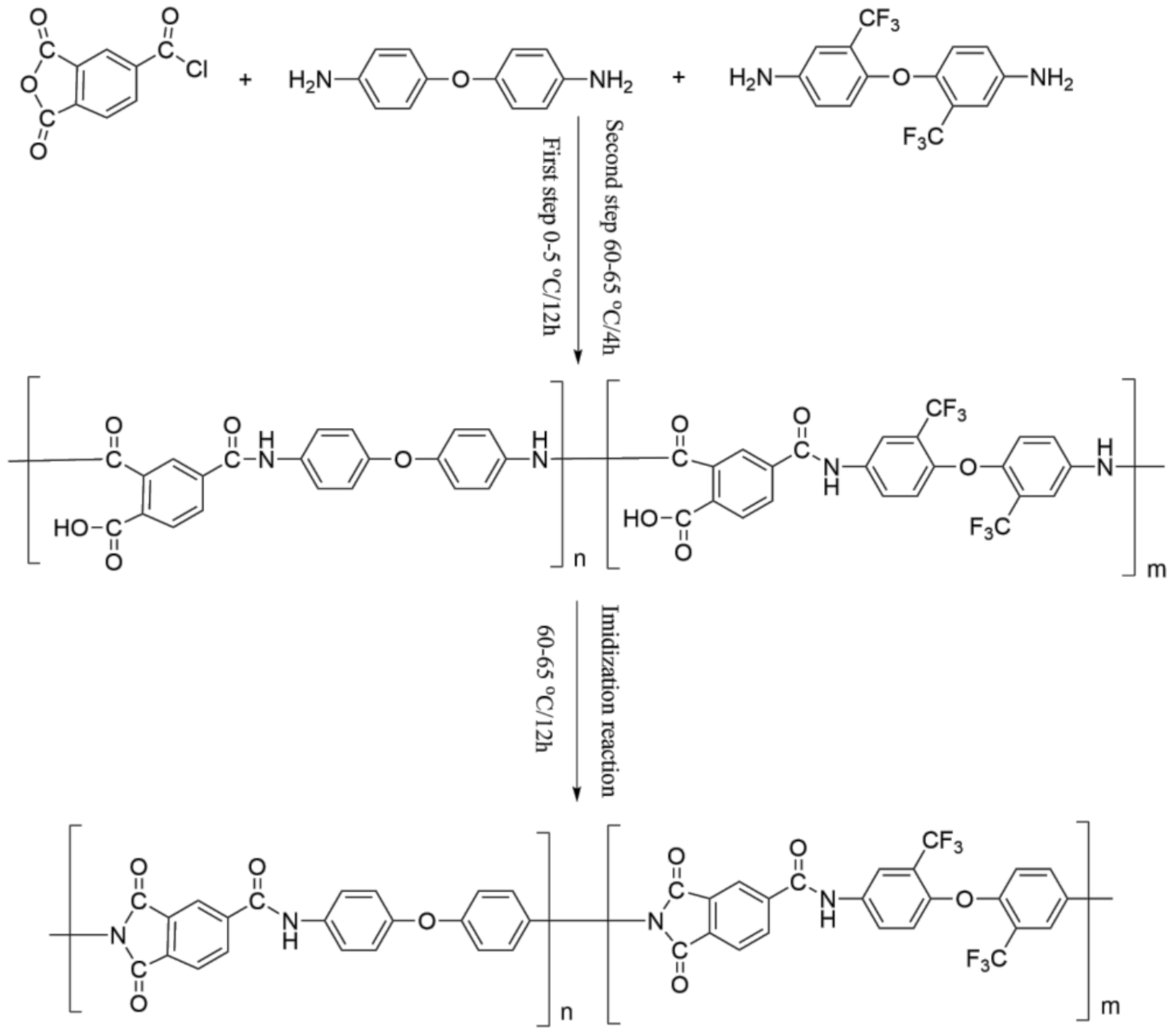
2.2. Preparation of PAI
- (1)
- Polymerization process
- (2)
- Post-processing process
- (3)
- Sample preparation method
2.3. Characterization
3. Results and Discussion
3.1. Structural Characterization of PAI
3.1.1. Infrared Spectroscopy (FT-IR)
3.1.2. Measurement of Molecular Weight and Polydispersity Index
3.2. Heat Resistance Test
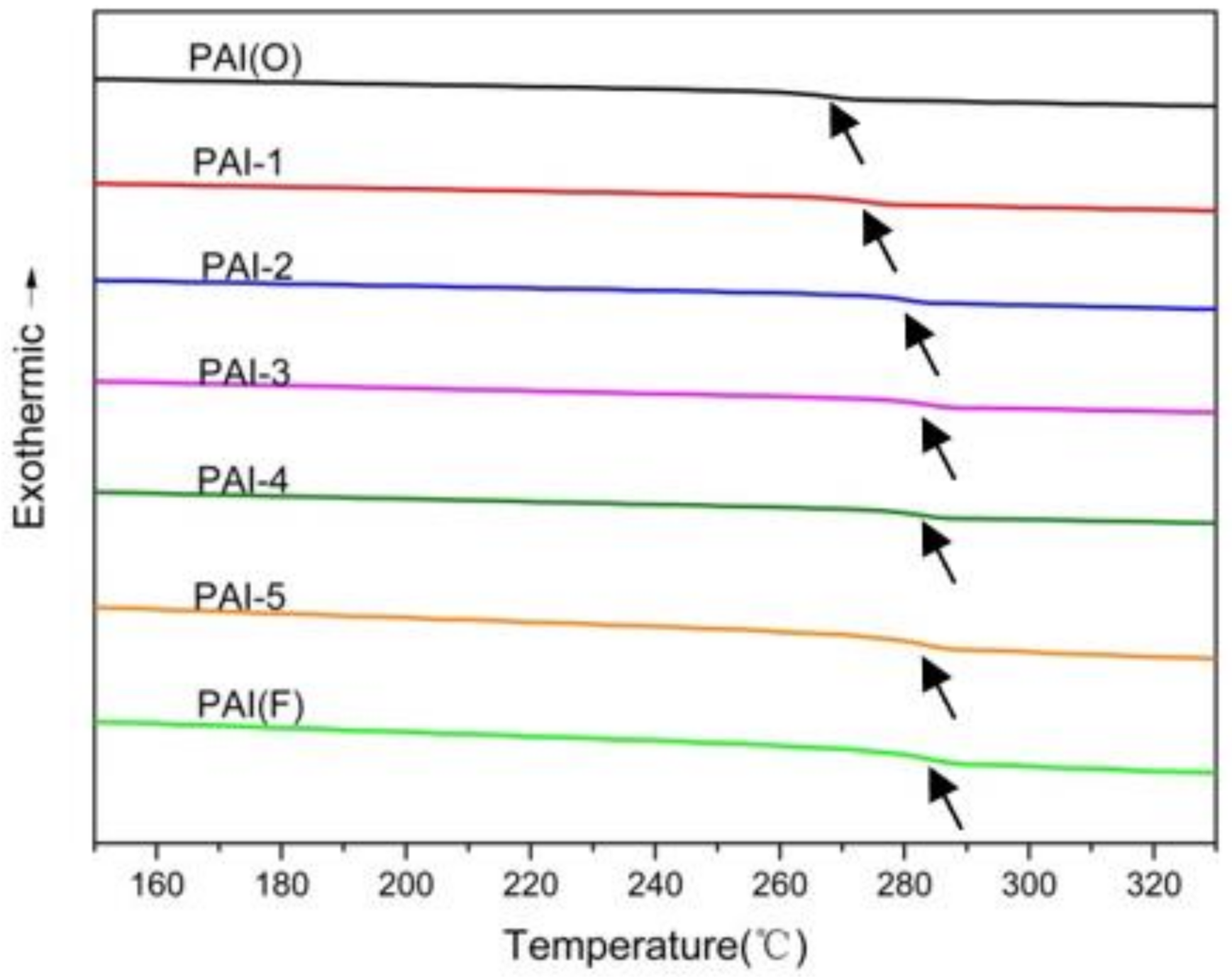
3.2.1. DSC Test Characterization
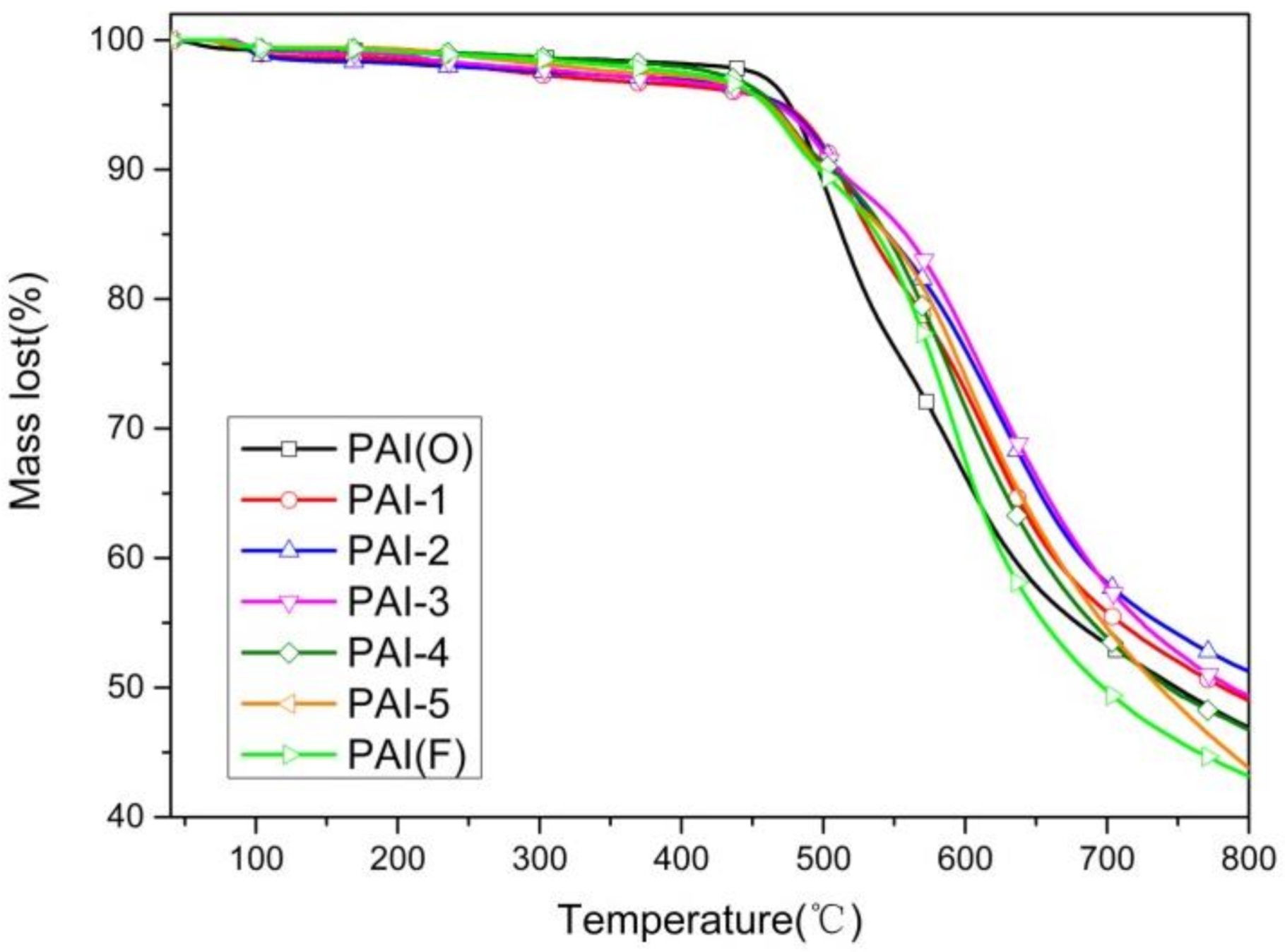
3.2.2. TGA Test Characterization
3.3. Tensile Property Test
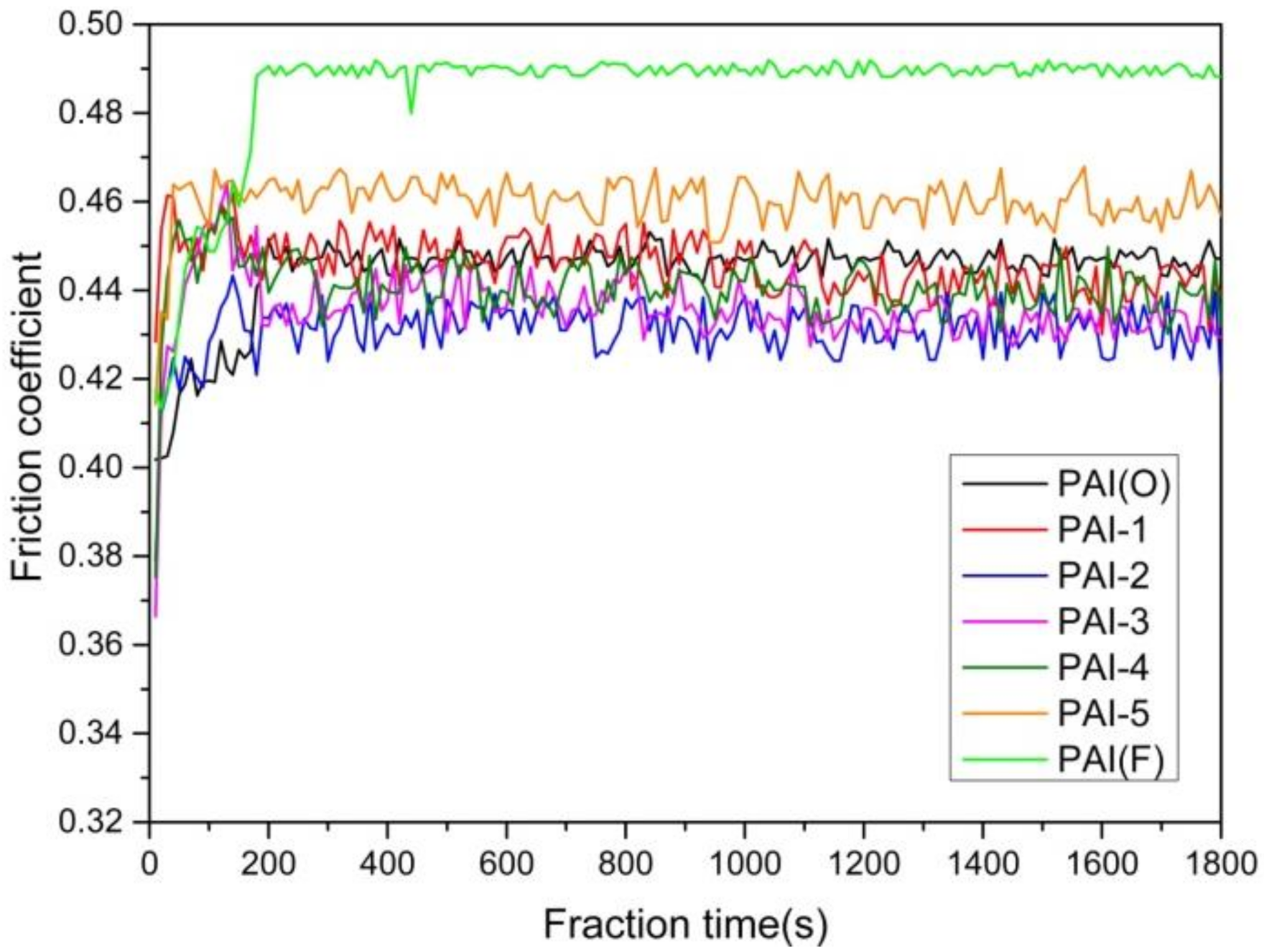
3.4. Characterization of Friction and Wear Properties
3.4.1. Friction Coefficient and Wear Test
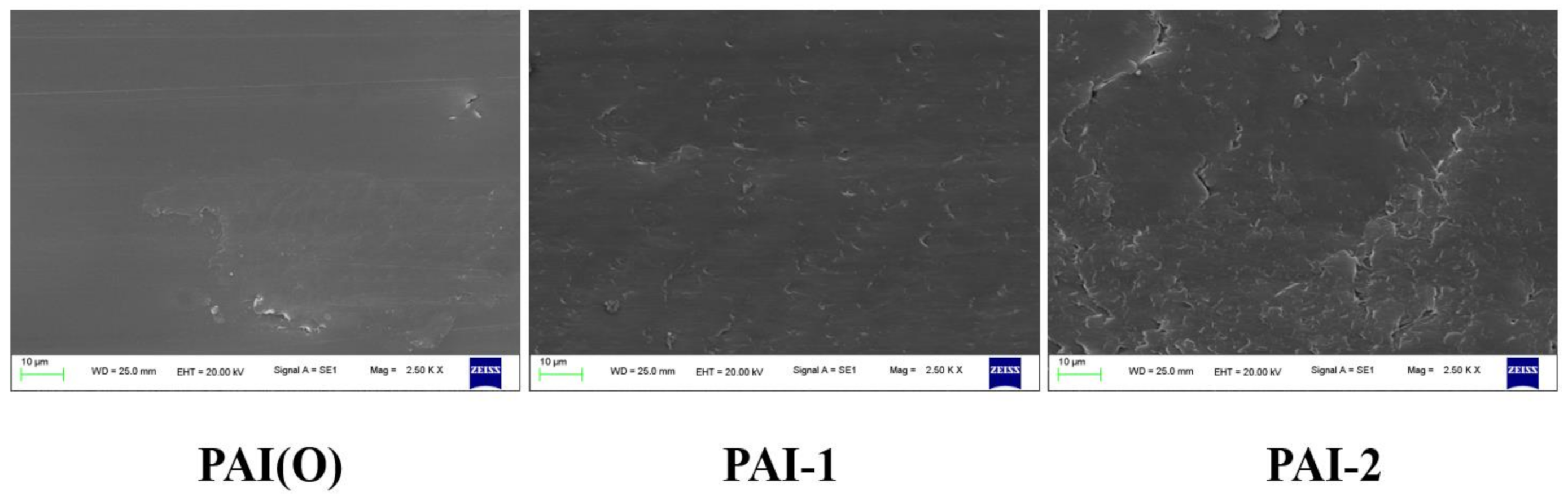
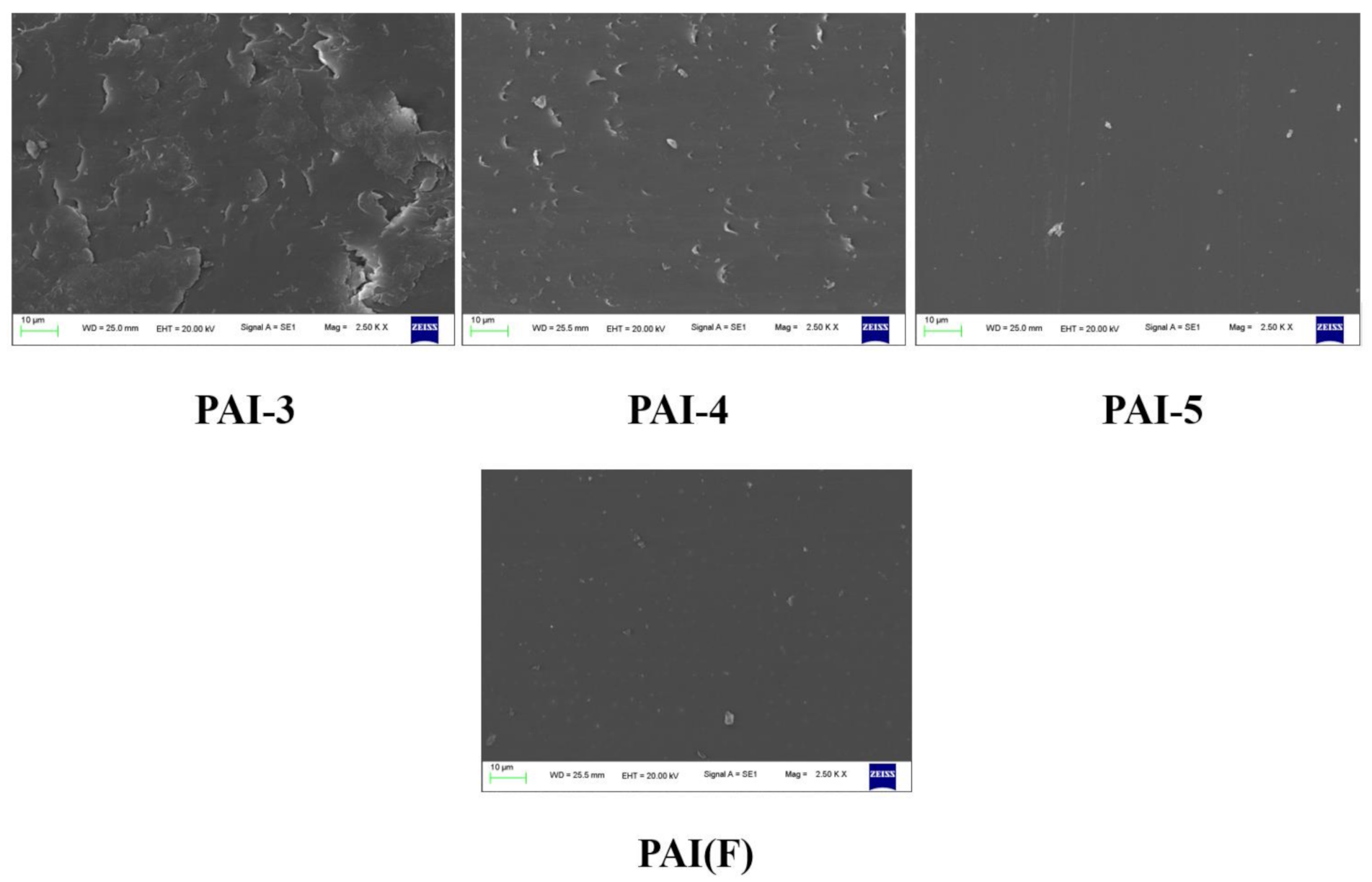
3.4.2. Scanning Electron Microscope Analysis
3.5. Hydrophobic Performance Test
4. Conclusions
- (1)
- The Tg of PAI polymer synthesized with 4,4’-diaminodiphenyl ether as diamine monomer was 266.5 °C. With the increase of the amount of 2,2’-bis (trifluoromethyl)-4,4’-diaminophenyl ether, the glass transition temperature of PAI terpolymer gradually increased. When the molar ratio of the two diamines was 5:5, the Tg of PAI terpolymer reached 282.9 °C, and then with the increase of the amount of 2,2’-bis (trifluoromethyl)-4,4’-diaminophenyl ether, the Tg changed little.
- (2)
- The Td5% of PAI copolymer synthesized by 4,4’-diaminodiphenyl ether and TMAC was 476.5 °C. With the increase of the dosage of 2,2’-bis (trifluoromethyl)-4,4’-diaminophenyl ether, the Td5% of PAI material gradually decreased. The Td5% of PAI copolymer synthesized by 2,2’-bis (trifluoromethyl)-4,4’-diaminophenyl ether reached the minimum value of 459.6 °C.
- (3)
- The tensile strength and elongation at break of PAI synthesized by 4,4’-diaminodiphenyl ether were the highest, which were 133.4 MPa and 9.46% respectively. With the increase of the dosage of 2,2’-bis (trifluoromethyl)-4,4’-diaminophenyl ether, the tensile strength and elongation at break of PAI materials decreased gradually. The tensile strength and elongation at break of PAI copolymer synthesized by 2,2’-bis (trifluoromethyl)-4,4’-diaminophenyl ether reached the minimum value of 61.3 MPa and 3.86%, respectively.
- (4)
- The wear mechanism of PAI varied with the content of trifluoromethyl. With the increase of the amount of fluorinated diamine monomer, the adhesive wear degree of PAI materials gradually increased, and reached the maximum when the molar ratio of the two monomers was 5:5, and then decreased gradually. Different trifluoromethyl content had little effect on friction coefficient, and the friction coefficient increased slightly when the molar ratio of 4,4 ′-diaminodiphenyl ether to 2,2′-bis (trifluoromethyl)-4,4 ′-diaminophenyl ether is 1:9. With the increase of trifluoromethyl content, the wear of PAI material would increase.
- (5)
- PAI material synthesized by 4,4’-diaminodiphenyl ether had the smallest water contact angle and the largest water absorption rate. With the increase of the amount of 2,2’-bis (trifluoromethyl)-4,4’-diaminophenyl ether, the water contact angle of PAI material increased and the water absorption decreased. The results showed that trifluoromethyl had good hydrophobic properties.
| PAI materials synthesized by 4,4′-diaminodiphenyl ether/2,2′-bis (trifluoromethyl)-4,4′-diaminophenyl ether and their properties Haiyang Yang, Duxin Li*, Jun Yang*, Jin Wang, Shunchang Gan | In this paper, 4,4′-diaminodiphenyl ether and 2,2′-bis (trifluoromethyl)-4,4′-diaminophenyl ether are selected for molecular structure design, and PAI materials are synthesized by acyl chloride method. The results of this study showed that the introduction of trifluoromethyl into the side chain provided an effective way to prepare PAI materials with low water absorption. Considering the comprehensive properties such as heat resistance, friction and wear, tensile properties, etc., the appropriate addition amount is 10–30%. |
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dinehart, R.A. Preparation and fabrication of aromatic polyimides. J. Appl. Polym. Sci. 2010, 11, 609–627. [Google Scholar] [CrossRef]
- Harrass, M.; Friedrich, K.; Almajid, A. Tribological behavior of selected engineering polymers under rolling contact. Tribol. Int. 2010, 43, 635–646. [Google Scholar] [CrossRef]
- Takizawa, K.; Wakita, J.; Kakiage, M.; Masunaga, H.; Ando, S. Molecular Aggregation Structures of Polyimide Films at Very High Pressure Analyzed by Synchrotron Wide-Angle X-ray Diffraction. Macromolecules 2010, 43, 2115–2117. [Google Scholar] [CrossRef]
- Li, Z.; Kou, K.; Zhang, J.; Zhang, Y.; Wang, Y.; Pan, C. Solubility, electrochemical behavior and thermal stability of polyimides synthesized from 1,3,5-triazine-based diamine. J. Mater. Sci. Mater. Electron. 2017, 28, 6079–6087. [Google Scholar] [CrossRef]
- Dutczak, S.M.; Cuperus, F.P.; Wessling, M.; Stamatialis, D.F. New crosslinking method of polyamide-imide membranes for potential application in harsh polar aprotic solvents. Sep. Purif. Technol. 2013, 102, 142–146. [Google Scholar] [CrossRef]
- Chen, L.W.; Ho, K.S. Synthesis of polyamide-imide by blocked-methylene diisocyanates. J. Polym. Sci. Part A Polym. Chem. 2015, 35, 1711–1717. [Google Scholar] [CrossRef]
- Kalinina, I.; Gumargalieva, K.; Shlyapnikov, Y.A. Effect of p-toluensulfonic acid on oxidation of polyethylene and polyethylene-poly-p-methylstyrene blends. Polym. Degrad. Stab. 1993, 40, 411–416. [Google Scholar] [CrossRef]
- Wang, C.S.; Yang, R.W. Synthesis and properties of flourine-containing polyimides. J. Appl. Polym. Sci. 1997, 66, 609–617. [Google Scholar] [CrossRef]
- Banerjee, S.; Madhra, M.K.; Kute, V. Polyimides 6: Synthesis, characterization, and comparison of properties of novel fluorinated poly(ether imides). J. Appl. Polym. Sci. 2004, 93, 821–832. [Google Scholar] [CrossRef]
- Maeda, N.; Chen, N.; Tirrell, M.; Israelachvili, J.N. Adhesion and friction mechanisms of polymer-on-polymer surfaces. Science 2002, 297, 379–382. [Google Scholar] [CrossRef] [PubMed]
- Cong, P.; Li, T.; Liu, X.; Zhang, X.; Xue, Q.; Li, J. Effect of temperature on the friction and wear properties of a crosslinked and a linear polyimide. Acta Polym. Sin. 1998, 556–561. [Google Scholar]
- Tian, J.; Wang, H.; Huang, Z.; Lu, R.; Cong, P.; Liu, X.; Li, T. Investigation on tribological properties of fluorinated polyimide. J. Macromol. Sci. B 2010, 49, 791–801. [Google Scholar] [CrossRef]
- Fusaro, R.L.; Hady, W.F. Low-wear partially fluorinated polyimides. ASLE Trans. 1985, 28, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.P.; Su, Y.Y.; Hsu, M.Y. Organo-soluble and lightly-colored fluorinated polyimides based on 2,2-bis [4-(3,4-dicarboxyphenoxy)phenyl] hexafluoropropane dianhydride and aromatic bis (ether amine) s bearing pendent trifluoromethyl groups. Polym. J. 2006, 38, 132–144. [Google Scholar] [CrossRef] [Green Version]
- Jonquières, A.; Vicherat, A.; Lochon, P. Synthesis and characterization of new polyamideimides with a highly flexible soft block. J. Polym. Sci. Part A Polym. Chem. 1999, 37, 2873–2889. [Google Scholar] [CrossRef]
- Xu, S.G.; Yang, M.J.; Bai, F.L. Optical behaviors and electroluminescence of a new polyimide. Synth. Met. 2003, 137, 1097–1098. [Google Scholar] [CrossRef]
- Yamaguchi, Y. Tribology of Plastic Materials; Elsevier: Amsterdam, The Netherlands, 1990. [Google Scholar]
- Tanaka, K.; Miyata, T. Studies on the friction and transfer of semicrystalline polymers. Wear 1977, 41, 383–398. [Google Scholar] [CrossRef]
- Jones, J.W.; Eiss, N.S.E., Jr. Effect of Chemical Structure on the Friction and Wear of Polyimide Thin Films. ACS Symp. Ser. 1985, 135–148. [Google Scholar]
- Chitsaz-Zadeh, M.; Eiss, N., Jr. Friction and wear of polyimide thin films. Wear 1986, 110, 359–368. [Google Scholar] [CrossRef]


| Sample Number | PAI(O) | PAI-1 | PAI-2 | PAI-3 | PAI-4 | PAI-5 | PAI(F) |
|---|---|---|---|---|---|---|---|
| Mn(105) | 2.82 | 2.52 | 2.51 | 2.78 | 2.59 | 2.58 | 2.52 |
| Mw(105) | 3.49 | 3.40 | 3.46 | 3.84 | 3.56 | 3.53 | 3.46 |
| Polydispersity index | 1.24 | 1.35 | 1.38 | 1.38 | 1.38 | 1.37 | 1.37 |
| Sample Number | PAI(O) | PAI-1 | PAI-2 | PAI-3 | PAI-4 | PAI-5 | PAI(F) |
|---|---|---|---|---|---|---|---|
| Tg/°C | 266.5 | 272.9 | 278.5 | 282.9 | 281.6 | 281.2 | 281.8 |
| Sample Number | PAI(O) | PAI-1 | PAI-2 | PAI-3 | PAI-4 | PAI-5 | PAI(F) |
|---|---|---|---|---|---|---|---|
| Td5%/°C | 476.5 | 472.1 | 470.1 | 467.2 | 465.1 | 461.3 | 459.6 |
| Sample Number | PAI(O) | PAI-1 | PAI-2 | PAI-3 | PAI-4 | PAI-5 | PAI(F) |
|---|---|---|---|---|---|---|---|
| Tensile strength/MPa | 133.4 | 119.0 | 95.3 | 87.1 | 72.5 | 71.8 | 61.3 |
| Elongation at break/% | 9.46 | 7.36 | 7.26 | 6.60 | 6.47 | 5.75 | 3.86 |
| Sample Number | PAI(O) | PAI-1 | PAI-2 | PAI-3 | PAI-4 | PAI-5 | PAI(F) |
|---|---|---|---|---|---|---|---|
| Friction factor | 0.4471 | 0.4453 | 0.4318 | 0.4356 | 0.4403 | 0.4604 | 0.4897 |
| Abrasion loss/mg | 1.5 | 1.9 | 2.3 | 2.4 | 3.5 | 3.7 | 3.8 |
| Sample Number | PAI(O) | PAI-1 | PAI-2 | PAI-3 | PAI-4 | PAI-5 | PAI(F) |
|---|---|---|---|---|---|---|---|
| Water contact angle/° | 77.7 | 85.4 | 89.2 | 90.4 | 91.5 | 92.0 | 92.3 |
| Water absorption/% | 2.79 | 2.18 | 2.01 | 2.00 | 1.86 | 1.72 | 1.42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, H.; Li, D.; Yang, J.; Wang, J.; Gan, S. PAI Materials Synthesized by 4,4′-Diaminodiphenyl ether/2,2′-Bis (trifluoromethyl)-4,4′-diaminophenyl ether and Their Properties. Materials 2021, 14, 6376. https://doi.org/10.3390/ma14216376
Yang H, Li D, Yang J, Wang J, Gan S. PAI Materials Synthesized by 4,4′-Diaminodiphenyl ether/2,2′-Bis (trifluoromethyl)-4,4′-diaminophenyl ether and Their Properties. Materials. 2021; 14(21):6376. https://doi.org/10.3390/ma14216376
Chicago/Turabian StyleYang, Haiyang, Duxin Li, Jun Yang, Jin Wang, and Shunchang Gan. 2021. "PAI Materials Synthesized by 4,4′-Diaminodiphenyl ether/2,2′-Bis (trifluoromethyl)-4,4′-diaminophenyl ether and Their Properties" Materials 14, no. 21: 6376. https://doi.org/10.3390/ma14216376
APA StyleYang, H., Li, D., Yang, J., Wang, J., & Gan, S. (2021). PAI Materials Synthesized by 4,4′-Diaminodiphenyl ether/2,2′-Bis (trifluoromethyl)-4,4′-diaminophenyl ether and Their Properties. Materials, 14(21), 6376. https://doi.org/10.3390/ma14216376





