Aesthetic Outcomes of Early Occlusal Loaded SLA Dental Implants with Hydroxyl Ion Modified Surface—A 12 Months Prospective Study
Abstract
:1. Introduction
Aim of the Study
2. Materials and Methods
2.1. Inclusion and Exclusion Criteria
- Group 1 (G2; n = 20 patients)—3.5 mm diameter implants were used
- Group 2 (G3; n = 20 patients)—4.0 mm diameter implants were used
- systemic or local diseases that could compromise healing or osteointegration,
- smoking,
- bruxism,
- pregnancy,
- breastfeeding.
2.2. Protocol of an Experiment
- Consultation visit: a precondition of the patient for the surgery, clinical and radiological examination CBCT (cone-beam computed tomography) (Galileos® D3437, Sirona Dental, Erlangen, Germany), approximal plaque index (API), height of keratinized tissue (HKT) assessment;
- Implantation: intraoperative and postoperative RVG (radiovisiography—Planmeca OY, Helsinki, Finland), gingival biotype assessment thick/thin;
- Four weeks after the implantation: intraoral scan, screw-retained prosthetic, RVG;
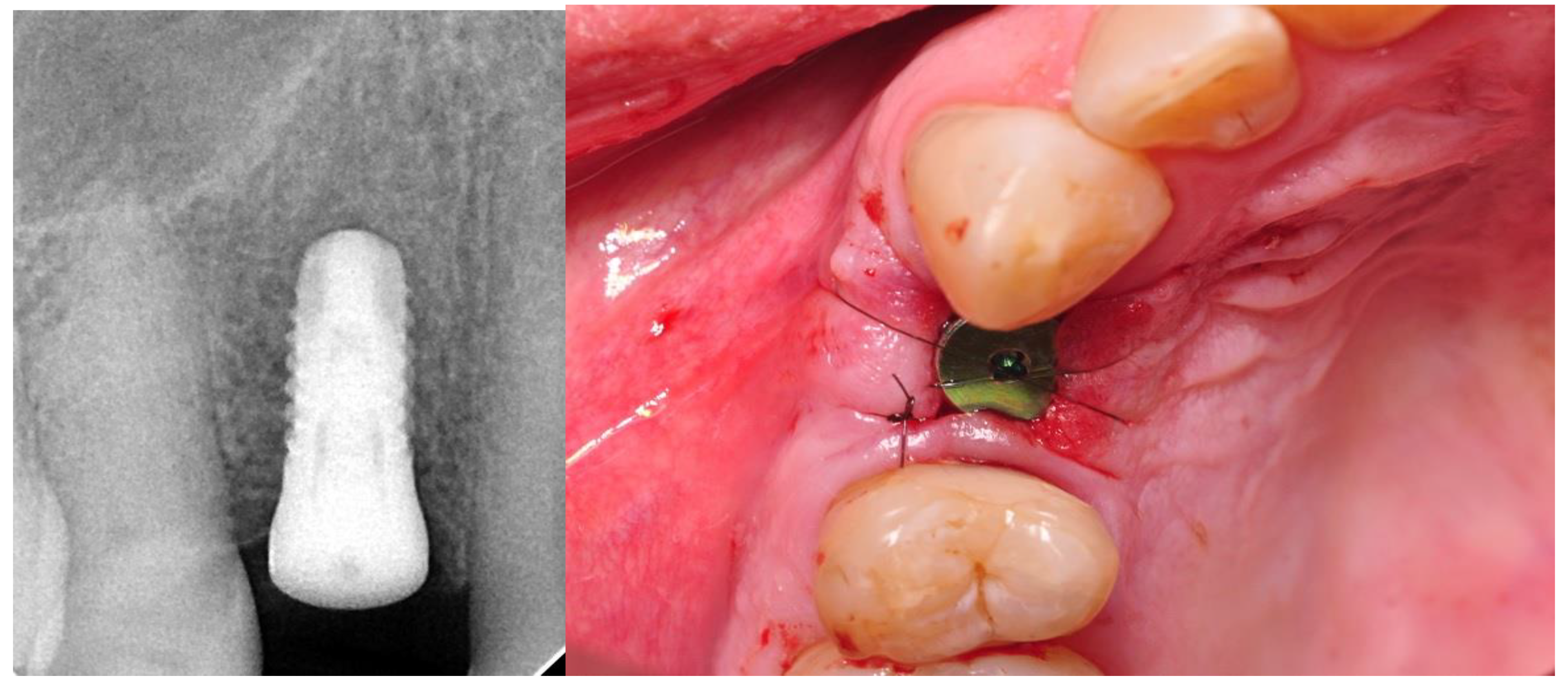
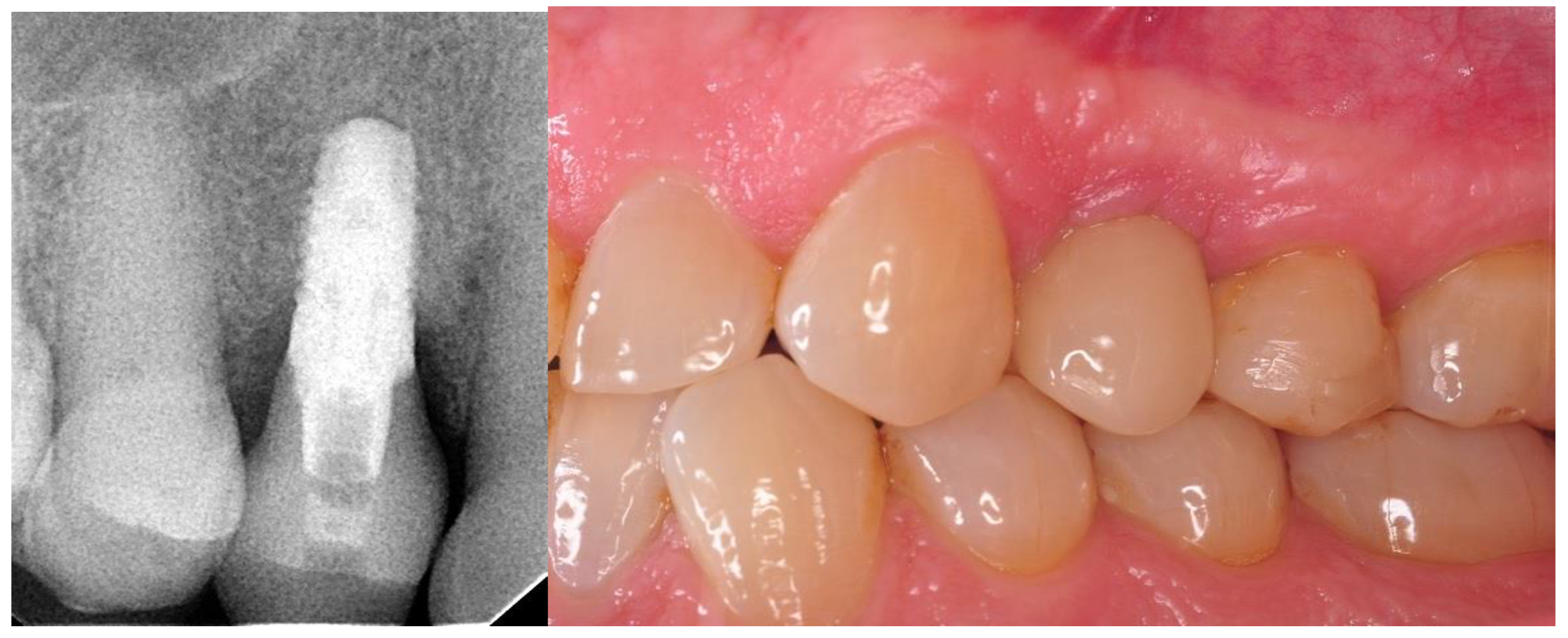
- Twelve months after the surgery: clinical evaluation (HKT, probing pocket depth (PPD), Visual Analogue Scale (VAS), pink esthetic score (PES) and white esthetic score (WES)) and radiological assessment (RVG and CBCT).
2.3. Implants
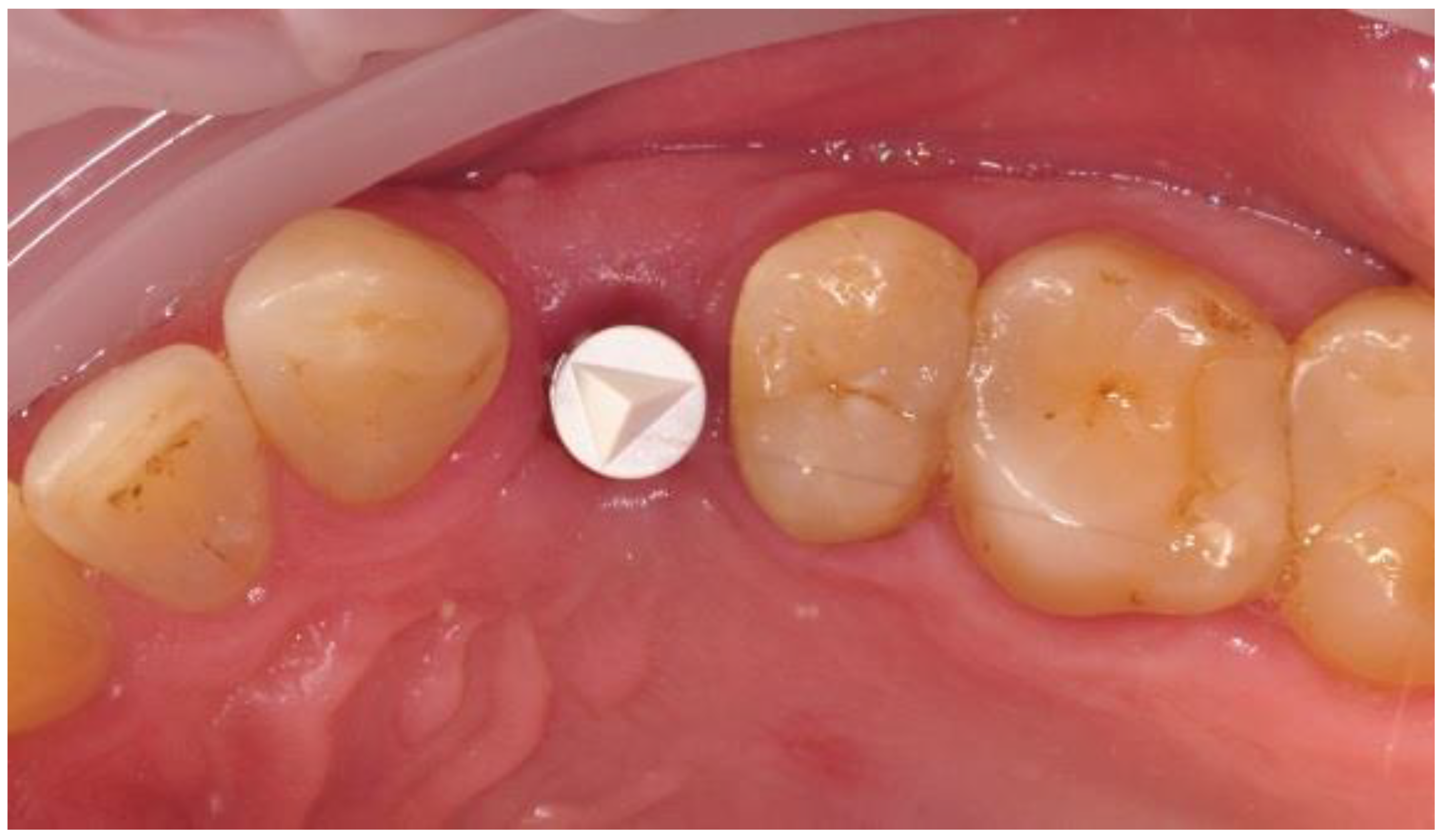
2.4. Surgical Phase
2.5. Prosthetic Phase
2.6. MBL (Marginal Bone Loss) Assessment Using the Radiological Examination
2.7. Periodontal Parameters Measurements
2.8. Aesthetic Evaluation
2.9. VAS (Visual Analogue Score) Assessment
2.10. Statistical Analyses
3. Results
3.1. General Data
3.2. Results of MBL
3.3. Results of Periodontal Parameters Measurements
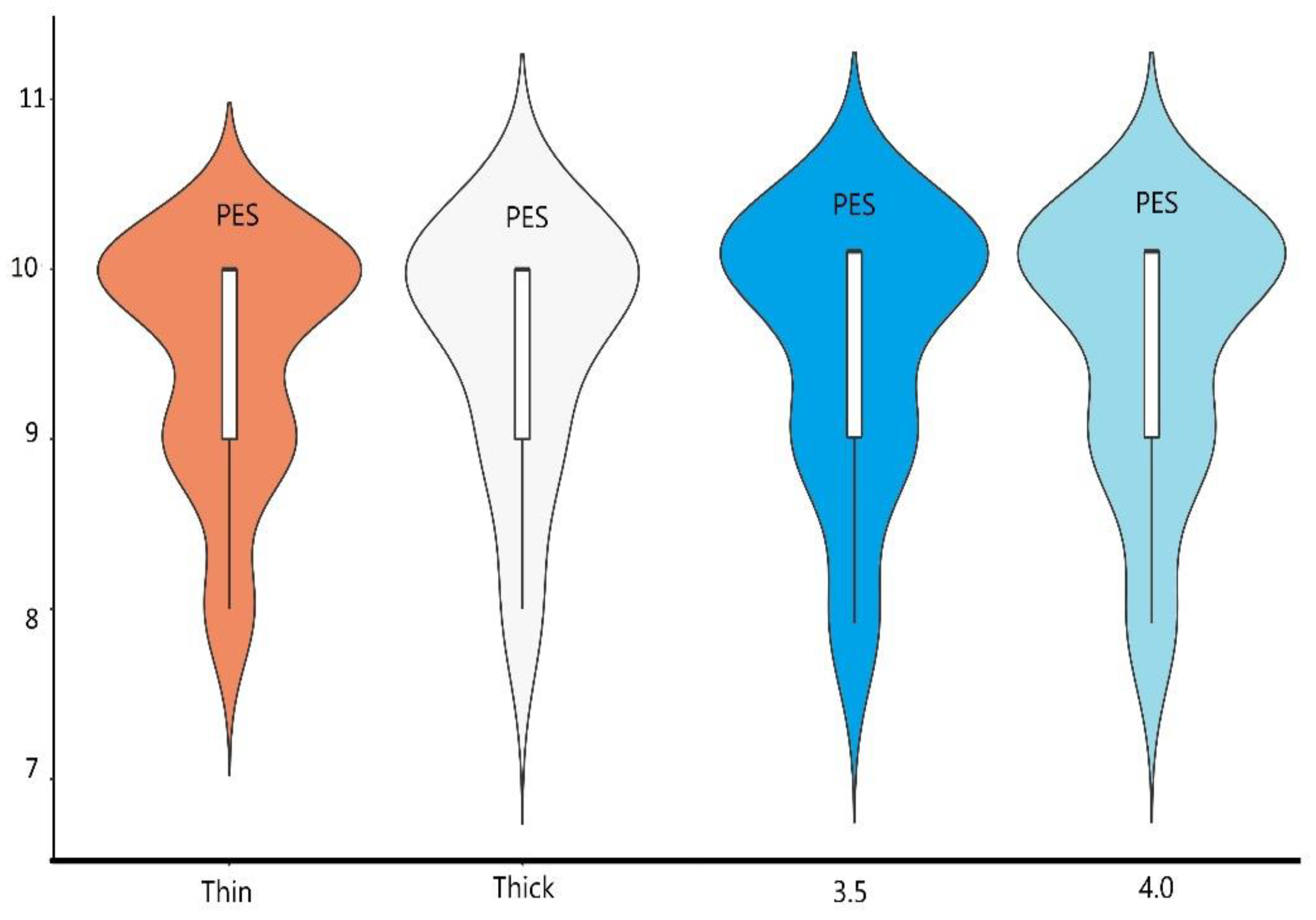
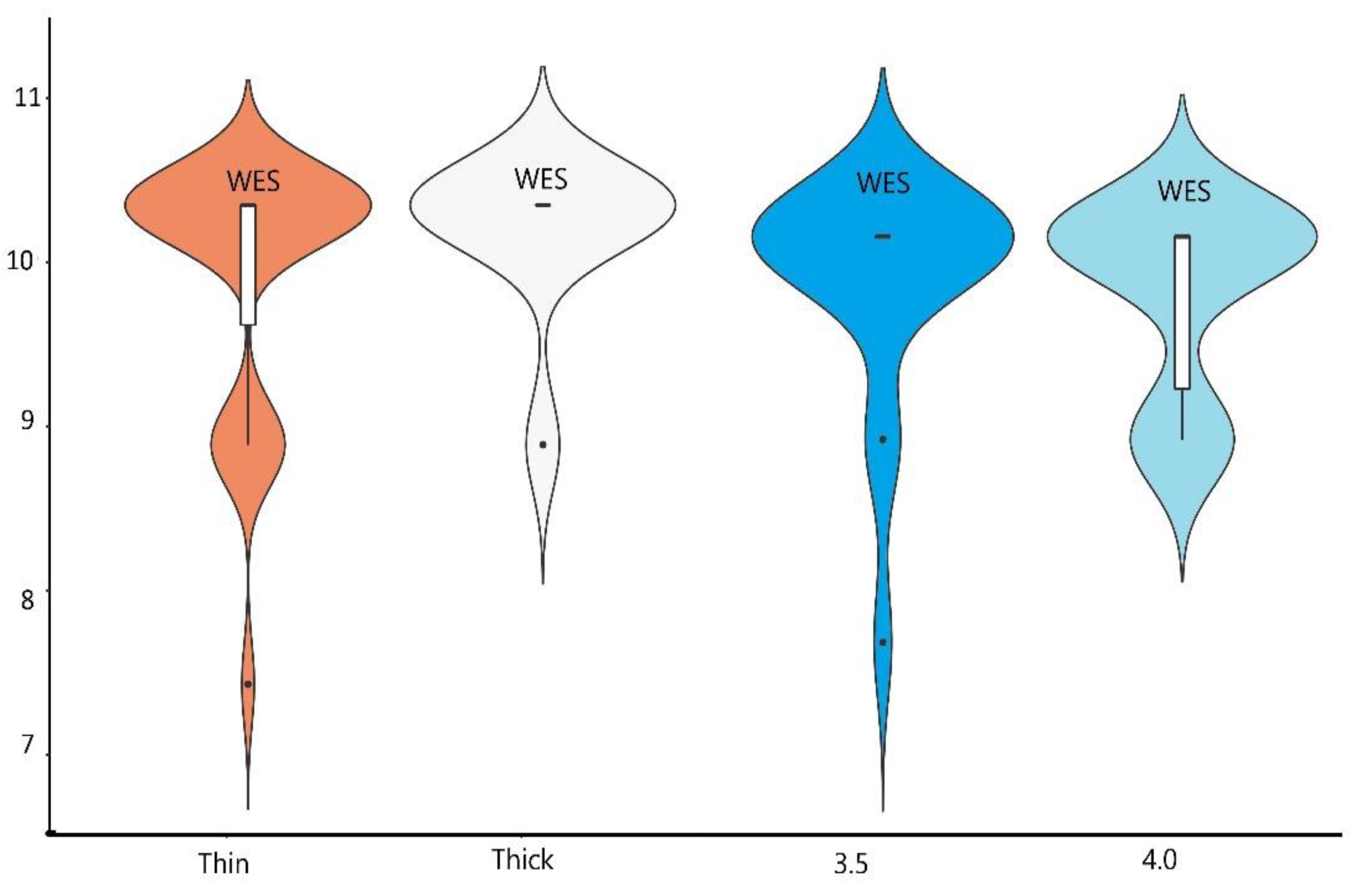
3.4. Results of Aesthetic Evaluation
3.5. Results of VAS Score
4. Discussion
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zukauskas, S.; Puisys, A.; Andrijauskas, P.; Zaleckas, L.; Vindasiute-Narbute, E.; Linkevičius, T. Influence of Implant Placement Depth and Soft Tissue Thickness on Crestal Bone Stability Around Implants with and Without Platform Switching: A Comparative Clinical Trial. Int. J. Periodontics Restor. Dent. 2021, 41, 347–355. [Google Scholar] [CrossRef]
- Sinjari, B.; D’Addazio, G.; Traini, T.; Varvara, G.; Scarano, A.; Murmura, G.; Caputi, S. A 10-year retrospective comparative human study on screw-retained versus cemented dental implant abutments. J. Boil. Regul. Homeost. Agents 2019, 33, 787–797. [Google Scholar]
- Esposito, M.; Grusovin, M.G.; Maghaireh, H.; Worthington, H.V. Interventions for replacing missing teeth: Different times for loading dental implants. Cochrane Database Syst. Rev. 2013, 2013, CD003878. [Google Scholar] [CrossRef] [Green Version]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwiatek, J.; Jaroń, A.; Trybek, G. Impact of the 25-Hydroxycholecalciferol Concentration and Vitamin D Deficiency Treatment on Changes in the Bone Level at the Implant Site during the Process of Osseointegration: A Prospective, Randomized, Controlled Clinical Trial. J. Clin. Med. 2021, 10, 526. [Google Scholar] [CrossRef] [PubMed]
- McCullough, J.J.; Klokkevold, P.R. The effect of implant macro-thread design on implant stability in the early post-operative period: A randomized, controlled pilot study. Clin. Oral Implant. Res. 2016, 28, 1218–1226. [Google Scholar] [CrossRef]
- Shen, X.; Ma, P.; Hu, Y.; Xu, G.; Zhou, J.; Cai, K. Mesenchymal stem cell growth behavior on micro/nano hierarchical surfaces of titanium substrates. Colloids Surfaces B Biointerfaces 2015, 127, 221–232. [Google Scholar] [CrossRef]
- Coelho, P.G.; Jimbo, R.; Tovar, N.; Bonfante, E.A. Osseointegration: Hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent. Mater. 2015, 31, 37–52. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.; Camacho, F.; Peñarrocha, D.; Tallarico, M.; Perez, S.; Canullo, L. Influence of plasma cleaning procedure on the interaction between soft tissue and abutments: A randomized controlled histologic study. Clin. Oral Implant. Res. 2016, 28, 1269–1277. [Google Scholar] [CrossRef]
- Att, W.; Hori, N.; Takeuchi, M.; Ouyang, J.; Yang, Y.; Anpo, M.; Ogawa, T. Time-dependent degradation of titanium osteoconductivity: An implication of biological aging of implant materials. Biomaterials 2009, 30, 5352–5363. [Google Scholar] [CrossRef] [PubMed]
- Krawiec, M.; Hadzik, J.; Dominiak, M.; Grzebieluch, W.; Błaszczyszyn, A.; Kubasiewicz-Ross, P. Early Loading of Titanium Dental Implants with Hydroxyl Ion Modified Surface: A 12-Month Prospective Clinical Trial. Appl. Sci. 2021, 11, 2958. [Google Scholar] [CrossRef]
- Misch, C.E.; Dietsh-Misch, F.; Hoar, J.; Beck, G.; Hazen, R.; Misch, C.M. A bone quality-based implant system: First year of prosthetic loading Crestal bone loss Radiographic changes Success rates Prospective study Implant success rate BioHorizon implants Bone quality Implant design. J. Oral Implantol. 1999, 25, 185–197. [Google Scholar] [CrossRef]
- Belser, U.C.; Grütter, L.; Vailati, F.; Bornstein, M.M.; Weber, H.-P.; Buser, D. Outcome Evaluation of Early Placed Maxillary Anterior Single-Tooth Implants Using Objective Esthetic Criteria: A Cross-Sectional, Retrospective Study in 45 Patients With a 2- to 4-Year Follow-Up Using Pink and White Esthetic Scores. J. Periodontol. 2009, 80, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Schnitman, P.A.; Wohrle, P.S.; Rubenstein, J.E. Immediate fixed interim prostheses supported by two-stage threaded implants: Methodology and results. J. Oral Implant. 1990, 16, 96–105. [Google Scholar]
- Chen, J.; Cai, M.; Yang, J.; Aldhohrah, T.; Wang, Y. Immediate versus early or conventional loading dental implants with fixed prostheses: A systematic review and meta-analysis of randomized controlled clinical trials. J. Prosthet. Dent. 2019, 122, 516–536. [Google Scholar] [CrossRef] [Green Version]
- Schnitman, P.A.; Wöhrle, P.S.; Rubenstein, J.E.; DaSilva, J.D.; Wang, N.H. Ten-year results for Brånemark implants immediately loaded with fixed prostheses at implant placement. Int. J. Oral Maxillofac. Implant. 1997, 12, 495–503. [Google Scholar]
- Papaspyridakos, P.; Chen, C.-J.; Chuang, S.-K.; Weber, H.-P.; Gallucci, G.O. A systematic review of biologic and technical complications with fixed implant rehabilitations for edentulous patients. Int. J. Oral Maxillofac. Implant. 2012, 27, 102–110. [Google Scholar]
- Linkevicius, T.; Apse, P.; Grybauskas, S.; Puisys, A. The influence of soft tissue thickness on crestal bone changes around implants: A 1-year prospective controlled clinical trial. Int. J. Oral Maxillofac. Implant. 2009, 24, 712–719. [Google Scholar]
- Puzio, M.; Hadzik, J.; Błaszczyszyn, A.; Gedrange, T.; Dominiak, M. Soft tissue augmentation around dental implants with connective tissue graft (CTG) and xenogenic collagen matrix (XCM). 1-year randomized control trail. Ann. Anat. Anat. Anz. 2020, 230, 151484. [Google Scholar] [CrossRef] [PubMed]
- Puzio, M.; Błaszczyszyn, A.; Hadzik, J.; Dominiak, M. Ultrasound assessment of soft tissue augmentation around implants in the aesthetic zone using a connective tissue graft and xenogeneic collagen matrix—1-year randomised follow-up. Ann. Anat. Anat. Anz. 2018, 217, 129–141. [Google Scholar] [CrossRef]
- Heinemann, F.; Grufferty, B.; Papavasiliou, G.; Dominiak, M.; García, J.J.; Trullenque-Eriksson, A.; Esposito, M. Immediate occluding definitive partial fixed prosthesis versus non-occluding provisional restorations—4-month post-loading results from a pragmatic multicenter randomised controlled trial. Eur. J. Oral Implant. 2016, 9, 47–56. [Google Scholar]
- Makowiecki, A.; Botzenhart, U.; Seeliger, J.; Heinemann, F.; Biocev, P.; Dominiak, M. A comparative study of the effectiveness of early and delayed loading of short tissue-level dental implants with hydrophilic surfaces placed in the posterior section of the mandible—A preliminary study. Ann. Anat. Anat. Anz. 2017, 212, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Makowiecki, A.; Hadzik, J.; Błaszczyszyn, A.; Gedrange, T.; Dominiak, M. An evaluation of superhydrophilic surfaces of dental implants—A systematic review and meta-analysis. BMC Oral Health 2019, 19, 79. [Google Scholar] [CrossRef]
- Chen, S.T.; Buser, D. Esthetic Outcomes Following Immediate and Early Implant Placement in the Anterior Maxilla—A Systematic Review. Int. J. Oral Maxillofac. Implant. 2014, 29, 186–215. [Google Scholar] [CrossRef] [Green Version]
- Arunyanak, S.P.; Pollini, A.; Ntounis, A.; Morton, D. Clinician assessments and patient perspectives of single-tooth implant restorations in the esthetic zone of the maxilla: A systematic review. J. Prosthet. Dent. 2017, 118, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Hof, M.; Umar, N.; Budas, N.; Seemann, R.; Pommer, B.; Zechner, W. Evaluation of implant esthetics using eight objective indices-Comparative analysis of reliability and validity. Clin. Oral Implant. Res. 2018, 29, 697–706. [Google Scholar] [CrossRef]
- Fürhauser, R.; Florescu, D.; Benesch, T.; Haas, R.; Mailath, G.; Watzek, G. Evaluation of soft tissue around single-tooth implant crowns: The pink esthetic score. Clin. Oral Implant. Res. 2005, 16, 639–644. [Google Scholar] [CrossRef]
- Choquet, V.; Hermans, M.; Adriaenssens, P.P.; Daelemans, P.; Tarnow, D.D.; Malevez, C. Clinical and Radiographic Evaluation of the Papilla Level Adjacent to Single-Tooth Dental Implants. A Retrospective Study in the Maxillary Anterior Region. J. Periodontol. 2001, 72, 1364–1371. [Google Scholar] [CrossRef]
- Tarnow, D.P.; Magner, A.W.; Fletcher, P. The Effect of the Distance from the Contact Point to the Crest of Bone on the Presence or Absence of the Interproximal Dental Papilla. J. Periodontol. 1992, 63, 995–996. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-K.; Kim, H.-S.; Yi, Y.-J.; Yun, P.-Y. Evaluation of subjective satisfaction of dental implant patients. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 130–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youk, S.-Y.; Lee, J.-H.; Park, J.-M.; Heo, S.J.; Roh, H.-K.; Park, E.-J.; Shin-Young, Y. A survey of the satisfaction of patients who have undergone implant surgery with and without employing a computer-guided implant surgical template. J. Adv. Prosthodont. 2014, 6, 395–405. [Google Scholar] [CrossRef] [PubMed]

| Variable | Group3,5 | Group 4,0 | t | p | d Cohena | ||
|---|---|---|---|---|---|---|---|
| M | SD | M | SD | ||||
| MBL | 0.26 | 0.31 | 0.14 | 0.24 | 1.30 | 0.203 | 0.43 |
| HKT 0 | 4.11 | 1.15 | 3.75 | 1.16 | 0.96 | 0.344 | 0.31 |
| HKT 1 | 4.00 | 1.30 | 3.85 | 0.88 | 0.43 | 0.671 | 0.14 |
| PPD | 2.17 | 0.53 | 2.04 | 0.37 | 0.82 | 0.417 | 0.27 |
| VAS | 1.53 | 0.96 | 1.75 | 1.33 | −0.60 | 0.554 | −0.19 |
| PES | 9.50 | 0.71 | 9.50 | 0.71 | 0.00 | 1.000 | 0.00 |
| WES | 9.78 | 0.55 | 9.72 | 0.46 | 0.33 | 0.744 | 0.12 |
| Variable | Biotype | |||||
|---|---|---|---|---|---|---|
| Thin (n = 27) | ||||||
| Mean rank | Me | IQR | M | SD | p | |
| MBL | 17.76 | 0.00 | 0.35 | 0.19 | 0.29 | 0.472 |
| PPD | 18.13 | 2.00 | 0.75 | 2.11 | 0.49 | 0.709 |
| PES | 18.20 | 10.00 | 1.00 | 9.48 | 0.7 | 0.736 |
| WES | 17.81 | 10.00 | 1.00 | 9.7 | 0.54 | 0.35 |
| HKT0 | 17.57 | 4.00 | 1.00 | 3.64 | 0.91 | 0.028 |
| HKT1 | 17.79 | 4.00 | 1.00 | 3.66 | 1.01 | 0.014 |
| VAS | 1.79 | 1 | 1 | 1.79 | 1.29 | 0.464 |
| Thick (n = 13) | ||||||
| Mean rank | Me | IQR | M | SD | p | |
| MBL | 20.72 | 0.30 | 0.45 | 0.24 | 0.24 | 0.472 |
| PPD | 19.61 | 2.25 | 0.56 | 2.1 | 0.36 | 0.709 |
| PES | 19.39 | 10.00 | 1.00 | 9.56 | 0.53 | 0.736 |
| WES | 20.56 | 10.00 | 0.00 | 9.89 | 0.33 | 0.35 |
| HKT0 | 26.18 | 5.00 | 2.00 | 4.64 | 1.43 | 0.028 |
| HKT1 | 27.64 | 5.00 | 1.00 | 4.64 | 1.02 | 0.014 |
| VAS | 1.29 | 1 | 1 | 1.27 | 0.47 | 0.464 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawiec, M.; Hadzik, J.; Olchowy, C.; Dominiak, M.; Kubasiewicz-Ross, P. Aesthetic Outcomes of Early Occlusal Loaded SLA Dental Implants with Hydroxyl Ion Modified Surface—A 12 Months Prospective Study. Materials 2021, 14, 6353. https://doi.org/10.3390/ma14216353
Krawiec M, Hadzik J, Olchowy C, Dominiak M, Kubasiewicz-Ross P. Aesthetic Outcomes of Early Occlusal Loaded SLA Dental Implants with Hydroxyl Ion Modified Surface—A 12 Months Prospective Study. Materials. 2021; 14(21):6353. https://doi.org/10.3390/ma14216353
Chicago/Turabian StyleKrawiec, Maciej, Jakub Hadzik, Cyprian Olchowy, Marzena Dominiak, and Paweł Kubasiewicz-Ross. 2021. "Aesthetic Outcomes of Early Occlusal Loaded SLA Dental Implants with Hydroxyl Ion Modified Surface—A 12 Months Prospective Study" Materials 14, no. 21: 6353. https://doi.org/10.3390/ma14216353
APA StyleKrawiec, M., Hadzik, J., Olchowy, C., Dominiak, M., & Kubasiewicz-Ross, P. (2021). Aesthetic Outcomes of Early Occlusal Loaded SLA Dental Implants with Hydroxyl Ion Modified Surface—A 12 Months Prospective Study. Materials, 14(21), 6353. https://doi.org/10.3390/ma14216353







