Diamond Coating Reduces Nuclear Fuel Rod Corrosion at Accidental Temperatures: The Role of Surface Electrochemistry and Semiconductivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diamond Coating
2.2. Scanning Electron Microscopy, Raman Spectroscopy
2.3. Oxidation Kinetics
2.4. Carrier Gas Hot Extraction
2.5. Electrochemical Impedance Spectroscopy
2.6. Ab Initio Calculations
3. Results
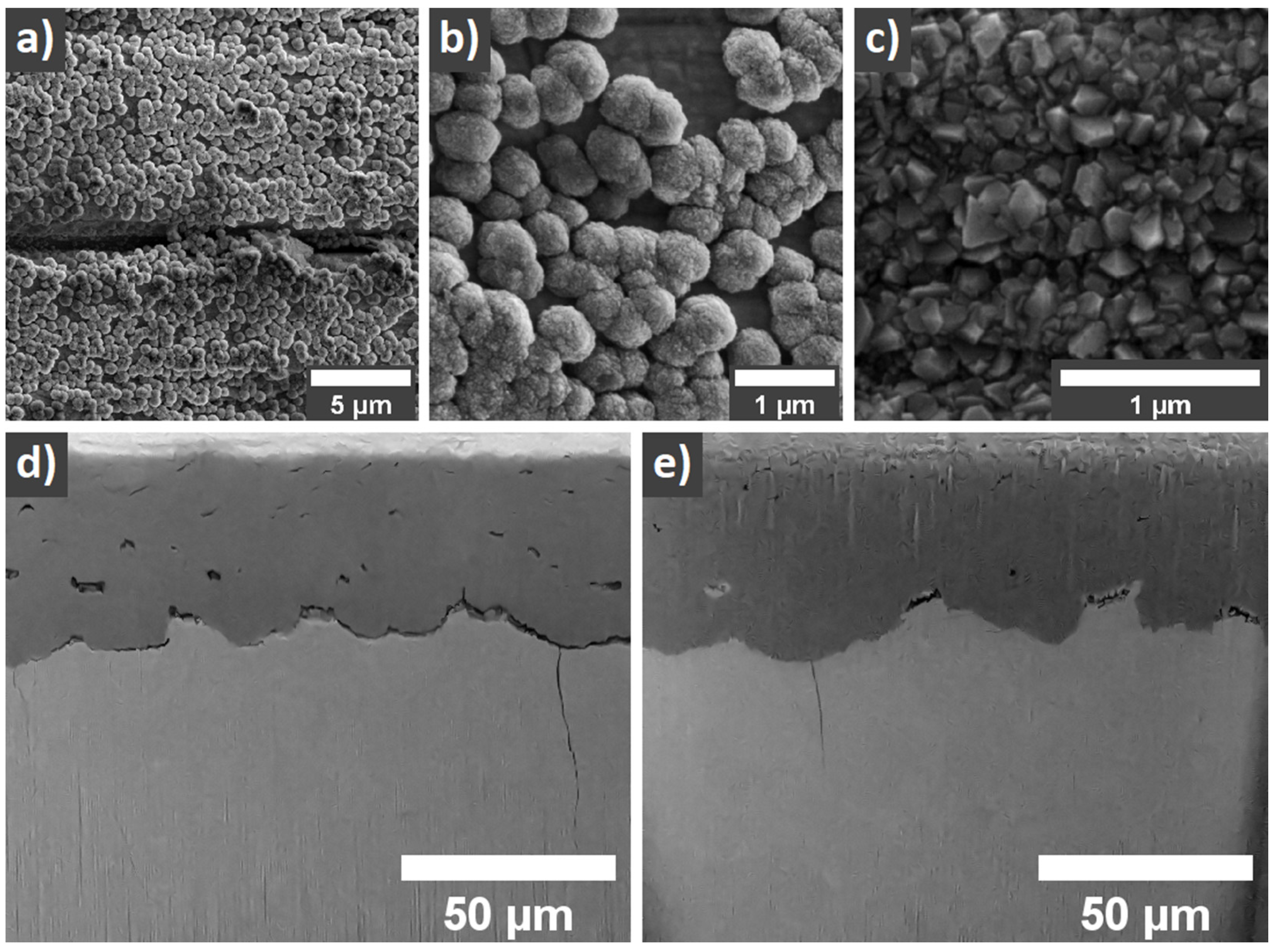
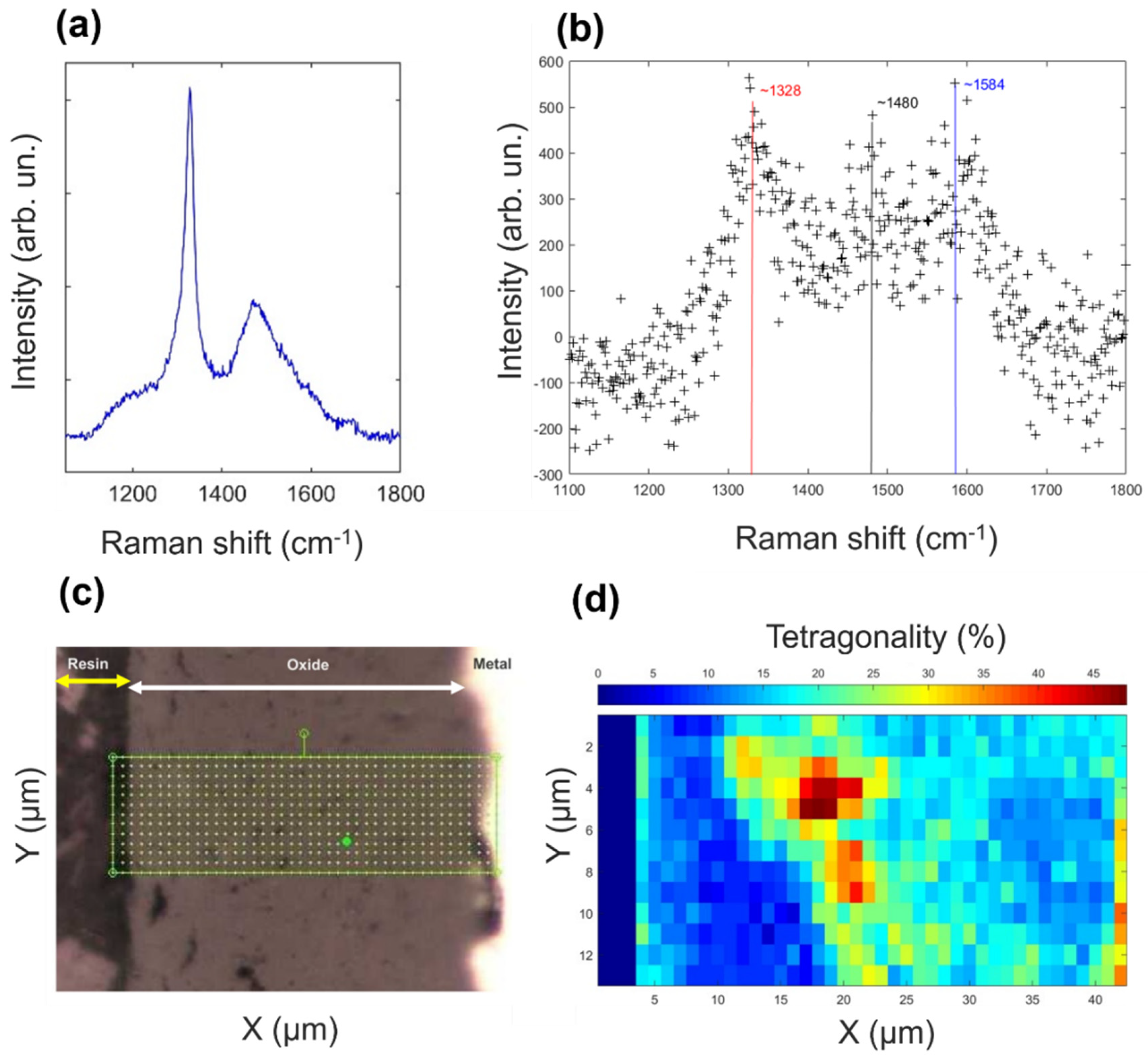
3.1. Scanning Electron Microscopy, Raman Spectroscopy
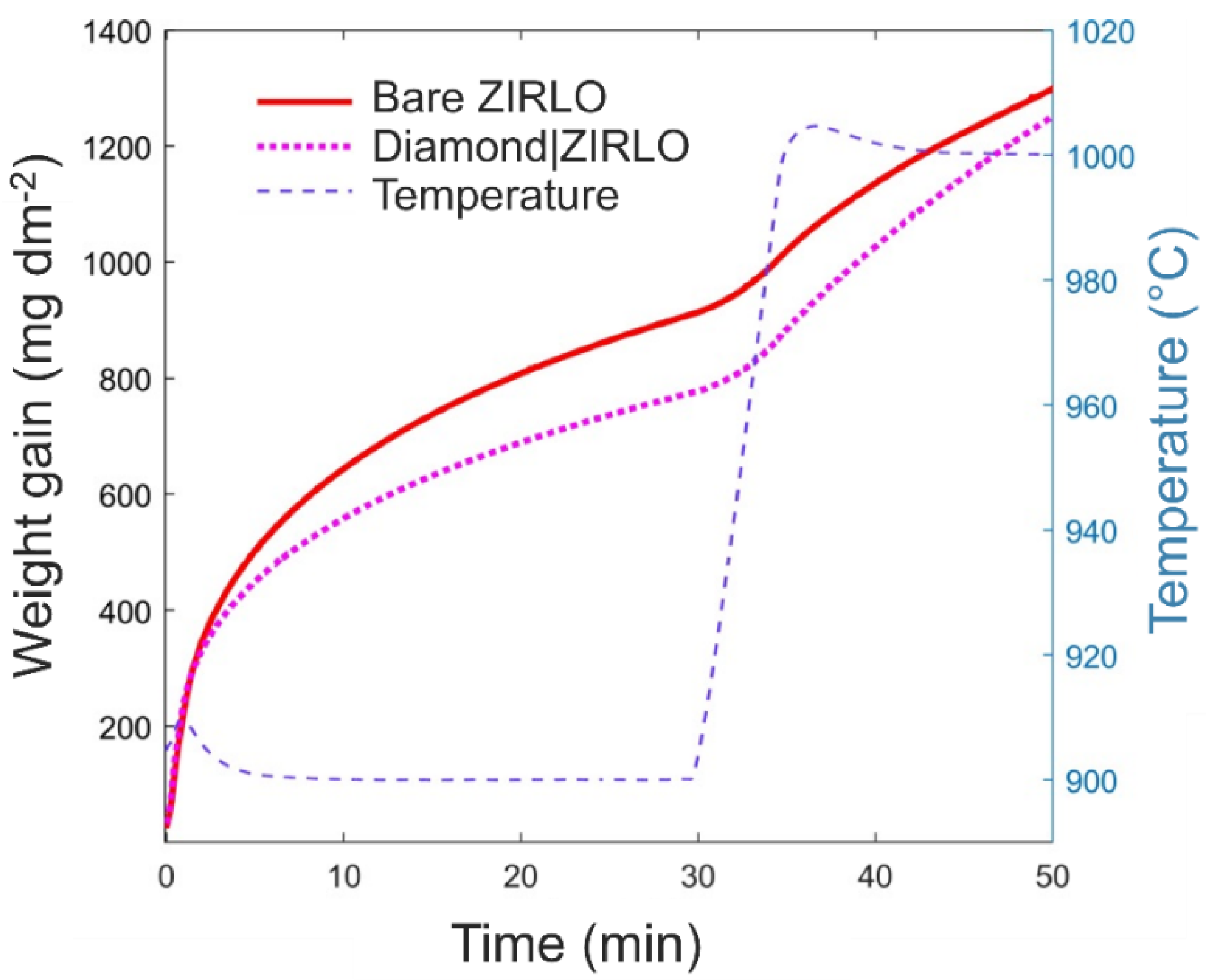
3.2. Oxidation Kinetics
3.3. Effect of the Diamond Coating on the Hydrogen Uptake by ZIRLO Surfaces
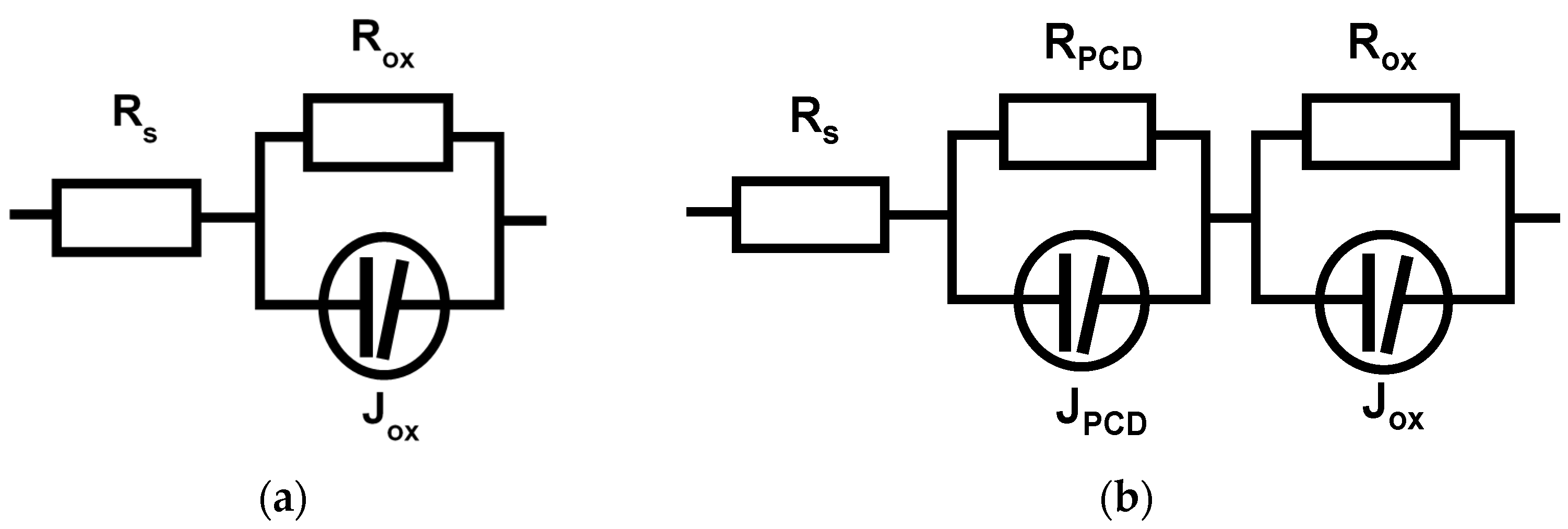
3.4. Electrochemical Impedance Spectroscopy
3.5. Ab Initio Calculations
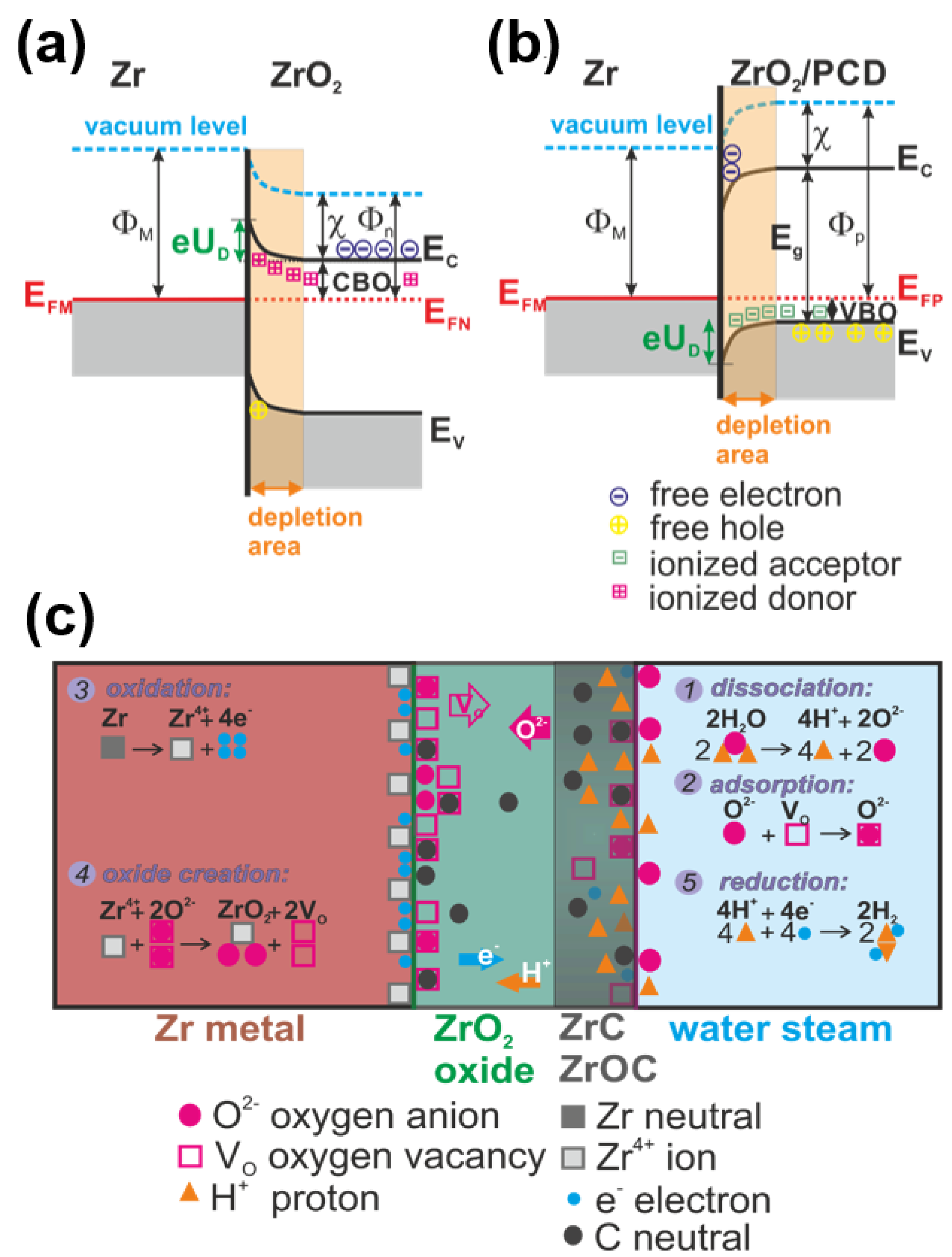
4. Discussion
Corrosion of the Diamond-Coated ZIRLO
5. Conclusions
- Carbon from diamond coating changes ZrO2 layer semi conductivity from the n-type to the n/p mixture type. Due to such material modification, the electric field at yhe Zr/ZrO2 interface is reduced and limits the Zr oxidation. After 20 min at 900 °C in a hot steam furnace, the oxidation of the diamond-coated ZIRLO rod was strongly affected by carbon diffusion: coated ZIRLO corrosion was reduced by ~20% compared to that of the uncoated ZIRLO rod treated under the same conditions. This protection is stronger with growing temperature and time when carbon atoms even from diamond nanocrystals are released and penetrate into the deeper parts of ZrO2.
- “Soft” carbon from diamond coatings supports establishment of ZrC and ZrOC complexes, which interact with H+. ZrC/H+ and ZrOC/H+ complexes disturb the water dissociation equilibrium (cathodic and anodic balance). However, the diamond coating protection declined at 1000 °C (oxidation of the diamond layer-coated ZIRLO rods was reduced just by 7% compared to that of the uncoated ZIRLO rods), and we suppose that this effect is strongly influenced by the complexes H+/ZrOC and H+/ZrC high temperature instability. At steam temperatures around 1000 °C, the H+/ZrOC and H+/ZrC complexes are massively destroyed, and better conditions for water dissociation on ZrOC and ZrC groups cause an increase of the ZIRLO corrosion.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudling, P.; Adamson, R.B. Performance and inspection of zirconium alloy fuel bundle components in light water reactors (LWRs). In Materials’ Ageing and Degradation in Light Water Reactors: Mechanisms and Management; Murty, K.L., Ed.; Woodhead Publ Ltd.: Cambridge, UK, 2013; pp. 246–283. [Google Scholar] [CrossRef]
- Allen, T.R.; Konings, R.J.M.; Motta, A.T. Corrosion of Zirconium Alloys. In Comprehensive Nuclear Materials; Elsevier Science Bv: Amsterdam, The Netherlands, 2012; pp. 49–68. [Google Scholar]
- Motta, A.T.; Couet, A.; Comstock, R.J. Corrosion of Zirconium Alloys Used for Nuclear Fuel Cladding. In Annual Review of Materials Research; Clarke, D.R., Ed.; Annual Reviews: San Mateo, CA, USA, 2015; Volume 45, pp. 311–343. [Google Scholar]
- Högberg, L. Root causes and impacts of severe accidents at large nuclear power plants. Ambio 2015, 42, 267–284. [Google Scholar] [CrossRef] [Green Version]
- Avincola, V.A.; Grosse, M.; Stegmaier, U.; Steinbrueck, M.; Seifert, H.J. Oxidation at high temperatures in steam atmosphere and quench of silicon carbide composites for nuclear application. Nucl. Eng. Des. 2015, 295, 468–478. [Google Scholar] [CrossRef]
- Cox, B. Some thoughts on the mechanisms of in-reactor corrosion of zirconium alloys. J. Nucl. Mater. 2005, 336, 331–368. [Google Scholar] [CrossRef]
- Saji, G. Root cause study on hydrogen generation and explosion through radiation-induced electrolysis in the Fukushima Daiichi accident. Nucl. Eng. Des. 2016, 307, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Fromhold, A.T. Parabolic oxidation of metals in homogeneous electric-fields. J. Phys. Chem. Solids 1972, 33, 95–120. [Google Scholar] [CrossRef]
- Tang, C.C.; Stueber, M.; Seifert, H.J.; Steinbrueck, M. Protective coatings on zirconium-based alloys as accident-tolerant fuel (ATF) claddings. Corros. Rev. 2017, 35, 141–165. [Google Scholar] [CrossRef]
- Ni, N.; Lozano-Perez, S.; Sykes, J.M.; Smith, G.D.W.; Grovenor, C.R.M. Focussed ion beam sectioning for the 3D characterisation of cracking in oxide scales formed on commercial ZIRLOTM alloys during corrosion in high temperature pressurised water. Corros. Sci. 2011, 53, 4073–4083. [Google Scholar] [CrossRef]
- Bell, B.D.C.; Murphy, S.T.; Burr, P.A.; Comstock, R.J.; Partezana, J.M.; Grimes, R.W.; Wenman, M.R. The influence of alloying elements on the corrosion of Zr alloys. Corros. Sci. 2016, 105, 36–43. [Google Scholar] [CrossRef] [Green Version]
- Yuan, G.H.; Cao, G.Q.; Yue, Q.; Yang, L.; Yun, Y.F.; Shao, G.S.; Hu, J.H. Formation and fine-structures of nano-precipitates in ZIRLO. J. Alloys Compd. 2016, 687, 451–457. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.Y.; Li, J.X.; Zhang, Y.; Zhang, L.C.; Lu, W.J.; Wang, L.Q.; Zhang, L.F.; Zhang, D. Zr-Sn-Nb-Fe-Si-O alloy for fuel cladding candidate: Processing, microstructure, corrosion resistance and tensile behavior. Corros. Sci. 2015, 100, 332–340. [Google Scholar] [CrossRef]
- Couet, A.; Motta, A.T.; Ambard, A. The coupled current charge compensation model for zirconium alloy fuel cladding oxidation: I. Parabolic oxidation of zirconium alloys. Corros. Sci. 2015, 100, 73–84. [Google Scholar] [CrossRef]
- Ensor, B.; Lucente, A.M.; Frederick, M.J.; Sutliff, J.; Motta, A.T. The role of hydrogen in zirconium alloy corrosion. J. Nucl. Mater. 2017, 496, 301–312. [Google Scholar] [CrossRef]
- Choudhry, K.I.; Mahboubi, S.; Botton, G.A.; Kish, J.R.; Svishchev, I.M. Corrosion of engineering materials in a supercritical water cooled reactor: Characterization of oxide scales on Alloy 800H and stainless steel 316. Corros. Sci. 2015, 100, 222–230. [Google Scholar] [CrossRef]
- Khatamian, D. Solubility and partitioning of hydrogen in metastable Zr-based alloys used in the nuclear industry. J. Alloys Compd. 1999, 293, 893–899. [Google Scholar] [CrossRef]
- Kratochvilova, I.; Skoda, R.; Skarohlid, J.; Ashcheulov, P.; Jager, A.; Racek, J.; Taylor, A.; Shao, L. Nanosized polycrystalline diamond cladding for surface protection of zirconium nuclear fuel rods. J. Mater. Process. Technol. 2014, 214, 2600–2605. [Google Scholar] [CrossRef]
- Skarohlid, J.; Ashcheulov, P.; Skoda, R.; Taylor, A.; Ctvrtlik, R.; Tomastik, J.; Fendrych, F.; Kopecek, J.; Chab, V.; Cichon, S.; et al. Nanocrystalline diamond protects Zr cladding surface against oxygen and hydrogen uptake: Nuclear fuel durability enhancement. Sci. Rep. 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashcheulov, P.; Skoda, R.; Skarohlid, J.; Taylor, A.; Fekete, L.; Fendrych, F.; Vega, R.; Shao, L.; Kalvoda, L.; Vratislav, S.; et al. Thin polycrystalline diamond films protecting zirconium alloys surfaces: From technology to layer analysis and application in nuclear facilities. Appl. Surf. Sci. 2015, 359, 621–628. [Google Scholar] [CrossRef]
- Kratochvilova, I.; Ashcheulov, P.; Skarohlid, J.; Skoda, R.; Kopecek, J.; Sajdl, P.; Macak, J.; Lajcinova, M.; Novakova, A.; Neethling, J.; et al. Zr alloy protection against high-temperature oxidation: Coating by a double-layered structure with active and passive functional properties. Corros. Sci. 2020, 163, 11. [Google Scholar] [CrossRef]
- Peng, D.Q.; Bai, X.D.; Chen, B.S. Corrosion behavior of carbon implanted ZIRLO alloy in 1 M H2SO4. J. Mater. Sci. 2005, 40, 1169–1175. [Google Scholar] [CrossRef]
- Sebera, J.; Nespurek, S.; Kratochvilova, I.; Zalis, S.; Chaidogiannos, G.; Glezos, N. Charge carrier mobility in sulphonated and non-sulphonated Ni phthalocyanines: Experiment and quantum chemical calculations. Eur. Phys. J. B 2009, 72, 385–395. [Google Scholar] [CrossRef]
- Škoda, R.; Škarohlíd, J.; Kratochvílová, I.; Taylor, A. Fendrych Nuclear Reactor Having a Layer Protecting the Surface of Zirconium Alloys and Nuclear Reactor Comprising Same. U.S. patent 10916352, 9 February 2021. [Google Scholar]
- Fendrych, F.; Taylor, A.; Peksa, L.; Kratochvilova, I.; Vlcek, J.; Rezacova, V.; Petrak, V.; Kluiber, Z.; Fekete, L.; Liehr, M.; et al. Growth and characterization of nanodiamond layers prepared using the plasma-enhanced linear antennas microwave CVD system. J. Phys. D Appl. Phys. 2010, 43, 374018. [Google Scholar] [CrossRef] [Green Version]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.J.; Refson, K.; Payne, M.C. First principles methods using CASTEP. Z. Für Krist. 2005, 220, 567–570. [Google Scholar] [CrossRef] [Green Version]
- Vanderbilt, D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism. Phys. Rev. B 1990, 41, 7892–7895. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamor, M.A.; Wu, C.H.; Carter, R.O.; Lindsay, N.E. Pendant benzene in hydrogenated diamond-like carbon. Appl. Phys. Lett. 1989, 55, 1388–1390. [Google Scholar] [CrossRef]
- Mosinska, L.; Popielarski, P.; Fabisiak, K.; Dychalska, A. Effects of hydrogen termination of CVD diamond layers. Opt. Mater. 2020, 101, 109676. [Google Scholar] [CrossRef]
- Marchon, B.; Gui, J.; Grannen, K.; Rauch, G.C.; Ager, J.W.; Silva, S.R.P.; Robertson, J. Photoluminescence and Raman spectroscopy in hydrogenated carbon films. IEEE Trans. Magn. 1997, 33, 3148–3150. [Google Scholar] [CrossRef] [Green Version]
- Efaw, C.M.; Vandegrift, J.L.; Reynolds, M.; McMurdie, S.; Jaques, B.J.; Hu, H.Q.; Xiong, H.; Hurley, M.F. Characterization of zirconium oxides part I: Raman mapping and spectral feature analysis. Nucl. Mater. Energy 2019, 21, 11. [Google Scholar] [CrossRef]
- Jonscher, A.K. A new understanding of the dielectric-relaxation of solids. J. Mater. Sci. 1981, 16, 2037–2060. [Google Scholar] [CrossRef]
- Krausova, A.; Macak, J.; Sajdl, P.; Novotny, R.; Renciukova, V.; Vrtilkova, V. In-situ electrochemical study of Zr1nb alloy corrosion in high temperature Li+ containing water. J. Nucl. Mater. 2015, 467, 302–310. [Google Scholar] [CrossRef]
- Renciukova, V.; Macak, J.; Sajdl, P.; Novotny, R.; Krausova, A. Corrosion of zirconium alloys demonstrated by using impedance spectroscopy. J. Nucl. Mater. 2018, 510, 312–321. [Google Scholar] [CrossRef]
- Kumar, M.K.P.; Laxmeesha, P.M.; Ray, S.; Srivastava, C. Enhancement in the corrosion resistance of nanocrystalline aluminium coatings by incorporation of graphene oxide. Appl. Surf. Sci. 2020, 533, 8. [Google Scholar] [CrossRef]
- Deo, Y.; Guha, S.; Sarkar, K.; Mohanta, P.; Pradhan, D.; Mondal, A. Electrodeposited Ni-Cu alloy coatings on mild steel for enhanced corrosion properties. Appl. Surf. Sci. 2020, 515, 12. [Google Scholar] [CrossRef]
- Perevalov, T.V.; Shaposhnikov, A.V.; Nasyrov, K.A.; Gritsenko, D.V.; Gritsenko, V.A.; Tapilin, V.M. Electronic structure of ZrO2 and HfO2. In Defects in High-K Gate Dielectric Stacks: Nano-Electronic Semiconductor Devices; Gusev, E., Ed.; Springer: Dordrecht, The Netherlands, 2006; Volume 220, pp. 423–434. [Google Scholar]
- Rebak, R.B.; Li, Y.-P.; Kim, Y.-J. Review on electrochemical corrosion of zirconium alloys in high temperature water. In Proceedings of the 14th International Conference on Environmental Degradation of Materials in Nuclear Power Systems, Virginia Beach, VA, USA, 23–27 August 2009; pp. 1400–1406. [Google Scholar]
- Landolt, D. Corrosion and Surface Chemistry of Metals; Taylor & Francis Group: New York, NY, USA, 2007. [Google Scholar] [CrossRef]
- Moya, J.S.; Diaz, M.; Bartolome, J.F.; Roman, E.; Sacedon, J.L.; Izquierdo, J. Zirconium oxide film formation on zircaloy by water corrosion. Acta Mater. 2000, 48, 4749–4754. [Google Scholar] [CrossRef]

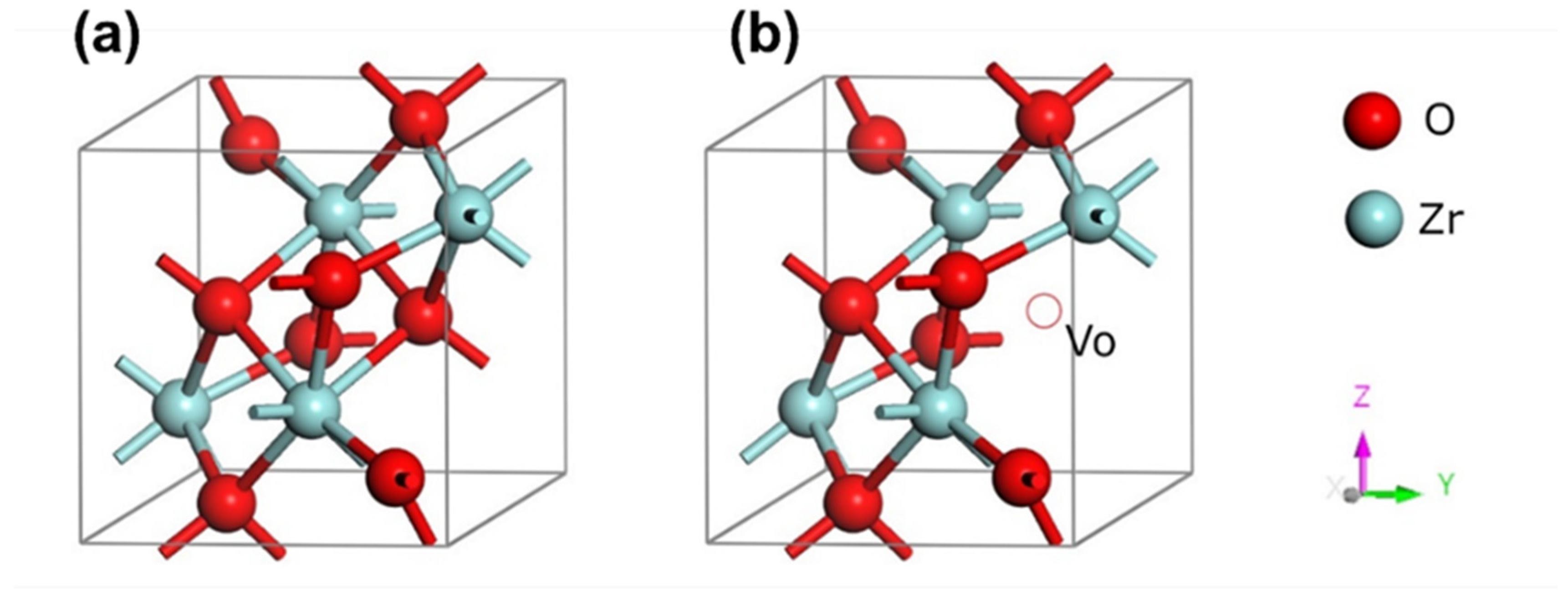
| ZIRLO Rod Sample | Relative Weight Gain (% of ZIRLO wg) 850 °C | Weight Gain (mg∙dm−2) 850 °C | Relative Weight Gain (% of ZIRLO wg) 900–1000 °C | Weight Gain (mg∙dm−2) 900–1000 °C |
|---|---|---|---|---|
| Coated | 84 | 700 | 93 | 1134 |
| Bare | 100 | 790 | 100 | 1220 |
| Scheme | Remark | Rdiam | Cdiam | Ddiam | εdiam | Rox | Cox | dox | εOX |
|---|---|---|---|---|---|---|---|---|---|
| (Ω·cm2) | (F·cm−2) | (µm) | (Ω·cm2) | (F·cm−2) | (µm) | ||||
| ZIRLO | bare | 4 × 107 | 1.7 × 10−6 | ||||||
| ZIRLO + diam | 0.5 μm diam | 32 | 2.2 × 10−8 | 0.5 | 12 | 9 × 107 | 2.2 × 10−7 | ||
| ZIRLO | 40 min, 850 °C | 1.2 × 109 | 1.8 × 10−9 | 25 | 54 | ||||
| ZIRLO + diam | 40 min, 850 °C | 1188 | 8.5 × 10−9 | 1–2 | 10–19 | 7.4 × 1010 | 9.5 × 10−10 | 22 | 21 |
| Surface Type | (eV) |
|---|---|
| ZrO2 | −0.31 |
| ZrOVO | −0.32 |
| ZrOC | −0.77 |
| ZrC | −0.55 |
| Surface Type | (eV) | (eV) |
|---|---|---|
| ZrO2 | −0.51 | −17.12 |
| ZrOVo | −0.63 | −16.87 |
| ZrOC | −1.59 | −18.14 |
| ZrC | −0.59 | −19.57 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Celbová, L.; Ashcheulov, P.; Klimša, L.; Kopeček, J.; Aubrechtová Dragounová, K.; Luštinec, J.; Macák, J.; Škoda, R.; Kratochvílová, I. Diamond Coating Reduces Nuclear Fuel Rod Corrosion at Accidental Temperatures: The Role of Surface Electrochemistry and Semiconductivity. Materials 2021, 14, 6315. https://doi.org/10.3390/ma14216315
Celbová L, Ashcheulov P, Klimša L, Kopeček J, Aubrechtová Dragounová K, Luštinec J, Macák J, Škoda R, Kratochvílová I. Diamond Coating Reduces Nuclear Fuel Rod Corrosion at Accidental Temperatures: The Role of Surface Electrochemistry and Semiconductivity. Materials. 2021; 14(21):6315. https://doi.org/10.3390/ma14216315
Chicago/Turabian StyleCelbová, Lucie, Petr Ashcheulov, Ladislav Klimša, Jaromír Kopeček, Kateřina Aubrechtová Dragounová, Jakub Luštinec, Jan Macák, Radek Škoda, and Irena Kratochvílová. 2021. "Diamond Coating Reduces Nuclear Fuel Rod Corrosion at Accidental Temperatures: The Role of Surface Electrochemistry and Semiconductivity" Materials 14, no. 21: 6315. https://doi.org/10.3390/ma14216315
APA StyleCelbová, L., Ashcheulov, P., Klimša, L., Kopeček, J., Aubrechtová Dragounová, K., Luštinec, J., Macák, J., Škoda, R., & Kratochvílová, I. (2021). Diamond Coating Reduces Nuclear Fuel Rod Corrosion at Accidental Temperatures: The Role of Surface Electrochemistry and Semiconductivity. Materials, 14(21), 6315. https://doi.org/10.3390/ma14216315








