Antimicrobial and Anticorrosion Activity of a Novel Composite Biocide against Mixed Bacterial Strains in Taiwanese Marine Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Mixed Strains and Culture Media
2.3. Mixed Strain Purification
2.4. Polymerase Chain Reaction and Strain Identification
2.5. MICs of Composite Biocides
2.6. Crystal Violet Assay for Biofilm Quantification
2.7. MIC Immersion and Electrochemical Measurements
2.8. Surface Morphology Examination
2.9. Biofilm Characterization
3. Results
3.1. Strain Purification and Classification
3.2. Determination of the MICs of Composite Biocides
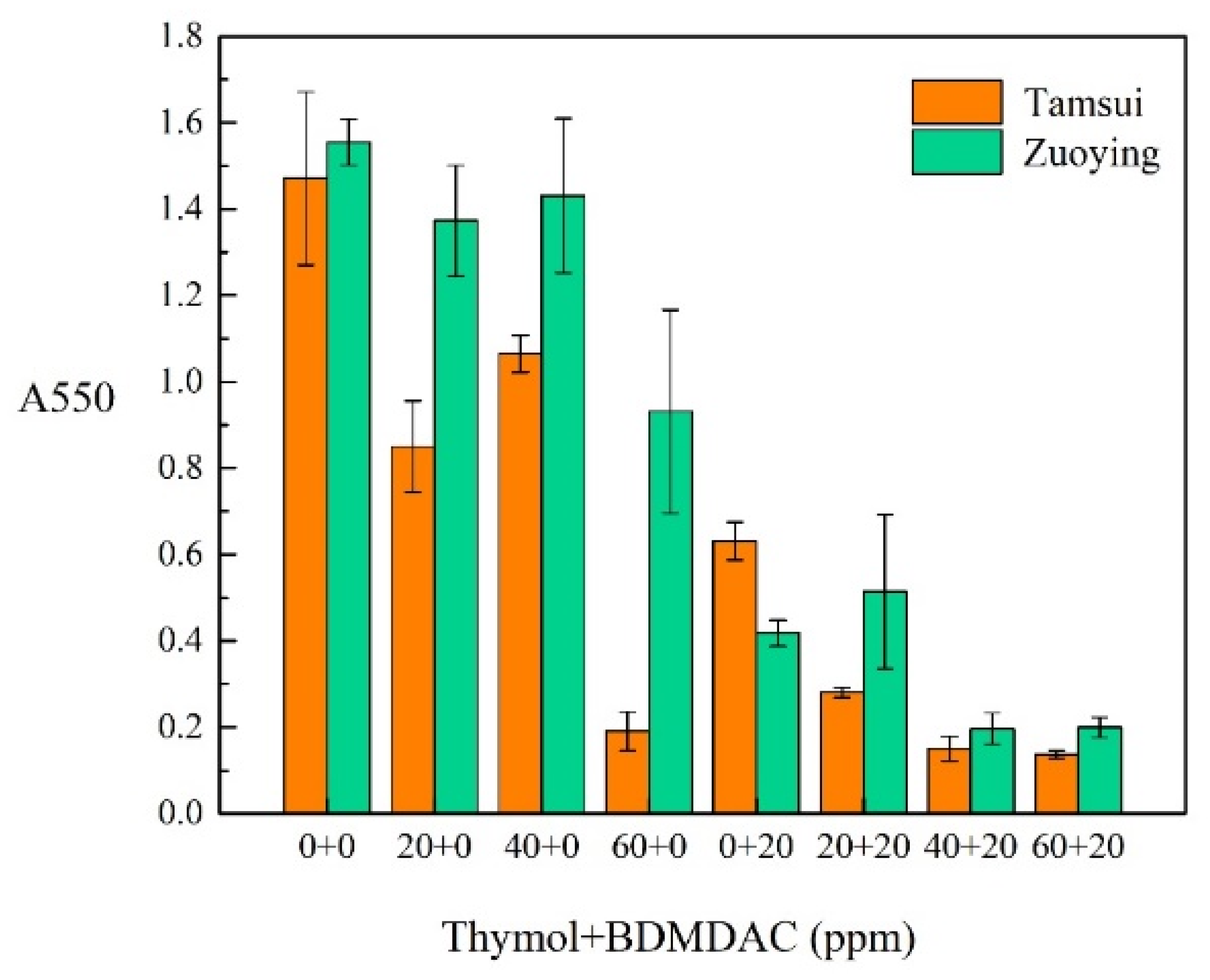
3.3. Effect of Composite Biocides on Biofilm Formation: CV Staining
3.4. Surface Morphology and Characterization
3.5. Characterization of Biofilm and Antimicrobial Activity
3.6. EIS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Xu, D.; Chen, C.; Li, X.; Jia, R.; Zhang, D.; Sand, W.; Wang, F.; Gu, T. Anaerobic microbiologically influenced corrosion mechanisms interpreted using bioenergetics and bioelectrochemistry: A review. J. Mater. Sci. Technol. 2018, 34, 1713–1718. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Liu, Z.; Li, Z.; Du, C.; Dong, C. Materials science: Share corrosion data. Nature 2015, 527, 441–442. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, Y.F. Microbial corrosion of initial perforation on abandoned pipelines in wet soil containing sulfate-reducing bacteria. Colloids Surf. B Biointerfaces 2020, 190, 110899. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xu, D.; Yang, K.; Liu, H.; Cheng, Y.F. Corrosion of antibacterial Cu-bearing 316L stainless steels in the presence of sulfate reducing bacteria. Corros. Sci. 2018, 132, 46–55. [Google Scholar] [CrossRef]
- Wu, T.; Xu, J.; Yan, M.; Sun, C.; Yu, C.; Ke, W. Synergistic effect of sulfate-reducing bacteria and elastic stress on corrosion of X80 steel in soil solution. Corros. Sci. 2014, 83, 38–47. [Google Scholar] [CrossRef]
- Yuan, S.; Pehkonen, S. Microbiologically influenced corrosion of 304 stainless steel by aerobic Pseudomonas NCIMB 2021 bacteria: AFM and XPS study. Colloids Surf. B Biointerfaces 2007, 59, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Chandrasatheesh, C.; Jayapriya, J.; George, R.; Mudali, U.K. Detection and analysis of microbiologically influenced corrosion of 316 L stainless steel with electrochemical noise technique. Eng. Fail. Anal. 2014, 42, 133–142. [Google Scholar] [CrossRef]
- Wang, H.; Ju, L.K.; Castaneda, H.; Cheng, G.; Newby, B.-M.Z. Corrosion of carbon steel C1010 in the presence of iron oxidizing bacteria Acidithiobacillus ferrooxidans. Corros. Sci. 2014, 89, 250–257. [Google Scholar] [CrossRef]
- Jia, R.; Yang, D.; Xu, D.; Gu, T. Electron transfer mediators accelerated the microbiologically influence corrosion against carbon steel by nitrate reducing Pseudomonas aeruginosa biofilm. Bioelectrochemistry 2017, 118, 38–46. [Google Scholar] [CrossRef]
- Halim, A.; Gubner, R.; Watkin, E. Preliminary Study on Nitrate Injection to Control Souring Problem in Oil Reservoir: Benefits And Side Effects On Steel Material (UNS S31603). In Proceedings of the CORROSION 2011, Houston, TX, USA, 13–17 March 2011; Volume 11229, pp. 3684–3698. [Google Scholar]
- Ozuolmez, D.; Na, H.; Lever, M.A.; Kjeldsen, K.U.; Jørgensen, B.B.; Plugge, C.M. Methanogenic archaea and sulfate reducing bacteria co-cultured on acetate: Teamwork or coexistence? Front. Microbiol. 2015, 6, 492. [Google Scholar] [CrossRef]
- Suarez, E.M.; Lepkova, K.; Kinsella, B.; Machuca, L.L. Aggressive corrosion of steel by a thermophilic microbial consortium in the presence and absence of sand. Int. Biodeterior. Biodegrad. 2019, 137, 137–146. [Google Scholar] [CrossRef]
- Gerald, O.J.; WenGe, L.; Zhang, L.; YuanTao, Z.; Long, L.C. Corrosion behaviour of 2205 duplex stainless steel in marine conditions containing Erythrobacter pelagi bacteria. Mater. Chem. Phys. 2020, 239, 122010. [Google Scholar] [CrossRef]
- Little, B.; Wagner, P.; Hart, K.; Ray, R.; Lavoie, D.; Nealson, K.; Aguilar, C. The role of metal-reducing bacteria in microbiologically influenced corrosion. In Proceedings of the Corrosion/97, New Orleans, LA, USA, 9–14 March 1997; Volume 215, pp. 9–14. [Google Scholar]
- Qu, Q.; Wang, L.; Li, L.; He, Y.; Yang, M.; Ding, Z. Effect of the fungus, Aspergillus niger, on the corrosion behaviour of AZ31B magnesium alloy in artificial seawater. Corros. Sci. 2015, 98, 249–259. [Google Scholar] [CrossRef]
- Hongwei, L.; Hongfang, L. Research Progress of Corrosion of Steels Induced by Iron Oxidizing Bacteria. J. Chin. Soc. Corros. Prot. 2017, 37, 195–206. [Google Scholar]
- Liu, H.; Fu, C.; Gu, T.; Zhang, G.; Lv, Y.; Wang, H.; Liu, H. Corrosion behavior of carbon steel in the presence of sulfate reducing bacteria and iron oxidizing bacteria cultured in oilfield produced water. Corros. Sci. 2015, 100, 484–495. [Google Scholar] [CrossRef]
- Halim, A.; Watkin, E.; Gubner, R. Short term corrosion monitoring of carbon steel by bio-competitive exclusion of thermophilic sulphate reducing bacteria and nitrate reducing bacteria. Electrochim. Acta 2012, 77, 348–362. [Google Scholar] [CrossRef]
- Voordouw, G.; Nemati, M.; Jenneman, G. Use of nitrate reducing, sulfide oxidizing bacteria to reduce souring in oil fields: Interactions with SRB and effects on corrosion. In Proceedings of the CORROSION 2002, Denver, CO, USA, 7–11 April 2002; Volume 34, pp. 1–6. [Google Scholar]
- Raman, R.S.; Banerjee, P.C.; Lobo, D.E.; Gullapalli, H.; Sumandasa, M.; Kumar, A.; Choudhary, L.; Tkacz, R.; Ajayan, P.M.; Majumder, M. Protecting copper from electrochemical degradation by graphene coating. Carbon 2012, 50, 4040–4045. [Google Scholar] [CrossRef]
- Duncan, K.E.; Perez-Ibarra, B.M.; Jenneman, G.; Harris, J.B.; Webb, R.; Sublette, K. The effect of corrosion inhibitors on microbial communities associated with corrosion in a model flow cell system. Appl. Microbiol. Biotechnol. 2014, 98, 907–918. [Google Scholar] [CrossRef]
- Kajiyama, F.; Okamura, K. Evaluating cathodic protection reliability on steel pipe in microbially active soils. Corrosion 1999, 55, 74–80. [Google Scholar] [CrossRef]
- Esquivel, R.G.; Olivares, G.Z.; Gayosso, M.H.; Trejo, A.G. Cathodic protection of XL 52 steel under the influence of sulfate reducing bacteria. Mater. Corros. 2011, 62, 61–67. [Google Scholar] [CrossRef]
- Guiamet, P.; de Saravia, S.G. Laboratory studies of biocorrosion control using traditional and environmentally friendly biocides: An overview. Lat. Am. Appl. Res. 2005, 35, 295–300. [Google Scholar]
- Bhola, S.M.; Alabbas, F.M.; Bhola, R.; Spear, J.R.; Mishra, B.; Olson, D.L.; Kakpovbia, A.E. Neem extract as an inhibitor for biocorrosion influenced by sulfate reducing bacteria: A preliminary investigation. Eng. Fail. Anal. 2014, 36, 92–103. [Google Scholar] [CrossRef]
- Liu, F.; Chang, X.; Yang, F.; Wang, Y.; Wang, F.; Dong, W.; Zhao, C. Effect of oxidizing and non-oxidizing biocides on biofilm at different substrate levels in the model recirculating cooling water system. World J. Microbiol. Biotechnol. 2011, 27, 2989–2997. [Google Scholar] [CrossRef]
- Khelissa, S.; Gharsallaoui, A.; Fadel, A.; Barras, A.; Jama, C.; Jbilou, F.; Chihib, N.-E. Microencapsulation of benzalkonium chloride enhanced its antibacterial and antibiofilm activities against Listeria monocytogenes and Escherichia coli. J. Appl. Microbiol. 2021, 131, 1136–1146. [Google Scholar] [CrossRef] [PubMed]
- Aikawa, T.; Yokota, K.; Kondo, T.; Yuasa, M. Intermolecular interaction between phosphatidylcholine and sulfobetaine lipid: A combination of lipids with antiparallel arranged headgroup charge. Langmuir 2016, 32, 10483–10490. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, C. The antibacterial finish of cotton via sols containing quaternary ammonium salts. J. Sol Gel Sci. Technol. 2009, 50, 15–21. [Google Scholar] [CrossRef]
- Hsu, C.W.; Chen, T.E.; Lo, K.Y.; Lee, Y.L. Inhibitive Properties of Benzyldimethyldodecylammonium Chloride on Microbial Corrosion of 304 Stainless Steel in a Desulfovibrio desulfuricans-Inoculated Medium. Materials 2019, 12, 307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steele, E.A.; Breen, C.; Campbell, E.; Martin, R. Food Regulations and Enforcement in the USA. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–13. [Google Scholar]
- Nicolaou, K.; Snyder, S.A. Chasing molecules that were never there: Misassigned natural products and the role of chemical synthesis in modern structure elucidation. Angew. Chem. Int. Ed. 2005, 44, 1012–1044. [Google Scholar] [CrossRef]
- Castillo, S.; Pérez-Alfonso, C.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. The essential oils thymol and carvacrol applied in the packing lines avoid lemon spoilage and maintain quality during storage. Food Control 2014, 35, 132–136. [Google Scholar] [CrossRef]
- Bauer, A.; Brönstrup, M. Industrial natural product chemistry for drug discovery and development. Nat. Prod. Rep. 2014, 31, 35–60. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Wiegand, I.; Hilpert, K.; Hancock, R.E. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- McFarland, J. The nephelometer: An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J. Am. Med. Assoc. 1907, 49, 1176–1178. [Google Scholar] [CrossRef] [Green Version]
- Chang, S.Y.; Chang, J.Y.; Xie, T.C.; Lo, K.Y.; Lee, Y.L. Evaluation of thymol as a biocide against microbiogically influenced corrosion: Taking Tamsui river bacteria strain as example. J. Taiwan Soc. Nav. Archit. Mar. Eng. 2018, 37, 159–167. [Google Scholar]
- Lv, M.; Du, M.; Li, X.; Yue, Y.; Chen, X. Mechanism of microbiologically influenced corrosion of X65 steel in seawater containing sulfate-reducing bacteria and iron-oxidizing bacteria. J. Mater. Res. Technol. 2019, 8, 4066–4078. [Google Scholar] [CrossRef]
- Wen, Y.; Zhang, X.R.; Chen, M.; Wu, Z.C.; Wang, Z.W. Characterization of antibiofouling behaviors of PVDF membrane modified by quaternary ammonium compound-combined use of QCM-D, FCM, and CLSM. J. Water Reuse Desalination 2018, 9, 18–30. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, D.; Shahzad, M.B.; Kang, Q.; Sun, Y.; Sun, Z.; Zhang, S.; Ren, L.; Yang, C.; Yang, K. Effect of surface passivation on corrosion resistance and antibacterial properties of Cu-bearing 316L stainless steel. Appl. Surf. Sci. 2016, 386, 371–380. [Google Scholar] [CrossRef]
- Liamleam, W.; Annachhatre, A.P. Electron donors for biological sulfate reduction. Biotechnol. Adv. 2007, 25, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Greene, E.A.; Brunelle, V.; Jenneman, G.E.; Voordouw, G. Synergistic inhibition of microbial sulfide production by combinations of the metabolic inhibitor nitrite and biocides. Appl. Environ. Microbiol. 2006, 72, 7897–7901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Cheng, Y.F. Mechanistic aspects of microbially influenced corrosion of X52 pipeline steel in a thin layer of soil solution containing sulphate-reducing bacteria under various gassing conditions. Corros. Sci. 2018, 133, 178–189. [Google Scholar] [CrossRef]
- Le Borgne, S.; Romero, J.; Garcia-Villalobos, J.; Videla, H.A. Detecting and monitoring bacteria in seawater injection systems. Mater. Perform. 2007, 46, 52–56. [Google Scholar]
- Herrera, L.K.; Videla, H.A. Role of iron-reducing bacteria in corrosion and protection of carbon steel. Int. Biodeterior. Biodegrad. 2009, 63, 891–895. [Google Scholar] [CrossRef]
- Coyer, J.A.; Cabello-Pasini, A.; Swift, H.; Alberte, R.S. N2 fixation in marine heterotrophic bacteria: Dynamics of environmental and molecular regulation. Proc. Natl. Acad. Sci. USA 1996, 93, 3575–3580. [Google Scholar] [CrossRef] [Green Version]
- Gaylarde, C.C.; Videla, H.A. Localised corrosion induced by a marine vibrio. Int. Biodeterior. 1987, 23, 91–104. [Google Scholar] [CrossRef]
- Ferreira, C.; Pereira, A.; Pereira, M.; Melo, L.; Simões, M. Physiological changes induced by the quaternary ammonium compound benzyldimethyldodecylammonium chloride on Pseudomonas fluorescens. J. Antimicrob. Chemother. 2011, 66, 1036–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa-Silva, M.; Simões, M.; Melo, L.; Machado, I. Pseudomonas fluorescens tolerance to benzyldimethyldodecyl ammonium chloride: Altered phenotype and cross-resistance. J. Glob. Antimicrob. Resist. 2018, 15, 188–195. [Google Scholar] [CrossRef]
- Engel, J.B.; Heckler, C.; Tondo, E.C.; Daroit, D.J.; da Silva Malheiros, P. Antimicrobial activity of free and liposome-encapsulated thymol and carvacrol against Salmonella and Staphylococcus aureus adhered to stainless steel. Int. J. Food Microbiol. 2017, 252, 18–23. [Google Scholar] [CrossRef]
- Wang, Y.; Yam, K.L. Inhibitory effect of thymol via different modes of delivery on growth of Escherichia coli DH5α. Food Packag. Shelf Life 2018, 16, 92–96. [Google Scholar] [CrossRef]
- Walsh, S.E.; Maillard, J.-Y.; Russell, A.; Catrenich, C.; Charbonneau, D.; Bartolo, R. Development of bacterial resistance to several biocides and effects on antibiotic susceptibility. J. Hosp. Infect. 2003, 55, 98–107. [Google Scholar] [CrossRef]
- Fu, E.; McCue, K.; Boesenberg, D. Chemical Disinfection of Hard Surfaces–Household, Industrial and Institutional Settings. In Handbook for Cleaning/Decontamination of Surfaces; Elsevier: Amsterdam, The Netherlands, 2007; pp. 573–592. [Google Scholar]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action, synergies, and interactions with food matrix components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.J.; Turner, R.J.; Joo, D.A.; Stan, M.A.; Chan, C.S.; Allan, N.D.; Vrionis, H.A.; Olson, M.E.; Ceri, H. Copper and quaternary ammonium cations exert synergistic bactericidal and antibiofilm activity against Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2008, 52, 2870–2881. [Google Scholar] [CrossRef] [Green Version]
- Hamoud, R.; Zimmermann, S.; Reichling, J.; Wink, M. Synergistic interactions in two-drug and three-drug combinations (thymol, EDTA and vancomycin) against multi drug resistant bacteria including E. coli. Phytomedicine 2014, 21, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.L. Clinical Microbiology Procedures Handbook; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Sader, H.S.; Fritsche, T.R.; Jones, R.N. Daptomycin bactericidal activity and correlation between disk and broth microdilution method results in testing of Staphylococcus aureus strains with decreased susceptibility to vancomycin. Antimicrob. Agents Chemother. 2006, 50, 2330–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]












| Tamsui Strains | Microorganism |
|---|---|
| IST1 | Oceanimonas doudoroffii [39] |
| IST2 | Shewanella algae [39] |
| Shewanella haliotis [39] | |
| IST3 | Shewanella baltica [39] |
| IST4 | Vibrio neocaledonicus [39] |
| IST5 | Acinetobacter johnsonii [39] |
| Acinetobacter tjernbergiae [39] | |
| IST6 | Oceanisphaera donghaensis [39] |
| Zuoying Strains | Microorganism |
| ISZ1 | Vibrio alginolyticus |
| ISZ2 | Vibrio harveyi |
| Vibrio natriegens | |
| ISZ3 | Vibrio algnolyticus |
| ISZ4 | Shewanella algae |
| ISZ5 | Oceanimonas baumannii. |
| Tamsui | Element (wt %) | Fe | Cr | Ni | O | S | P |
| 12 h | Spectrum 1 | 74.36 | 21.11 | 4.53 | |||
| Spectrum 2 | 62.39 | 19.48 | 8.32 | 7.78 | 2.03 | ||
| Spectrum 3 | 47.40 | 15.83 | 5.81 | 25.91 | 5.06 | ||
| 336 h | Spectrum 4 | 71.87 | 21.61 | 6.52 | |||
| Spectrum 5 | 35.51 | 11.20 | 1.83 | 36.13 | 15.33 | ||
| Spectrum 6 | 41.65 | 7.31 | 1.54 | 31.05 | 18.45 | ||
| Zuoying | Element (wt %) | Fe | Cr | Ni | O | S | P |
| 12 h | Spectrum 7 | 70.61 | 21.57 | 7.82 | |||
| Spectrum 8 | 54.78 | 16.99 | 3.91 | 17.02 | 1.28 | 6.03 | |
| Spectrum 9 | 61.36 | 17.50 | 5.10 | 12.48 | 0.70 | 2.87 | |
| 336 h | Spectrum 10 | 74.81 | 18.05 | 7.14 | |||
| Spectrum 11 | 59.86 | 18.91 | 8.04 | 10.10 | 3.08 | ||
| Spectrum 12 | 53.64 | 13.42 | 3.68 | 22.12 | 7.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.-Y.; Huang, S.-Y.; Chu, Y.-R.; Jian, S.-Y.; Lo, K.-Y.; Lee, Y.-L. Antimicrobial and Anticorrosion Activity of a Novel Composite Biocide against Mixed Bacterial Strains in Taiwanese Marine Environments. Materials 2021, 14, 6156. https://doi.org/10.3390/ma14206156
Chang S-Y, Huang S-Y, Chu Y-R, Jian S-Y, Lo K-Y, Lee Y-L. Antimicrobial and Anticorrosion Activity of a Novel Composite Biocide against Mixed Bacterial Strains in Taiwanese Marine Environments. Materials. 2021; 14(20):6156. https://doi.org/10.3390/ma14206156
Chicago/Turabian StyleChang, Soul-Yi, Shih-Yen Huang, Yu-Ren Chu, Shun-Yi Jian, Kai-Yin Lo, and Yueh-Lien Lee. 2021. "Antimicrobial and Anticorrosion Activity of a Novel Composite Biocide against Mixed Bacterial Strains in Taiwanese Marine Environments" Materials 14, no. 20: 6156. https://doi.org/10.3390/ma14206156
APA StyleChang, S.-Y., Huang, S.-Y., Chu, Y.-R., Jian, S.-Y., Lo, K.-Y., & Lee, Y.-L. (2021). Antimicrobial and Anticorrosion Activity of a Novel Composite Biocide against Mixed Bacterial Strains in Taiwanese Marine Environments. Materials, 14(20), 6156. https://doi.org/10.3390/ma14206156






