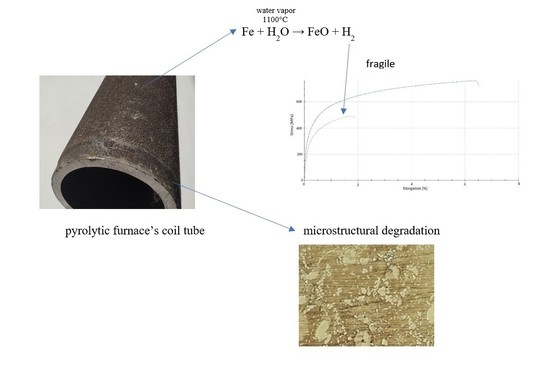
Investigation of the 0.4C-35Cr-45Ni-Nb Alloy after Service in High-Temperature Steam and Hydrocarbons Environment
Abstract
:1. Introduction
2. Materials and Methods
- Metallographic investigations were carried out using the metallographic microscope Nikon MA-200 (Nikon Corporation, Tokyo, Japan). The samples were prepared by grinding with abrasive paper of gradation up to 4000 (average particle diameter 2.5 μm) and then by polishing with diamond paste of gradation down to 1 μm. The etching involved a freshly prepared mixture of 10 cm3 of 65% nitric acid (HNO3), 20 cm3 of 40% hydrofluoric acid (HF) and 30 cm3 of glycerin (C3H5(OH)3). All solutions were prepared with ACS grade purity reagents from Sigma-Aldrich.
- Scanning electron microscopy (SEM) measurements were performed with the S-3400 N microscope manufactured by Hitachi (Schaumburg, IL, USA). The micrographs were taken in the secondary electron mode for 20 kV accelerating voltage. The imaging involved the backscattered electron (BSE) mode. The microscope was equipped with an EDX detector by ThermoFisher Scientific (Waltham, MA, USA).
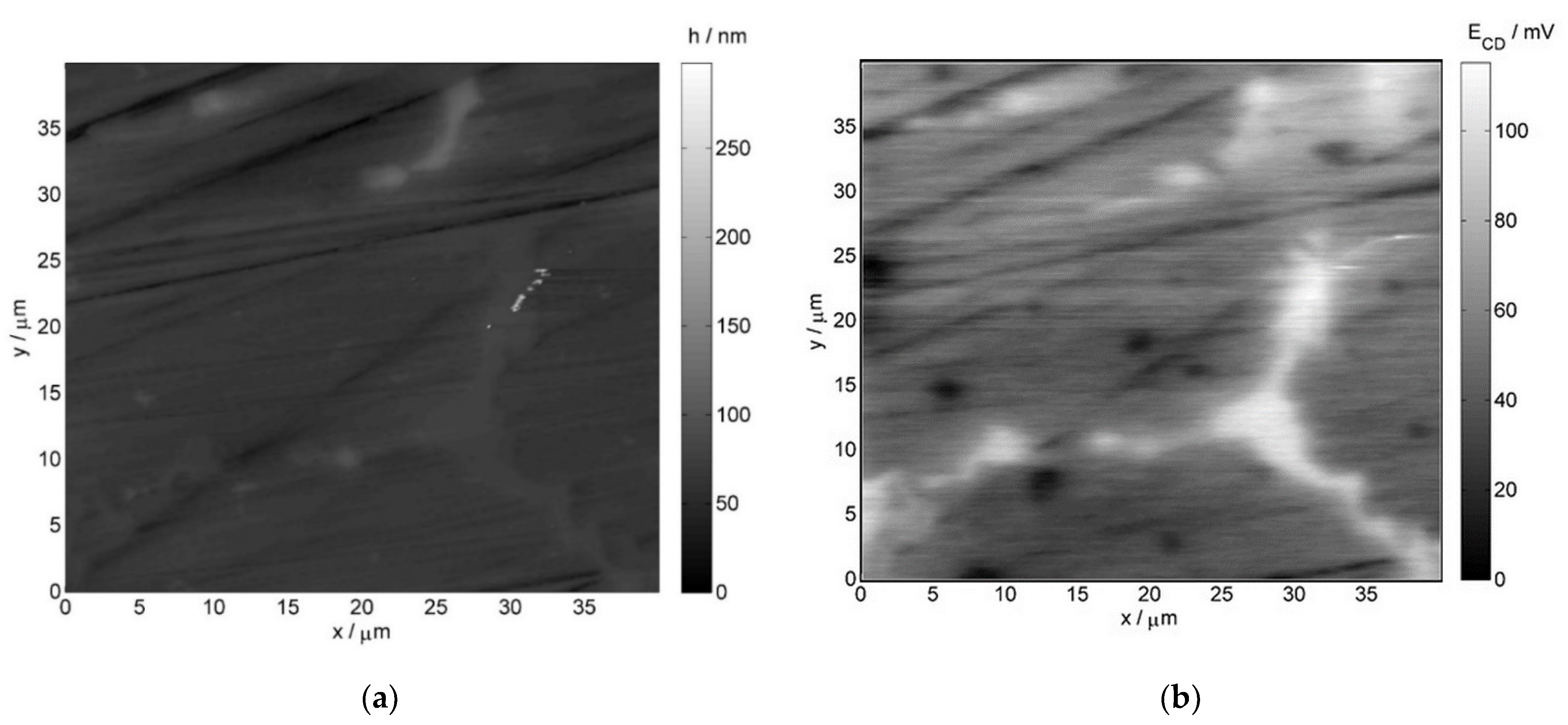
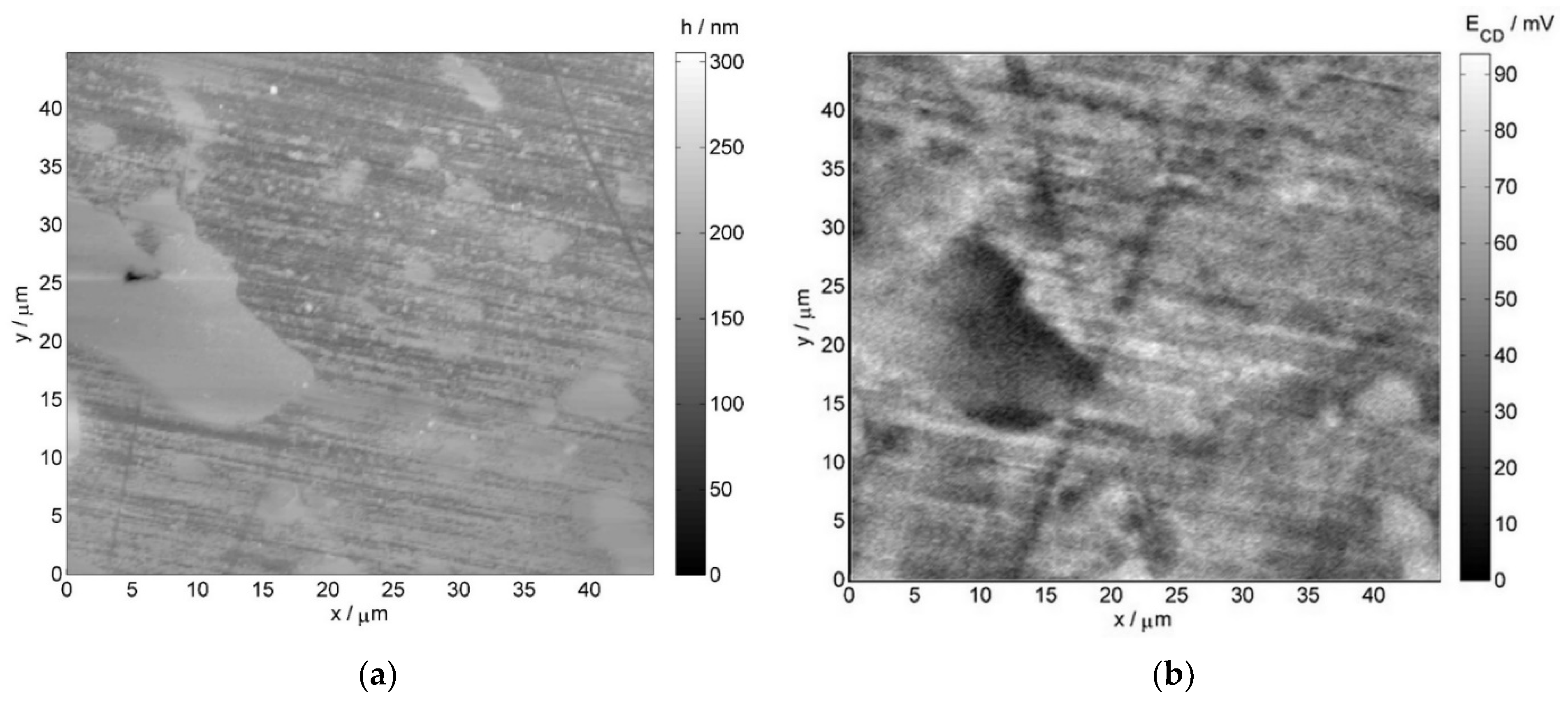
- Atomic force microscopy (AFM) and scanning Kelvin probe force microscopy (SKPFM) measurements employed the NTEGRA Aura microscope by NT-MDT (Moscow, Russia), with the head dedicated to Kelvin probe measurement mode. Commercially available NSG01 tips by NT-MDT were utilized for imaging. Additionally, they were vacuum sublimation coated with gold, the thickness of which was 100 nm. The scan rate was equal to 1 line per second. The operating point during topographic scan constituted half of the amplitude of free oscillations at a resonant frequency of the probe. During potential profile recording, the constant height component was 100 nm in order to minimize the influence of short-range interatomic forces.
- The temperature vacuum extraction method was used for the evaluation of hydrogen and methane trapped inside the material. The alloy samples were shredded into small pieces by a slow rotating lathe. Obtained alloy chips were kept in an ultrasonic bath with distilled water for 5 min and then dried at 1100 °C for 1 h. In the next step, the alloy chips were placed under 800 mbar vacuum and heated up to 810 °C. Evolved hydrogen and methane were measured using the Agilent 6890 gas chromatograph (Santa Clara, CA, USA).
- Mechanical tests were performed using the Zwick Z030 tensile testing machine (ZwickRoell GmbH & Co. KG, Ulm, Germany). Figure 1 illustrates the scheme and dimensions of the samples employed in the mechanical investigations. Stress-strain curves were obtained for three service exposed and three non-exposed samples. The strain increment rate was 1 mm/min.
3. Results and Discussion
- Central part, which was subjected to high-temperature impact;
- External part, which was in contact with high temperatures and flue gases originating from combustion of natural gas;
- Internal part, which was subjected to the influence of high temperatures, dilution steam and cracked hydrocarbon feeds.
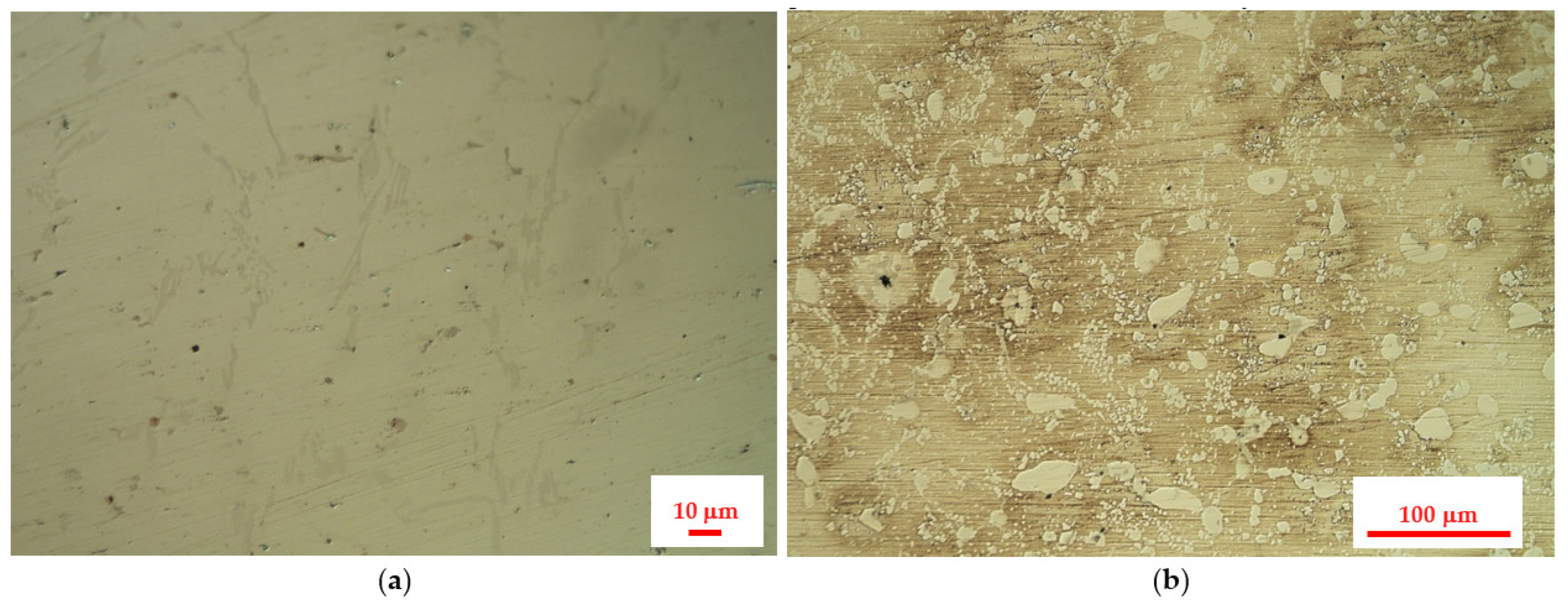
3.1. Results of the Investigation on the Central Part of the Furnace’s Coil Tube
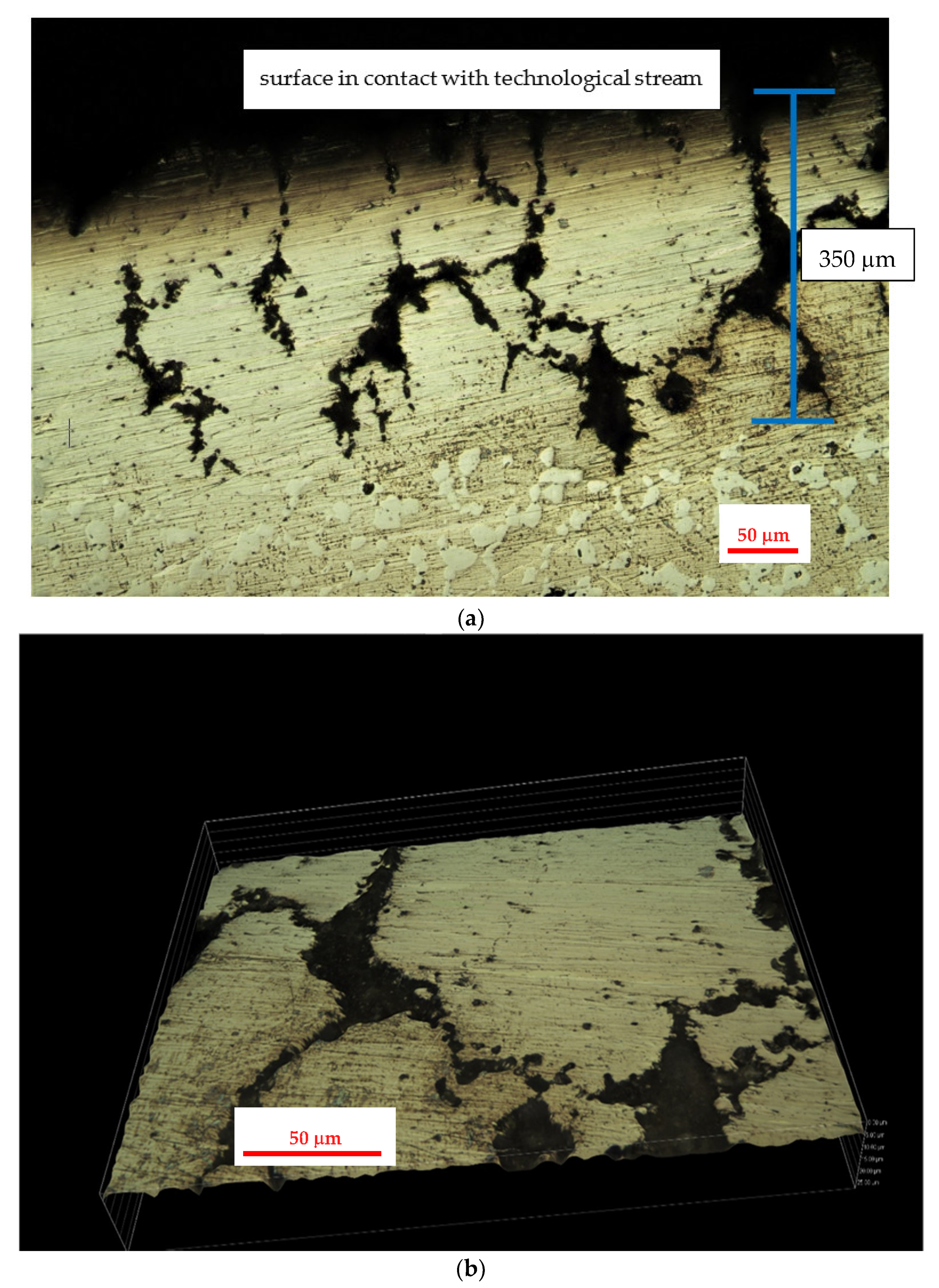
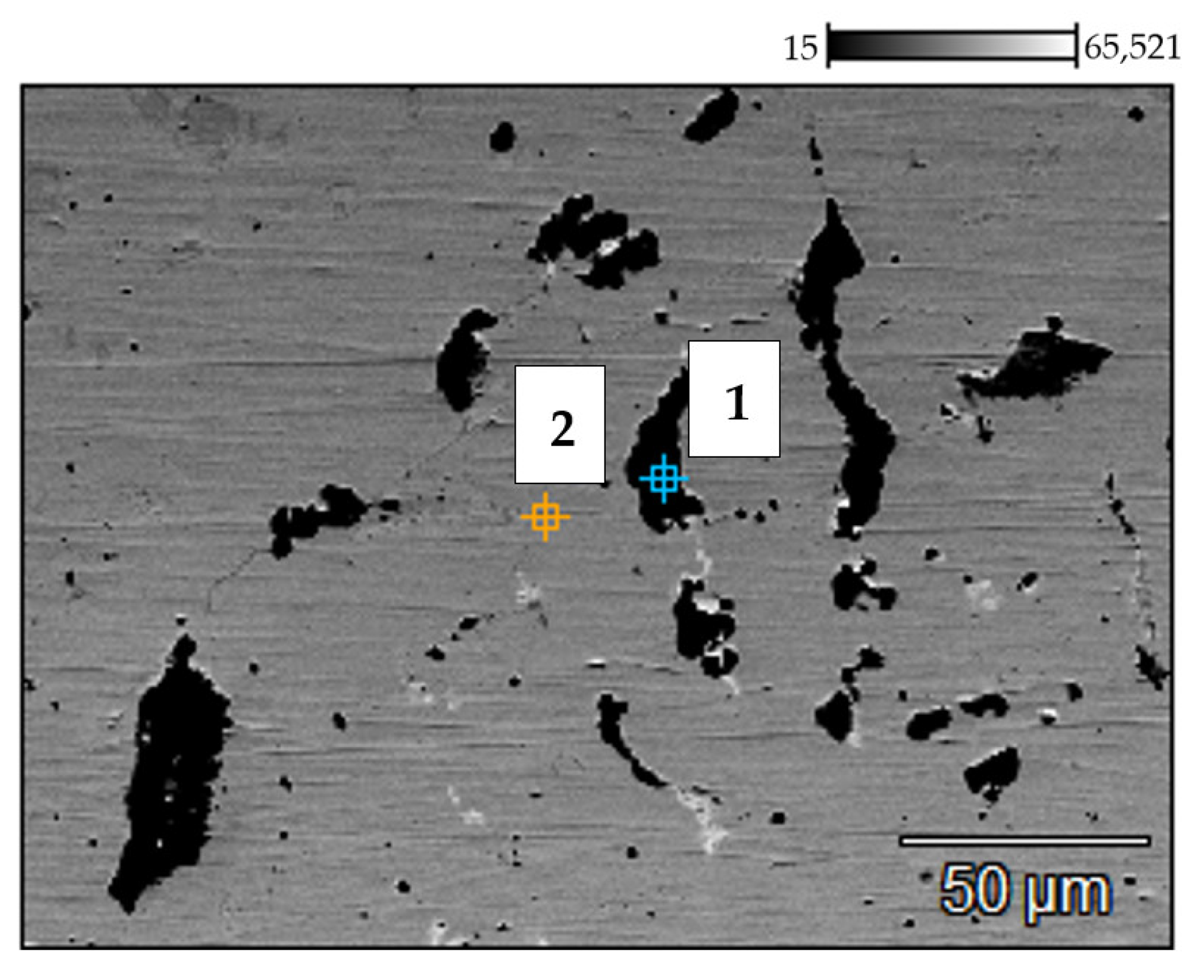
3.2. Results of the Investigation of the Internal Part of the Furnace’s Coil Tube (the Side of the Technological Stream)
3.3. Results of the Investigation of the External Part of the Furnace’s Coil Tube (from the Side in Contact with the Flue Gas)
3.4. Results of the Investigation of the Hydrogen and Methane Content in the Alloy Structure
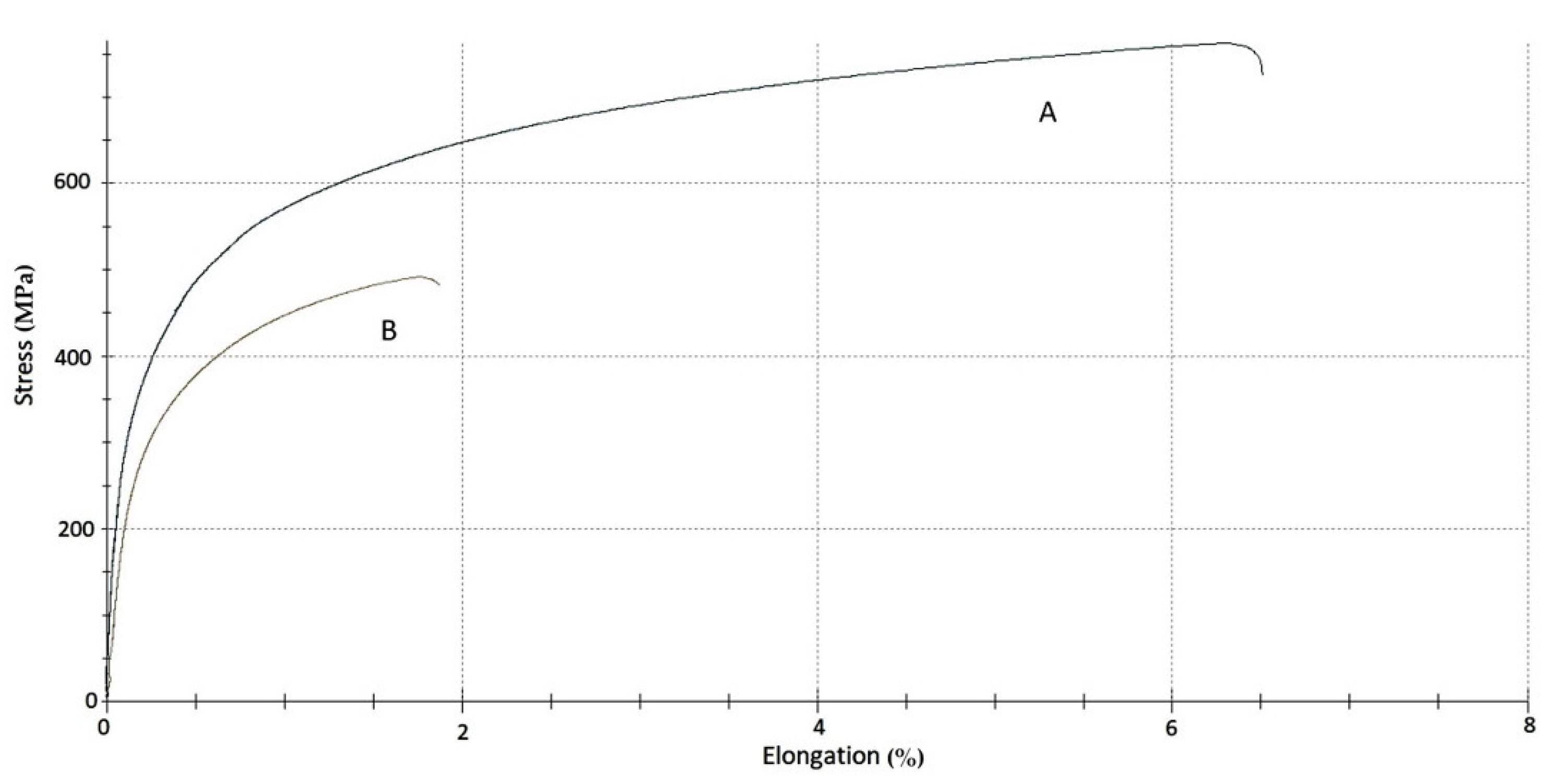
3.5. Results of the Mechanical Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kirchheiner, R.; Woelpert, P. Niobium in centrifugally cast tubes for petrochemical applications. In Proceedings of the International Symposium: Niobium, Science and Technology, Orlando, FL, USA, 2–5 December 2001. [Google Scholar]
- Ul-Hamid, A.; Tawancy, H.M.; Al-Jaroudi, S.S.; Mohammed, A.I.; Abbas, N.M. Carburization of Fe-Ni-Cr alloys at high temperatures. Mater. Sci.-Poland 2006, 24, 319–331. [Google Scholar]
- API 571. Damage Mechanisms Affecting Fixed Equipment in the Refining Industry; American Petroleum Institute: Washington, DC, USA, 2020. [Google Scholar]
- Hau, J.L.; Seijas, A. Furnace tube life assessment as impacted by the methodology used. In Proceedings of the NACE International: CORROSION 2008, New Orleans, LO, USA, 16–20 March 2008. [Google Scholar]
- Hill, T. Heater tube life management. In Proceedings of the National Petroleum Refiners Association Plant Maintenance Conference, Houston, TX, USA, 22–25 May 2000. [Google Scholar]
- Voicu, R.; Andrieu, E.; Poquillon, D.; Furtado, J.; Lacaze, J. Microstructure evolution of HP40-Nb alloys during aging under air at 1000 °C. Mater. Charact. 2009, 60, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Srisuwan, N.; Eidhed, K.; Kreatsereekul, N.; Yingsamphanchareon, T.; Kaewvilai, A. The study of heat treatment effects on chromium carbide precipitation of 35Cr-45Ni-Nb alloy for repairing furnace tubes. Metals 2016, 6, 26. [Google Scholar] [CrossRef] [Green Version]
- Sustaita-Torres, I.A.; Haro-Rodriguez, S.; Guerrero-Mata, M.P.; de la Garza, M.; Valdes, E.; Deschaux-Beaume, F.; Colas, R. Aging of a cast 35Cr–45Ni heat resistant alloy. Mater. Chem. Phys. 2012, 133, 1018–1023. [Google Scholar] [CrossRef]
- de Almeida Soares, G.D.; de Almeida, L.H.; da Silveira, T.L.; May, I.L. Niobium additions in HP heat-resistant cast stainless steels. Mater. Charact. 1992, 29, 387–396. [Google Scholar] [CrossRef]
- Wang, F.; Northwood, D.O. The effect of carbon content on the microstructure of an experimental heat-resistant steel. Mater. Charact. 1993, 31, 3–10. [Google Scholar] [CrossRef]
- Singh, S.P. International Conference & Expo On Corrosion 2017. In Proceedings of the Remaining Life Assessment of Outlet Radiant Heater Tube of Naphtha Cracking Unit—A Case Study, Mumbai, India, 17–20 September 2017; Available online: http://icspl.org/Corcon%202017/html/PDF/CR22.pdf (accessed on 16 October 2020).
- Tillack, D.J.; Guthrie, J.E. Wrought and Cast Heat-Resistant Stainless Steels and Nickel Alloys for the Refining and Petrochemical Industries; Technical Series No. 10071; Nickel Development Institute: Toronto, ON, Canada, 1998. [Google Scholar]
- Baker, B.A.; Smith, G.D. Metal dusting behavior of high-temperature alloys. In Proceedings of the NACE International CORROSION 99, San Antonio, TX, USA, 25–30 April 1999. [Google Scholar]
- Agarwal, D.C.; Kloewer, J.; Brill, U. Recent results on metal dusting of nickel base alloys and some applications. In Proceedings of the NACE International CORROSION 2001, Houston TX, USA, 11–16 March 2001. [Google Scholar]
- Bsat, S.; Huang, X. Corrosion behaviour 310 stainless steel in superheated steam. Oxid. Met. 2015, 84, 621–631. [Google Scholar] [CrossRef]
- Bischoff, J.; Motta, A.T.; Eichfeld, C.; Comstock, R.J.; Cao, G.; Allen, T.R. Corrosion of ferritic–martensitic steels in steam and supercritical water. J. Nucl. Mater. 2013, 441, 604–611. [Google Scholar] [CrossRef]





| C (%) | Ni (%) | Cr (%) | Fe (%) | Mn (%) | Si (%) | Nb (%) |
|---|---|---|---|---|---|---|
| 0.39 | 44.92 | 37.32 | 13.6 | 1.11 | 1.83 | 0.83 |
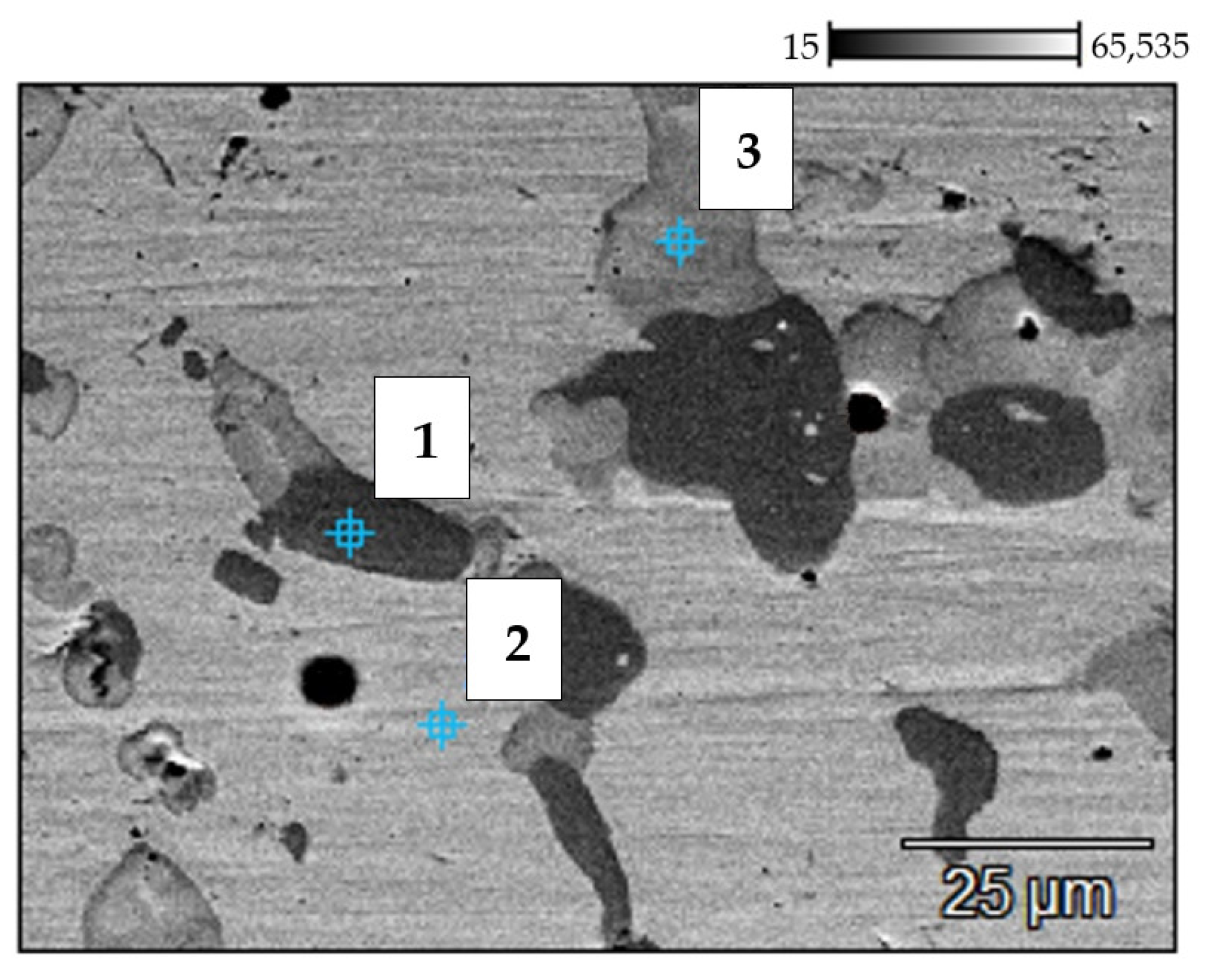
| No. | C (%) | Si (%) | V (%) | Cr (%) | Mn (%) | Fe (%) | Ni (%) | Nb (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.53 | 0.17 | 0.24 | 85.71 | 0.00 | 3.73 | 5.40 | 0.23 |
| 2 | 4.73 | 0.65 | 0.48 | 37.14 | 1.25 | 4.59 | 5.77 | 45.39 |
| No. | C (%) | Si (%) | V (%) | Cr (%) | Mn (%) | Fe (%) | Ni (%) | Nb (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.16 | 0.20 | 0.00 | 87.62 | 0.00 | 2.32 | 4.70 | 0.00 |
| 2 | 2.03 | 1.04 | 0.00 | 35.10 | 1.62 | 15.27 | 44.93 | 0.00 |
| 3 | 3.24 | 7.07 | 0.32 | 44.31 | 0.00 | 1.05 | 34.34 | 9.69 |
| No. | C (%) | Si (%) | V (%) | Cr (%) | Mn (%) | Fe (%) | Ni (%) | Nb (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 2.73 | 28.62 | 34.80 | 12.92 | 0.09 | 5.36 | 15.44 | 0.00 |
| 2 | 2.42 | 0.00 | 0.66 | 32.53 | 0.00 | 15.86 | 48.22 | 0.32 |
| No. | C (%) | Si (%) | V (%) | Cr (%) | Mn (%) | Fe (%) | Ni (%) | Nb (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | 4.73 | 7.42 | 0.48 | 34.14 | 0.00 | 2.07 | 5.77 | 45.39 |
| Alloy Sample | Hydrogen Content (ppm) | Methane Content (ppm) |
|---|---|---|
| service exposed | 77.1 | 134.3 |
| reference | 32.8 | 40.0 |
| Alloy Sample | σ for 0.2% Plastic Deformation (MPa) | Young’s Modulus (GPa) | σmax (MPa) | ε for σmax (%) |
|---|---|---|---|---|
| service exposed | 356 | 150 | 491 | 1.8 |
| reference | 323 | 163 | 559 | 6.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlikowski, J.; Szociński, M.; Zygmuntowicz, J.; Gajewski, G.; Filipkowski, W.; Darowicki, K. Investigation of the 0.4C-35Cr-45Ni-Nb Alloy after Service in High-Temperature Steam and Hydrocarbons Environment. Materials 2021, 14, 6139. https://doi.org/10.3390/ma14206139
Orlikowski J, Szociński M, Zygmuntowicz J, Gajewski G, Filipkowski W, Darowicki K. Investigation of the 0.4C-35Cr-45Ni-Nb Alloy after Service in High-Temperature Steam and Hydrocarbons Environment. Materials. 2021; 14(20):6139. https://doi.org/10.3390/ma14206139
Chicago/Turabian StyleOrlikowski, Juliusz, Michał Szociński, Janusz Zygmuntowicz, Gabriel Gajewski, Wojciech Filipkowski, and Kazimierz Darowicki. 2021. "Investigation of the 0.4C-35Cr-45Ni-Nb Alloy after Service in High-Temperature Steam and Hydrocarbons Environment" Materials 14, no. 20: 6139. https://doi.org/10.3390/ma14206139
APA StyleOrlikowski, J., Szociński, M., Zygmuntowicz, J., Gajewski, G., Filipkowski, W., & Darowicki, K. (2021). Investigation of the 0.4C-35Cr-45Ni-Nb Alloy after Service in High-Temperature Steam and Hydrocarbons Environment. Materials, 14(20), 6139. https://doi.org/10.3390/ma14206139