Advances in Sintering Techniques for Calcium Phosphates Ceramics
Abstract
:1. Introduction
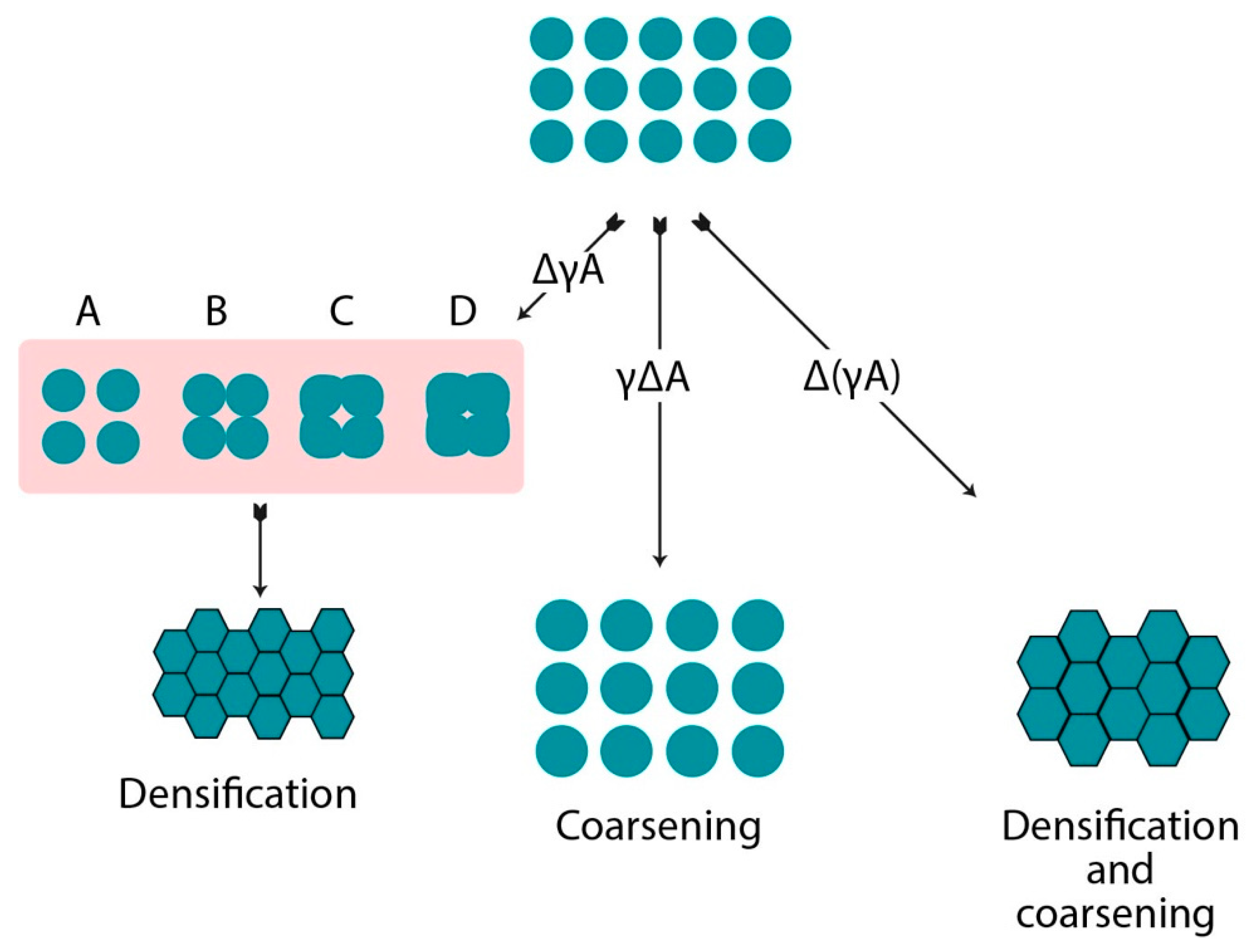
2. Sintering Process
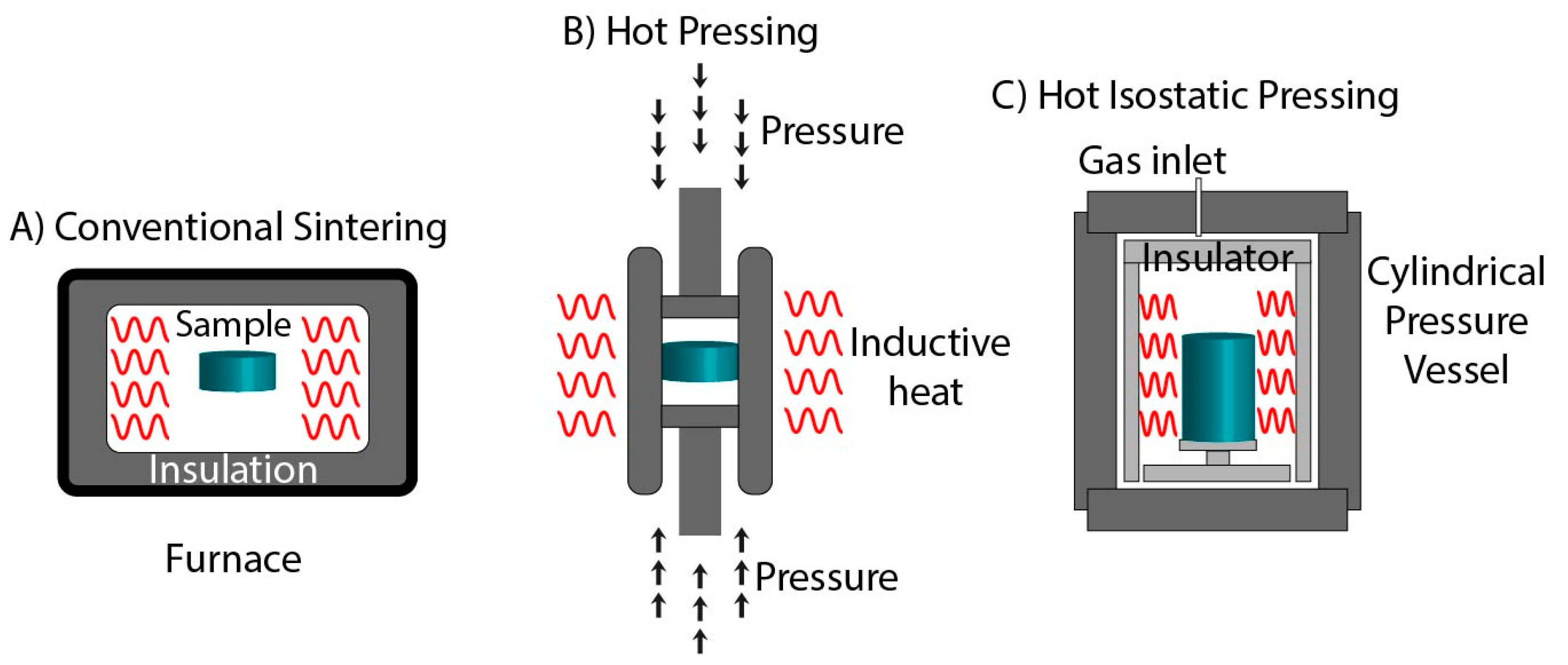
3. Conventional Sintering
3.1. Hot Pressing
3.2. Hot Isostatic Pressing
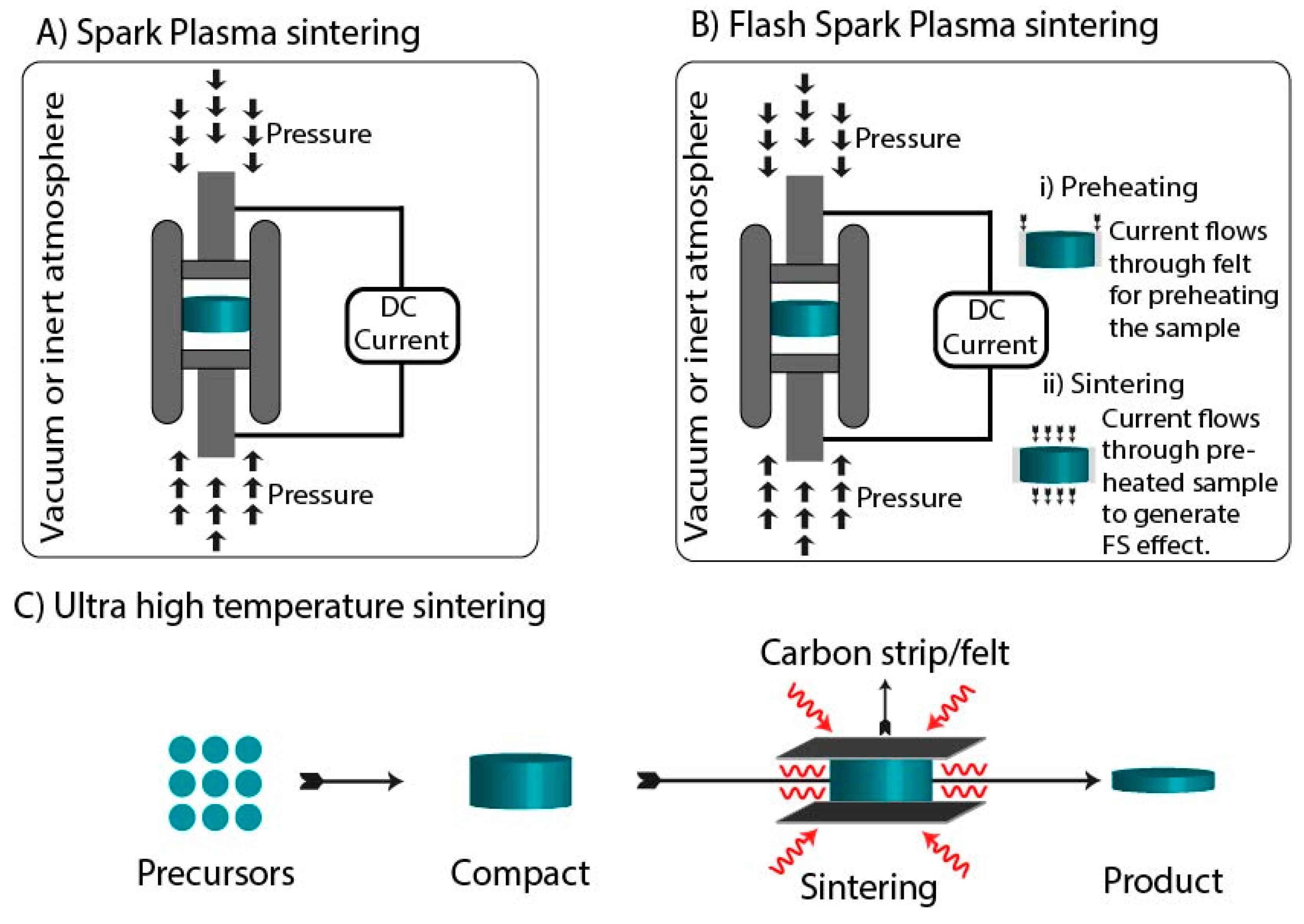
3.3. Spark Plasma Sintering
3.4. Flash Spark Plasma Sintering
3.5. Ultrafast High-Temperature Sintering
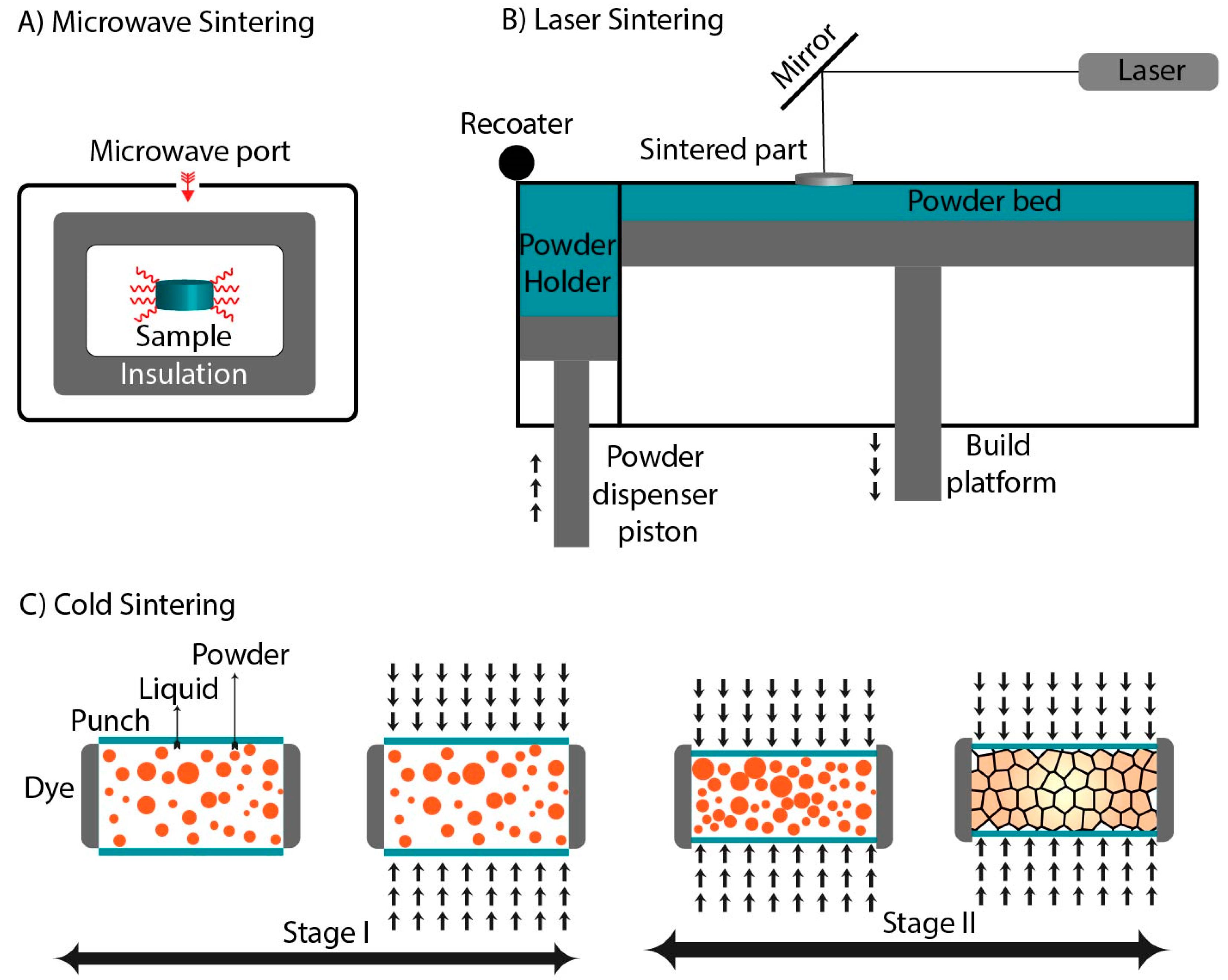
3.6. Microwave Sintering
3.7. Laser Sintering
3.8. Cold Sintering
4. Discussion
5. Summary and Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| CaP | Calcium Phosphate |
| HAp | Hydroxyapatite |
| TTCaP | Tetra-calcium phosphate |
| α-TCP | Alpha-tricalcium phosphate |
| β-TCP | Beta-tricalcium phosphate |
| OCP | Octa-calcium Phosphate |
| ACP | Amorphous calcium phosphate |
| DCPD | Dicalcium Phosphate dihydrate |
| CDHA | Calcium deficient hydroxyapatite |
| XRD | X-ray diffraction |
| SSA | Specific surface area |
| MPa | Mega Pascal |
| HP | Hot pressing |
| HIP | Hot isostatic pressing |
| SPS | Spark Plasma sintering |
| PECS | Pulsed electric current sintering |
| FAST | Field-assisted sintering technique |
| DC | Direct current |
| FS | Flash sintering |
| UHS | Ultrafast high-temperature sintering |
| MW | Microwave |
| MWS | Microwave sintering |
| LS | Laser sintering |
| CAM | Computer-aided manufacturing |
| CAD | Computer-aided design |
| 3D | Three dimension |
| CS | Cold sintering |
References
- German, R.M. The Emergence of Quantitative Sintering Theory from 1945 to 1955. JOM 2017, 69, 630–634. [Google Scholar] [CrossRef]
- Exner, H.; Arzt, E. Sintering Processes. In Physical Metallurgy; Elsevier: North Holland, The Netherlands, 1996; pp. 2627–2662. ISBN 9780444898753. [Google Scholar] [CrossRef]
- Kang, S.-J.L. Sintering Processes: Densification, Grain Growth and Microstructure. In Sintering; Butterworth-Heinemann: Oxford, UK, 2005; pp. 3–8. [Google Scholar] [CrossRef]
- Zimina, A.; Senatov, F.; Choudhary, R.; Kolesnikov, E.; Anisimova, N.; Kiselevskiy, M.; Orlova, P.; Strukova, N.; Generalova, M.; Manskikh, V.; et al. Biocompatibility and Physico-Chemical Properties of Highly Porous PLA/HA Scaffolds for Bone Reconstruction. Polymers 2020, 12, 2938. [Google Scholar] [CrossRef]
- He, Z.; Zhai, Q.; Hu, M.; Cao, C.; Wang, J.; Yang, H.; Li, B. Bone cements for percutaneous vertebroplasty and balloon kyphoplasty: Current status and future developments. J. Orthop. Transl. 2015, 3, 1–11. [Google Scholar] [CrossRef] [Green Version]
- LeGeros, R.Z. Calcium Phosphate-Based Osteoinductive Materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- De Sant’Anna, G.R.; Dos Santos, E.A.P.; Soares, L.E.S.; Santo, A.M.D.E.; Martin, A.; Duarte, D.A.; Pacheco-Soares, C.; Brugnera, A. Dental Enamel Irradiated with Infrared Diode Laser and Photo-Absorbing Cream: Part 2—EDX Study. Photomed. Laser Surg. 2009, 27, 771–782. [Google Scholar] [CrossRef] [Green Version]
- Dorozhkin, S.V. Calcium orthophosphates (CaPO4): Occurrence and properties. Prog. Biomater. 2016, 5, 9–70. [Google Scholar] [CrossRef] [Green Version]
- Chow, L.C. Next generation calcium phosphate-based biomaterials. Dent. Mater. J. 2009, 28, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Thomas, S.; Harshita; Mishra, P.K.; Talegaonkar, S. Ceramic Nanoparticles: Fabrication Methods and Applications in Drug Delivery. Curr. Pharm. Des. 2015, 21, 6165–6188. [Google Scholar] [CrossRef]
- Nikolenko, M.V.; Vasylenko, K.V.; Myrhorodska, V.D.; Kostyniuk, A.; Likozar, B. Synthesis of Calcium Orthophosphates by Chemical Precipitation in Aqueous Solutions: The Effect of the Acidity, Ca/P Molar Ratio, and Temperature on the Phase Composition and Solubility of Precipitates. Processes 2020, 8, 1009. [Google Scholar] [CrossRef]
- Tung, M.S. Calcium Phosphates: Structure, Composition, Solubility, and Stability. In Calcium Phosphates in Biological and Industrial Systems; Springer: Boston, MA, USA, 1998; pp. 1–19. [Google Scholar] [CrossRef]
- Dorozhkin, S.V. Calcium Orthophosphates in Nature, Biology and Medicine. Materials 2009, 2, 399–498. [Google Scholar] [CrossRef] [Green Version]
- Moseke, C.; Gbureck, U. Tetracalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2010, 6, 3815–3823. [Google Scholar] [CrossRef]
- Samavedi, S.; Whittington, A.; Goldstein, A.S. Calcium phosphate ceramics in bone tissue engineering: A review of properties and their influence on cell behavior. Acta Biomater. 2013, 9, 8037–8045. [Google Scholar] [CrossRef]
- Graça, M.P.F.; Gavinho, S.R. Calcium Phosphate Cements in Tissue Engineering. In Contemporary Topics about Phosphorus in Biology and Materials; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Canillas, M.; Pena, P.; de Aza, A.H.; Rodríguez, M.A. Calcium phosphates for biomedical applications. Boletín Soc. Española Cerámica y Vidr. 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef] [Green Version]
- Lane, J.M.; Mait, J.E.; Unnanuntana, A.; Hirsch, B.P.; Shaffer, A.D.; Shonuga, O.A. Materials in Fracture Fixation. Compr. Biomater. 2011, 6, 219–235. [Google Scholar] [CrossRef]
- Huang, J. Design and Development of Ceramics and Glasses. In Biology and Engineering of Stem Cell Niches; Elsevier: Cambridge, MA, USA, 2017; pp. 315–329. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Hong, K.S.; Lee, J.-K.; Kim, D.J.; Lee, J.H.; Chang, B.-S.; Lee, D.-H.; Lee, C.-K.; Chung, S.-S. Magnesia-doped HA/β-TCP ceramics and evaluation of their biocompatibility. Biomaterials 2004, 25, 393–401. [Google Scholar] [CrossRef]
- Duncan, J.; Macdonald, J.F.; Hanna, J.V.; Shirosaki, Y.; Hayakawa, S.; Osaka, A.; Skakle, J.M.; Gibson, I. The role of the chemical composition of monetite on the synthesis and properties of α-tricalcium phosphate. Mater. Sci. Eng. C 2014, 34, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eanes, E.D. Thermochemical studies on amorphous calcium phosphate. Calcif. Tissue Int. 1970, 5, 133–145. [Google Scholar] [CrossRef]
- Biesuz, M.; Galotta, A.; Motta, A.; Kermani, M.; Grasso, S.; Vontorová, J.; Tyrpekl, V.; Vilémová, M.; Sglavo, V.M. Speedy bioceramics: Rapid densification of tricalcium phosphate by ultrafast high-temperature sintering. Mater. Sci. Eng. C 2021, 127, 112246. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Loh, N.; Khor, K.; Tor, S.; Cheang, P. Spark plasma sintering of hydroxyapatite powders. Biomaterials 2002, 23, 37–43. [Google Scholar] [CrossRef]
- Walker, P.; Tarn, W.H. Handbook of Metal Etchants; CRC Press: Boca Raton, FL, USA, 1990. [Google Scholar]
- German, R. Sintering. In Encyclopedia of Materials: Science and Technology; Elsevier: Oxford, UK, 2001; pp. 8641–8643. [Google Scholar] [CrossRef]
- Pask, J.A. Thermodynamics and Mechanisms of Sintering. Available online: https://escholarship.org/content/qt67t140k9/qt67t140k9.pdf (accessed on 29 September 2021).
- Champion, E. Sintering of calcium phosphate bioceramics. Acta Biomater. 2013, 9, 5855–5875. [Google Scholar] [CrossRef]
- Salem, S.; Salem, A. Mechanisms of Momentum Transport in Viscous Flow Sintering. In Sintering Applications; IntechOpen: London, UK, 2013. [Google Scholar]
- Terwilliger, G.R.; Lange, F.F. Pressureless sintering of Si3N4. J. Mater. Sci. 1975, 10, 1169–1174. [Google Scholar] [CrossRef]
- Ramakumar, S.; Deviannapoorani, C.; Dhivya, L.; Shankar, L.S.; Murugan, R. Lithium garnets: Synthesis, structure, Li+ conductivity, Li+ dynamics and applications. Prog. Mater. Sci. 2017, 88, 325–411. [Google Scholar] [CrossRef]
- Nygren, M.; Shen, Z. Hot Pressing and Spark Plasma Sintering. In Ceramics Science and Technology; Wiley-VCH: Weinheim, Germany, 2012; pp. 189–214. ISBN 9783527311576. [Google Scholar]
- Hostaša, J. Ceramics for Laser Technologies. In Encyclopedia of Materials: Technical Ceramics and Glasses; Elsevier: Amsterdam, The Netherlands, 2021; pp. 110–124. [Google Scholar]
- Petrakova, N.V.; Lysenkov, A.S.; Ashmarin, A.A.; Egorov, A.A.; Fedotov, A.Y.; Shvorneva, L.I.; Komlev, V.S.; Barinov, S.M. Effect of hot pressing temperature on the microstructure and strength of hydroxyapatite ceramic. Inorg. Mater. Appl. Res. 2013, 4, 362–367. [Google Scholar] [CrossRef]
- Halouani, R.; Bernache-Assolant, D.; Champion, E.; Ababou, A. Microstructure and related mechanical properties of hot pressed hydroxyapatite ceramics. J. Mater. Sci. Mater. Electron. 1994, 5, 563–568. [Google Scholar] [CrossRef]
- Hosoi, K.; Hashida, T.; Takahashi, H.; Yamasaki, N.; Korenaga, T. New Processing Technique for Hydroxyapatite Ceramics by the Hydrothermal Hot-Pressing Method. J. Am. Ceram. Soc. 2005, 79, 2771–2774. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Zhu, K.; Fujino, T.; Onda, A.; Kajiyoshi, K.; Ioku, K. Preparation of Hydroxyapatite Ceramics by Hydrothermal Hot-Pressing Technique. Key Eng. Mater. 2006, 309–311, 57–60. [Google Scholar] [CrossRef]
- Brook, R.J.; Cahn, R.W.; Bever, M.B. Concise Encyclopedia of Advanced Ceramic Materials; Elsevier: Oxford, UK, 1991. [Google Scholar]
- Technologies and Applications||KOBE STEEL, Ltd. Available online: https://www.kobelco.co.jp/english/products/ip/technology/hip.html (accessed on 16 January 2021).
- Olmedo, D.G.; Tasat, D.R.; Duffó, G.; Guglielmotti, M.B.; Cabrini, R.L. The issue of corrosion in dental implants: A review. Acta Odontol Lat. 2009, 22, 3–9. [Google Scholar]
- Fu, Y.; Batchelor, A.W.; Khori, K.A. Hot Isostatic Pressing of Hydroxyapatite Coating for Improved Fretting Wear Resistance. J. Mater. Sci. Lett. 1998, 17, 1695–1696. [Google Scholar] [CrossRef]
- Harun, W.S.W.; Asri, R.I.M.; Sulong, A.B.; Ghani, S.A.C.; Ghazalli, Z. Hydroxyapatite-Based Coating on Biomedical Implant. In Hydroxyapatite-Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets; IntechOpen: Rijeka, Croatia, 2018; pp. 69–88. [Google Scholar]
- Bose, S.; Tarafder, S.; Bandyopadhyay, A. Hydroxyapatite coatings for metallic implants. In Hydroxyapatite (HAp) for Biomedical Applications; Woodhead Publishing: Cambridge, UK, 2015; pp. 143–157. [Google Scholar]
- Suárez, M.; Fernández, A.; Menéndez, J.L.; Torrecillas, R.; Kessel, H.U.; Hennicke, J.; Kirchner, R.; Kessel, T. Challenges and Opportunities for Spark Plasma Sintering: A Key Technology for a New Generation of Materials. In Sintering Applications; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Wang, L.; Pouchly, V.; Maca, K.; Shen, Z.; Xiong, Y. Intensive particle rearrangement in the early stage of spark plasma sintering process. J. Asian Ceram. Soc. 2015, 3, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Hu, Z.-Y.; Zhang, Z.-H.; Cheng, X.-W.; Wang, F.-C.; Zhang, Y.-F.; Li, S.-L. A review of multi-physical fields induced phenomena and effects in spark plasma sintering: Fundamentals and applications. Mater. Des. 2020, 191, 108662. [Google Scholar] [CrossRef]
- Zhou, M.; Ren, L.; Quan, G.; Gupta, M. Solid Phase Processing of Metal Matrix Composites. In Encyclopedia of Materials: Composites; Elsevier: Amsterdam, The Netherlands, 2021; pp. 173–196. [Google Scholar] [CrossRef]
- Cavaliere, P.; Sadeghi, B.; Shabani, A. Spark Plasma Sintering: Process Fundamentals. In Spark Plasma Sintering of Materials: Advances in Processing and Applications; Cavaliere, P., Ed.; Springer: Cham, Switzerland, 2019; pp. 3–20. [Google Scholar] [CrossRef]
- Leriche, A.; Cambier, F.; Hampshire, S. Sintering of Ceramics. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Pazhouhanfar, Y.; Delbari, S.A.; Asl, M.S.; Shaddel, S.; Pazhouhanfar, M.; Van Le, Q.; Shokouhimehr, M.; Mohammadi, M.; Namini, A.S. Characterization of spark plasma sintered TiC–Si3N4 ceramics. Int. J. Refract. Met. Hard Mater. 2021, 95, 105444. [Google Scholar] [CrossRef]
- Jarcho, M.; Bolen, C.H.; Thomas, M.B.; Bobick, J.; Kay, J.F.; Doremus, R.H. Hydroxylapatite synthesis and characterization in dense polycrystalline form. J. Mater. Sci. 1976, 11, 2027–2035. [Google Scholar] [CrossRef]
- Kawagoe, D.; Ioku, K.; Fujimori, H.; Goto, S. Transparent. BETA.-Tricalcium Phosphate Ceramics Prepared by Spark Plasma Sintering. J. Ceram. Soc. Jpn. 2004, 112, 462–463. [Google Scholar] [CrossRef] [Green Version]
- Grossin, D.; Rollin-Martinet, S.; Estournès, C.; Rossignol, F.; Champion, E.; Combes, C.; Rey, C.; Geoffroy, C.; Drouet, C. Biomimetic apatite sintered at very low temperature by spark plasma sintering: Physico-chemistry and microstructure aspects. Acta Biomater. 2010, 6, 577–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, J. The scientific questions and technological opportunities of flash sintering: From a case study of ZnO to other ceramics. Scr. Mater. 2018, 146, 260–266. [Google Scholar] [CrossRef]
- Du, B.; Gucci, F.; Porwal, H.; Grasso, S.; Mahajan, A.; Reece, M.J. Flash spark plasma sintering of magnesium silicide stannide with improved thermoelectric properties. J. Mater. Chem. C 2017, 5, 1514–1521. [Google Scholar] [CrossRef] [Green Version]
- Manière, C.; Lee, G.; Olevsky, E.A. All-Materials-Inclusive Flash Spark Plasma Sintering. Sci. Rep. 2017, 7, 15071. [Google Scholar] [CrossRef]
- Cologna, M.; Rashkova, B.; Raj, R. Flash Sintering of Nanograin Zirconia in <5 s at 850 °C. J. Am. Ceram. Soc. 2010, 93, 3556–3559. [Google Scholar] [CrossRef]
- Hwang, C.; Yun, J. Flash sintering of hydroxyapatite ceramics. J. Asian Ceram. Soc. 2021, 9, 304–311. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Frasnelli, M.; Sglavo, V.M. Flash sintering of tricalcium phosphate (TCP) bioceramics. J. Eur. Ceram. Soc. 2018, 38, 279–285. [Google Scholar] [CrossRef]
- Wang, C.; Ping, W.; Bai, Q.; Cui, H.; Hensleigh, R.; Wang, R.; Brozena, A.H.; Xu, Z.; Dai, J.; Pei, Y.; et al. A general method to synthesize and sinter bulk ceramics in seconds. Science 2020, 368, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. The Application of Microwave Technology in Chemistry and Chemical Engineering. In Proceedings of the Proceedings of the 2016 International Conference on Engineering Management (Iconf-EM 2016), Guangzhou, China, 26–27 November 2016. [Google Scholar]
- Oghbaei, M.; Mirzaee, O. Microwave versus conventional sintering: A review of fundamentals, advantages and applications. J. Alloys Compd. 2010, 494, 175–189. [Google Scholar] [CrossRef]
- Borrell, A.; Salvador, M.D. Advanced Ce ramic Materials Sintered by Microwave Technology. In Sinter Technology Method and Application; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Chelladurai, S.J.S.; Mayilswamy, S.; Balakrishnan, A.S.; Gnanasekaran, S. Green Materials and Advanced Manufacturing Technology: Concepts and Applications; CRC Press: Boca Raton, FL, USA, 2020. [Google Scholar]
- Hao, H.; Xu, L.; Huang, Y.; Zhang, X.; Xie, Z. Kinetics mechanism of microwave sintering in ceramic materials. Sci. China Ser. E Technol. Sci. 2008, 52, 2727–2731. [Google Scholar] [CrossRef]
- Fang, Y.; Agrawal, D.K.; Roy, D.M.; Roy, R. Microwave sintering of hydroxyapatite ceramics. J. Mater. Res. 1994, 9, 180–187. [Google Scholar] [CrossRef] [Green Version]
- Mirhadi, B. Microwave sintering of nano size powder β-TCP bioceramics. Sci. Sinter. 2014, 46, 185–193. [Google Scholar] [CrossRef] [Green Version]
- Bourell, D.L. Sintering in Laser Sintering. JOM 2016, 68, 885–889. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Kruth, J.; Mercelis, P.; Van Vaerenbergh, J.; Froyen, L.; Rombouts, M. Binding mechanisms in selective laser sintering and selective laser melting. Rapid Prototyp. J. 2005, 11, 26–36. [Google Scholar] [CrossRef] [Green Version]
- Kruth, J.P.; Wang, X.; Laoui, T.; Froyen, L. Lasers and materials in selective laser sintering. Assem. Autom. 2003, 23, 357–371. [Google Scholar] [CrossRef]
- Rho, J.-W.; Kim, J.-H.; Lee, C.-K. An Overview of Selective Laser Sintering. J. Weld. Join. 2008, 26, 34–37. [Google Scholar] [CrossRef]
- Simchi, A. Direct laser sintering of metal powders: Mechanism, kinetics and microstructural features. Mater. Sci. Eng. A 2006, 428, 148–158. [Google Scholar] [CrossRef]
- Qian, B.; Shen, Z. Laser sintering of ceramics. J. Asian Ceram. Soc. 2013, 1, 315–321. [Google Scholar] [CrossRef]
- Qin, T.; Li, X.; Long, H.; Bin, S.; Xu, Y. Bioactive Tetracalcium Phosphate Scaffolds Fabricated by Selective Laser Sintering for Bone Regeneration Applications. Materials 2020, 13, 2268. [Google Scholar] [CrossRef] [PubMed]
- Bulina, N.V.; Titkov, A.I.; Baev, S.G.; Makarova, S.V.; Khusnutdinov, V.R.; Bessmeltsev, V.P.; Lyakhov, N.Z. Laser sintering of hydroxyapatite for potential fabrication of bioceramic scaffolds. Mater. Today Proc. 2021, 37, 4022–4026. [Google Scholar] [CrossRef]
- Qingxi, H.; Baigong, M.; Liulan, L.; Minglun, F. Selective laser sintering of -tricalcium phosphate for bionic scaffold. In Proceedings of the International Technology and Innovation Conference 2006 (ITIC 2006), Hangzhou, China, 6–7 November 2006; Institution of Engineering and Technology (IET): Zhejiang, China, 2006; pp. 2–7. [Google Scholar]
- Grasso, S.; Biesuz, M.; Zoli, L.; Taveri, G.; Duff, A.I.; Ke, D.; Jiang, A.; Reece, M.J. A review of cold sintering processes. Adv. Appl. Ceram. 2020, 119, 115–143. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Guo, H.; Baker, A.L.; Lanagan, M.T.; Kupp, E.R.; Messing, G.L.; Randall, C.A. Cold Sintering: A Paradigm Shift for Processing and Integration of Ceramics. Angew. Chem. Int. Ed. 2016, 55, 11457–11461. [Google Scholar] [CrossRef]
- Maria, J.-P.; Kang, X.; Floyd, R.; Dickey, E.; Guo, H.; Guo, J.; Baker, A.; Funihashi, S.; Randall, C.A. Cold sintering: Current status and prospects. J. Mater. Res. 2017, 32, 3205–3218. [Google Scholar] [CrossRef] [Green Version]
- Riquet, G.; Marinel, S.; Bréard, Y.; Harnois, C. Sintering mechanism and grain growth in CaCu3Ti4O12 ceramics. Ceram. Int. 2019, 45, 9185–9191. [Google Scholar] [CrossRef]
- Biesuz, M.; Taveri, G.; Duff, A.I.; Olevsky, E.; Zhu, D.; Hu, C.; Grasso, S. A theoretical analysis of cold sintering. Adv. Appl. Ceram. 2020, 119, 75–89. [Google Scholar] [CrossRef]
- Rubenis, K.; Zemjane, S.; Vecstaudza, J.; Bitenieks, J.; Locs, J. Densification of amorphous calcium phosphate using principles of the cold sintering process. J. Eur. Ceram. Soc. 2021, 41, 912–919. [Google Scholar] [CrossRef]
- Hassan, M.U.; Akmal, M.; Ryu, H.J. Cold sintering of as-dried nanostructured calcium hydroxyapatite without using additives. J. Mater. Res. Technol. 2021, 11, 811–822. [Google Scholar] [CrossRef]
- German, R.M. Sintering With External Pressure. In Sintering: From Empirical Observations to Scientific Principles; Butterworth-Heinemann: Oxford, UK, 2014; pp. 305–354. [Google Scholar] [CrossRef]
- Fang, Z.; Wang, H. Sintering of ultrafine and nanosized ceramic and metallic particles. Ceramic Nanocomposites 2013, 431–473. [Google Scholar] [CrossRef]
- Kang, S.-J.L. Intermediate and Final Stage Sintering. In Sintering; Elsevier: Oxford, UK, 2005; pp. 57–87. [Google Scholar]

| Commercial Products | Implant Form | Biological Occurrence | Synthesis | Density (g/cm3) | −log Ksp at 25 °C | Melting Point (°C) | Ca/P Molar Ratio | Crystal Structure | Chemical Formula | Phase |
|---|---|---|---|---|---|---|---|---|---|---|
| Bone source (TTCaP and DCP) | Cement | - | Solid-state reaction at temperature 1450–1500 °C for 6–12 h. | 3.05 | 38 | Decomposes and transforms to HAp | 2 | Monoclinic | Ca4(PO4)2O | Tetra-calcium phosphate (TTCaP) |
| Ostim (HA) Graftys HBS (HA and TCP) Graftys quickset (HA and TCP) | Coating or deposited with polymer | Soft tissue calcification, enamel, dentin, bone, tooth, and urinary calculus | Precipitation performed at pH 9.5–12 at 90 °C | 3.16 | 58.4 | 1670 | 1.67 | Pseudohexagonal | Ca10(PO4)6(OH)2 | Hydroxyapatite (HAp) |
| Fracture grout (α-TCP and Calcium carbonate CaCO3) Biopex (α-TCP, DCPD, HA, and TTCaP) | Block | Dental and urinary calculus, salivary stones, tissue calcification, and milk Magnesium substituted β-TCP is identified in soft tissue calcification and dental calculus | Thermal Decomposition of β-TCP above 1125 °C | 2.86 | 25.5 | 1391 | 1.5 | Monoclinic | Ca3(PO4)2 | Tricalcium phosphate (a-TCP) |
| ChronOS Inkjet (β-TCP and DCPD) | Granules or block | a. Solid-state reaction b. Decomposition of calcium-deficient HAp above 750 °C c. Precipitation in an organic solvent | 3.08 | 28.9 | Decomposes and transforms α-TCP | 1.5 | Rhombohedral | Ca3(PO4)2 | Tricalcium phosphate (β-TCP) | |
| - | Granules | Dental and urinary calculus | Precipitation performed at pH 5.5–7.0 | 2.61 | 96.6 | Decomposes and transforms to HAp | 1.33 | Triclinic | Ca8H2(PO4)6·5H2O | Octa-calcium phosphate (OCP) |
| Embarc (ACP and DCPD) α−ΒΣΜ (AΧΠ ανδ ΔΧΠΔ) | Coating or granules | Kidney stone and heart calcification in uremic patients and soft tissue calcification | Precipitation performed at pH 5.0–12.0 | 0.92–1.75 | 26–33 | Transforms to a stable phase | 1.2–2.2 | Amorphous | Ca3(PO4)2 | Amorphous calcium phosphate (ACP) |
| Eurobone (DCPD and TCP) Calcibon (DCPD, α-TCP, CaCO3 and HA) | Powder | Dental calculi, chondrocalcinosis, crystalluria, and carious lesions | Precipitation performed below 80 °C under pH 2.0–6.5 | 2.32 | 6.59 | Decomposes and transforms to a stable phase | 1 | Monoclinic | CaHPO4·2H2O | Dicalcium phosphate dihydrate (DCPD) |
| Stage | Process | Densification | Coarsening | Loss in Total Specific Surface Area (SSA) |
|---|---|---|---|---|
| Initial | Neck growth formation | Trivial | Negligible | 50% |
| Intermediate | Elongation and rounding of pores | Substantial | Increase in grain and pore size | Complete loss of open porosity |
| Final | Closure of pores with final densification | Slow and minor change in density | Enormous pore and grain growth | Negligible |
| Technique | Temperature | Pressure | Current | Electromagnetic Radiations |
|---|---|---|---|---|
| Conventional sintering | ✓ | x | x | x |
| Hot Pressing | ✓ | ✓ | x | x |
| Hot Isostatic Pressing | ✓ | ✓ | x | x |
| Spark Plasma sintering | ✓ | ✓ | ✓ | x |
| Flash Spark Plasma sintering | ✓ | ✓ | ✓ | x |
| Ultrafast high-temperature sintering | ✓ | x | ✓ | x |
| Microwave sintering | ✓ | x | x | ✓ |
| Laser Sintering | ✓ | x | x | ✓ |
| Cold sintering | ✓ | ✓ | x | x |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Indurkar, A.; Choudhary, R.; Rubenis, K.; Locs, J. Advances in Sintering Techniques for Calcium Phosphates Ceramics. Materials 2021, 14, 6133. https://doi.org/10.3390/ma14206133
Indurkar A, Choudhary R, Rubenis K, Locs J. Advances in Sintering Techniques for Calcium Phosphates Ceramics. Materials. 2021; 14(20):6133. https://doi.org/10.3390/ma14206133
Chicago/Turabian StyleIndurkar, Abhishek, Rajan Choudhary, Kristaps Rubenis, and Janis Locs. 2021. "Advances in Sintering Techniques for Calcium Phosphates Ceramics" Materials 14, no. 20: 6133. https://doi.org/10.3390/ma14206133
APA StyleIndurkar, A., Choudhary, R., Rubenis, K., & Locs, J. (2021). Advances in Sintering Techniques for Calcium Phosphates Ceramics. Materials, 14(20), 6133. https://doi.org/10.3390/ma14206133







