Preliminary Study of the Influence of Supplementary Cementitious Materials on the Application of Electro Remediation Processes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Steel-Mortar Characterization
- fresh state characterization: workability: slump (EN 12350-2), density (EN 12350-6), and content of occluded air (EN 12350-7);
- chemical characterization by X-ray fluorescence (FRX); sample in pressed powder tablet;
- total porosity and pore size distribution by mercury intrusion porosimetry (MIP);
- pore solution composition by the so-called “pore pressing test,” applying ICP analysis;
- electrical resistivity using the so-called “direct method” (UNE 83988-1:2014);
- compressive and flexural strength (EN 12504-1:2009).
2.2.1. Corrosion Potential Ecorr
2.2.2. Concrete Resistivity (ρ) and Electrical Resistance (Re)
2.2.3. Corrosion Rate, Icorr
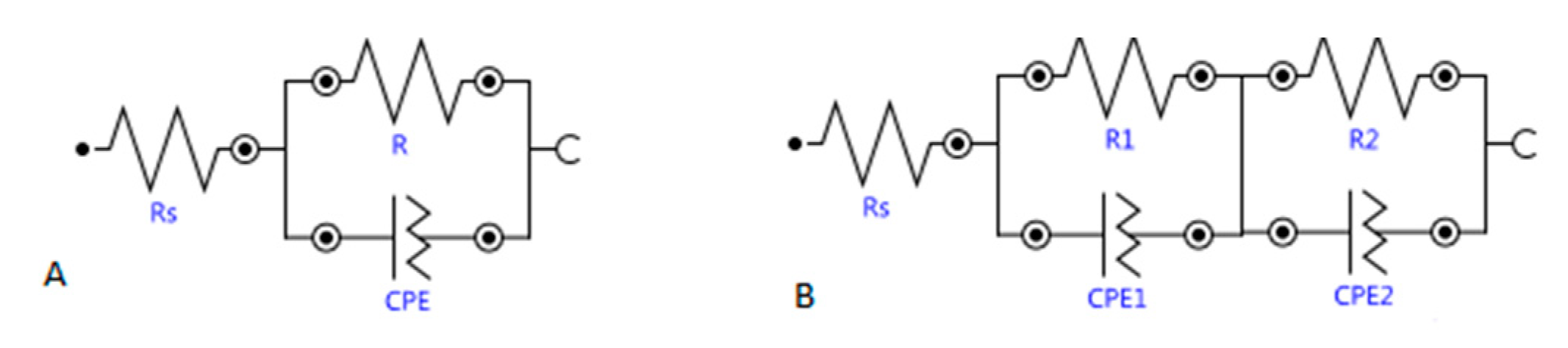
2.2.4. Electrochemical Impedance Spectroscopy (EIS)
2.3. Electrochemical Chloride Extraction (ECE): Application and Effectiveness Monitoring
- The initial voltage applied was 12 V until the accumulated charge reached 1500 Ah/m2.
- Then, the voltage applied was increased to 16 V until the accumulated charge was 2000 Ah/m2.
- The maximum voltage applied was 20 V. The treatment was stopped when it reached 4500 Ah/m2.
3. Results and Discussion
3.1. Mortar Characterization
3.1.1. Fresh Properties
3.1.2. Chemical Composition
3.1.3. Mechanical Resistance
3.1.4. Electrical Resistivity of the Mortars
3.2. Steel Electrochemical Characterization (before ECE)
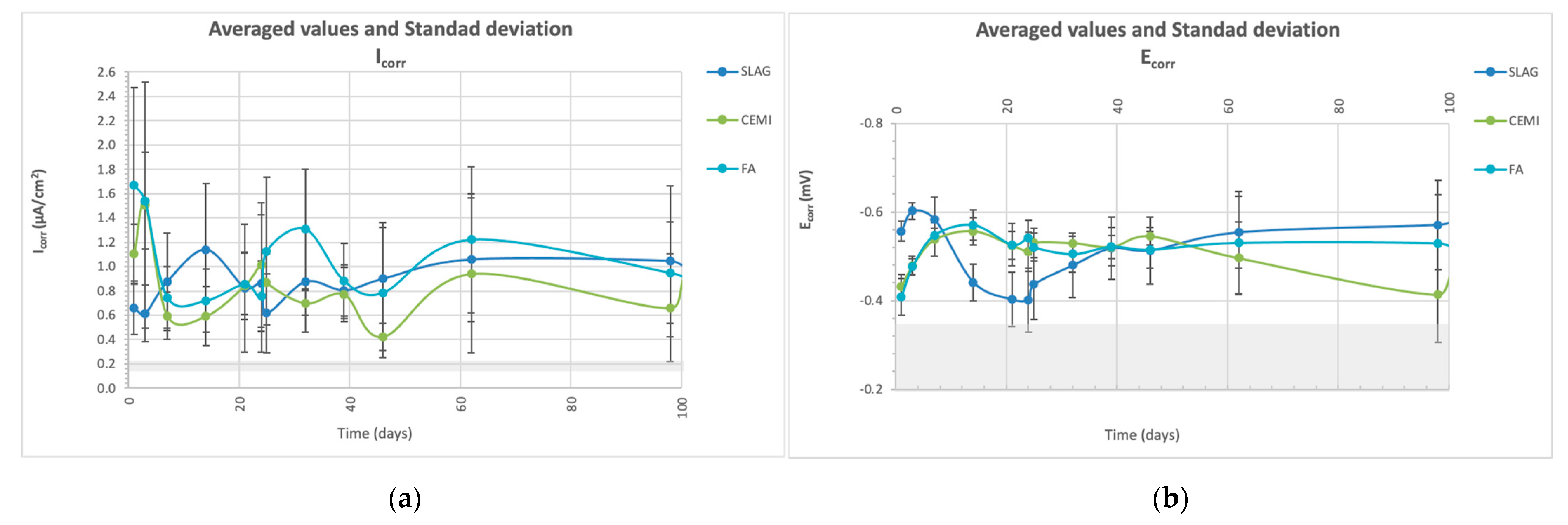
3.2.1. Corrosion Potential and Corrosion Rate Measured by LPR
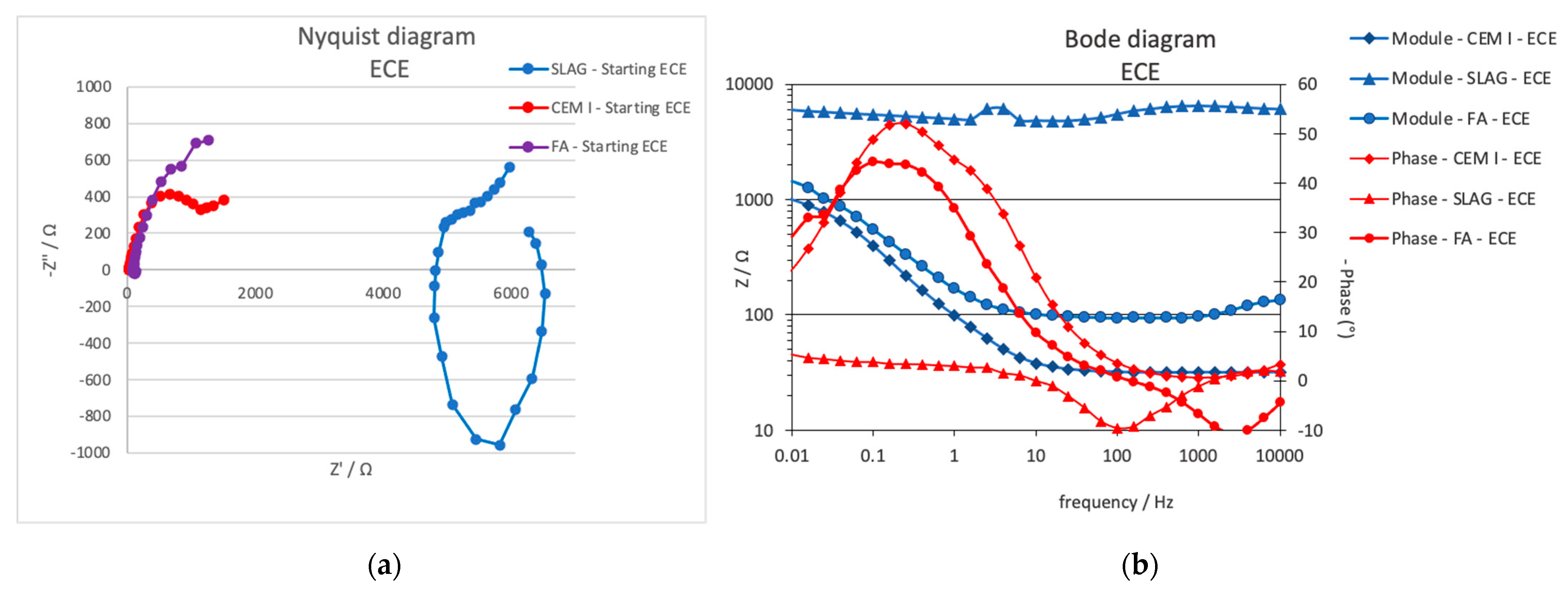
3.2.2. Electrochemical Impedance Spectroscopy, EIS
3.3. ECE Effects: Efficiency Indicators Evaluated during the Treatment
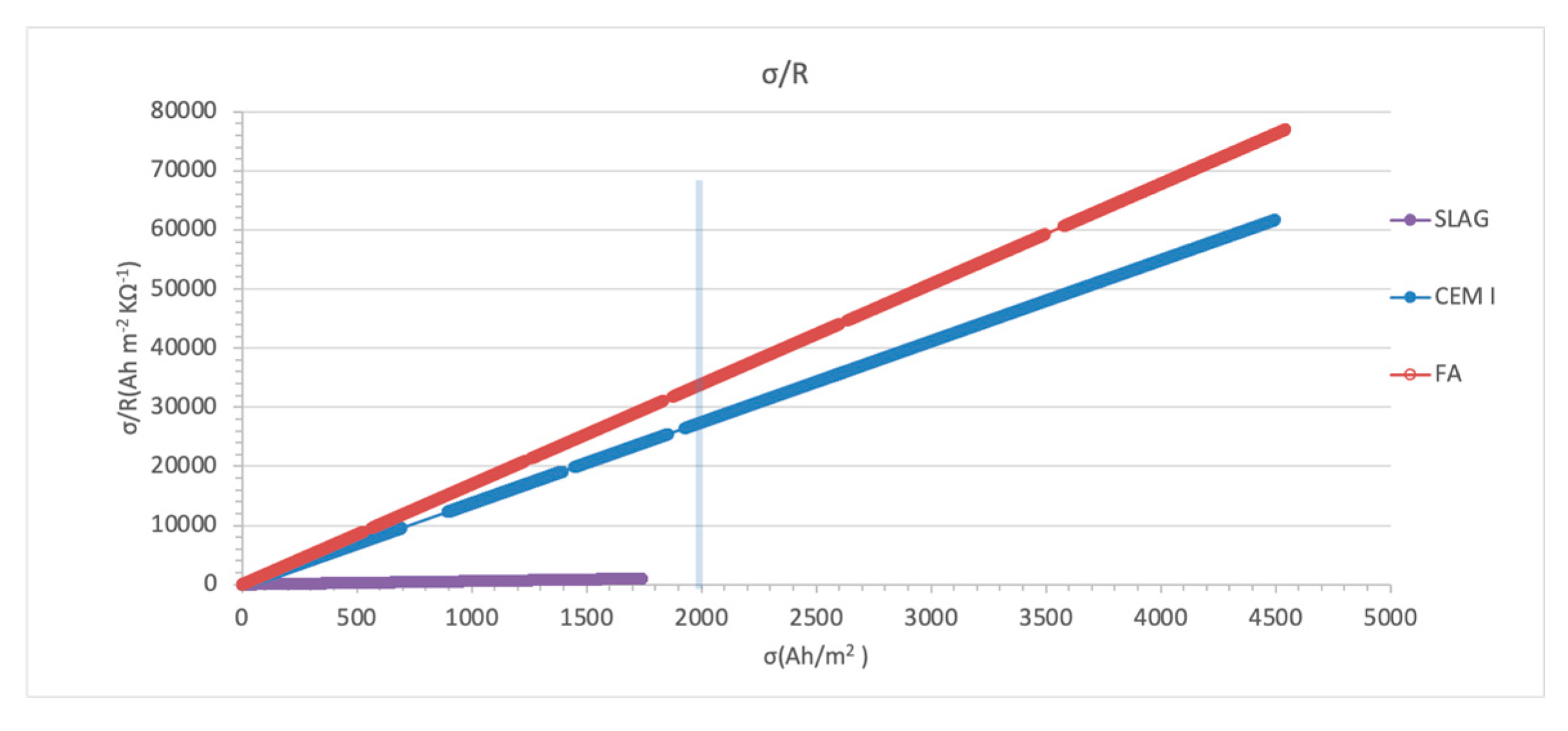
3.3.1. Surface Charged Density
3.3.2. Depolarization Records during ECE
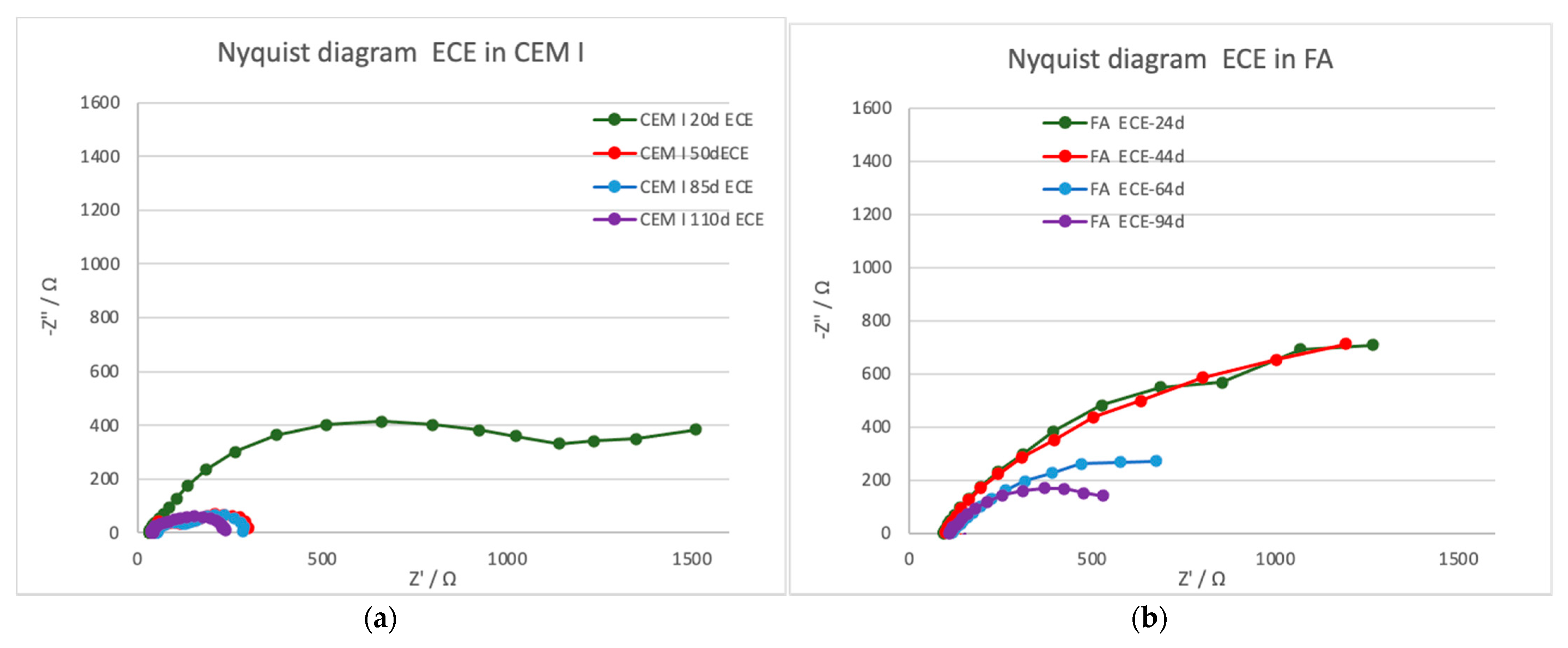
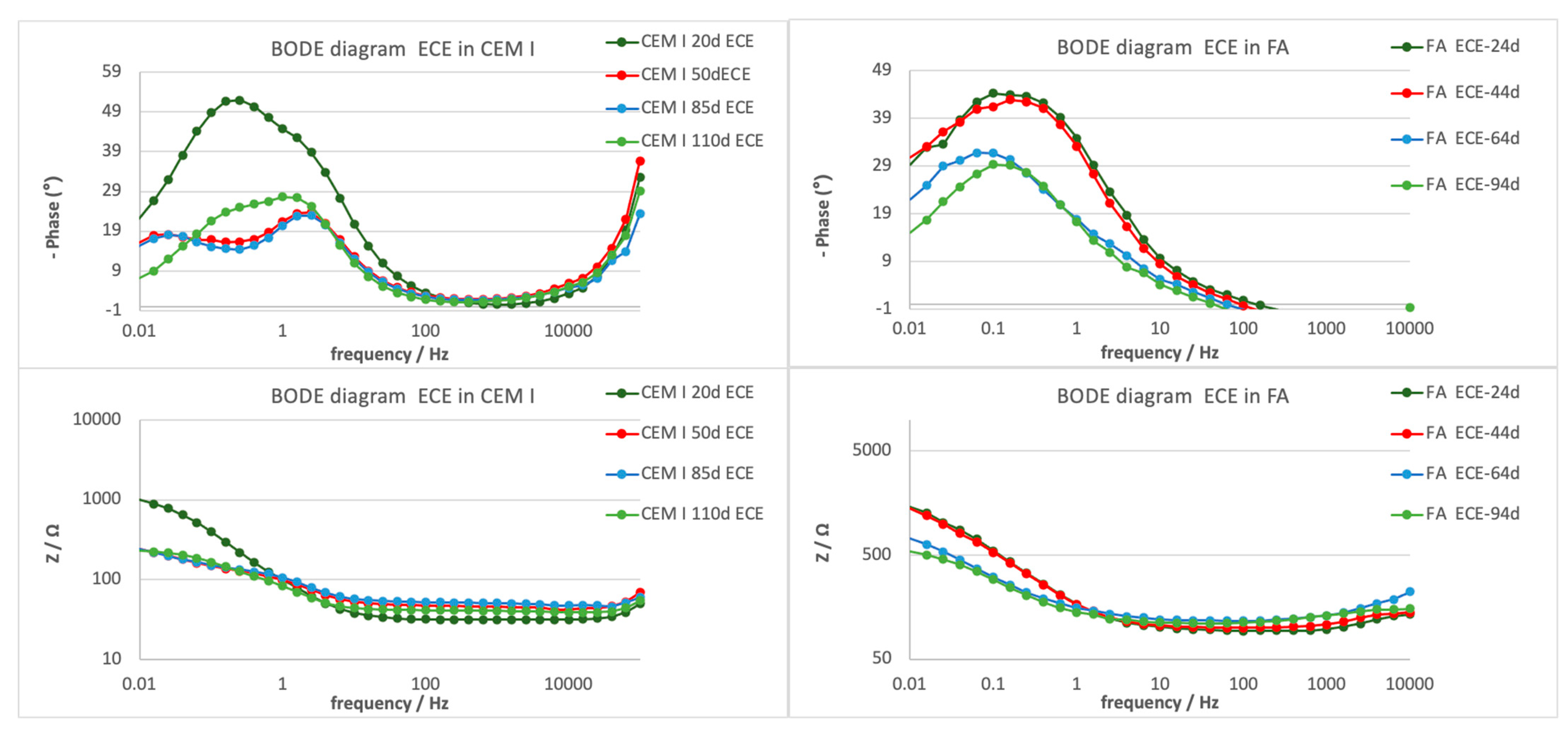
3.3.3. Electrochemical Impedance Spectroscopy
3.4. ECE Effects: Posttreatment Indicators
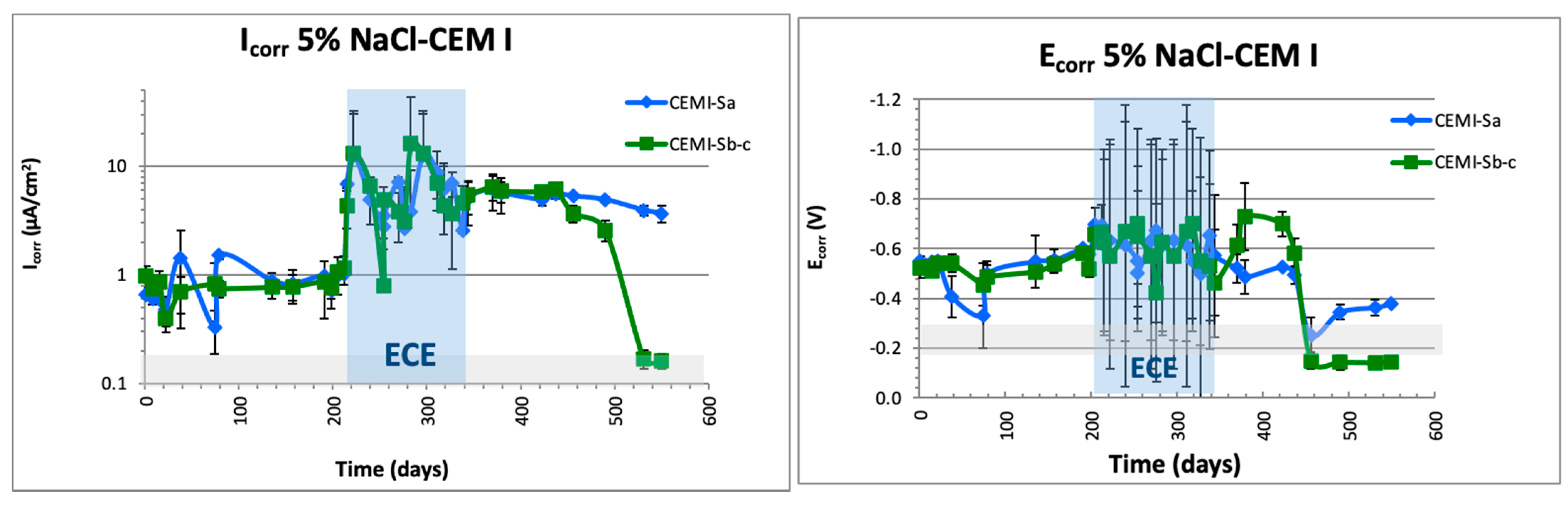
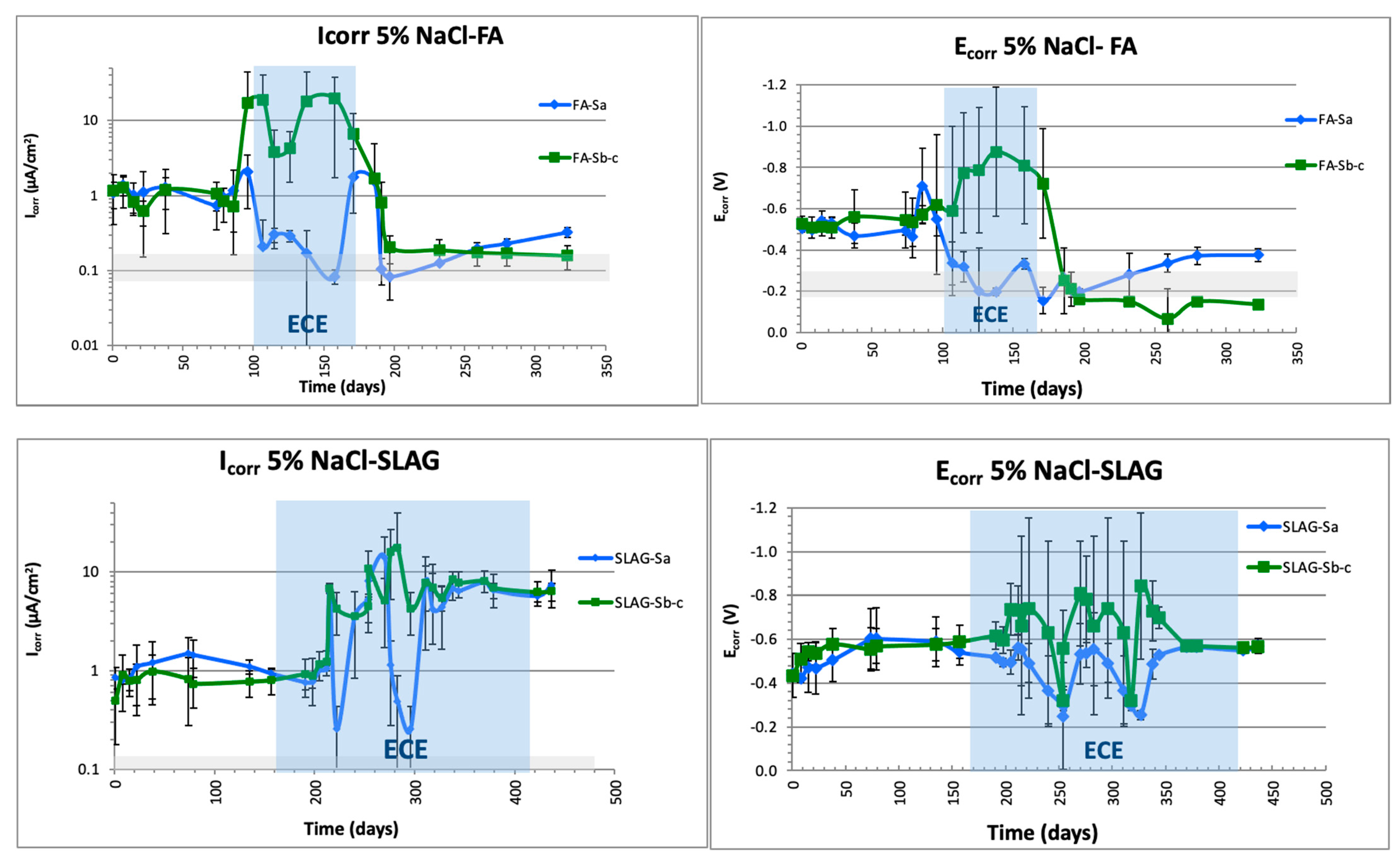
3.4.1. Electrochemical Characterization: Ecorr and Icorr (LPR and EIS Measurements)
3.4.2. Quantitative Chloride Profiles at the End of Treatment: Extraction Efficiency
3.5. ECE Additional Effects
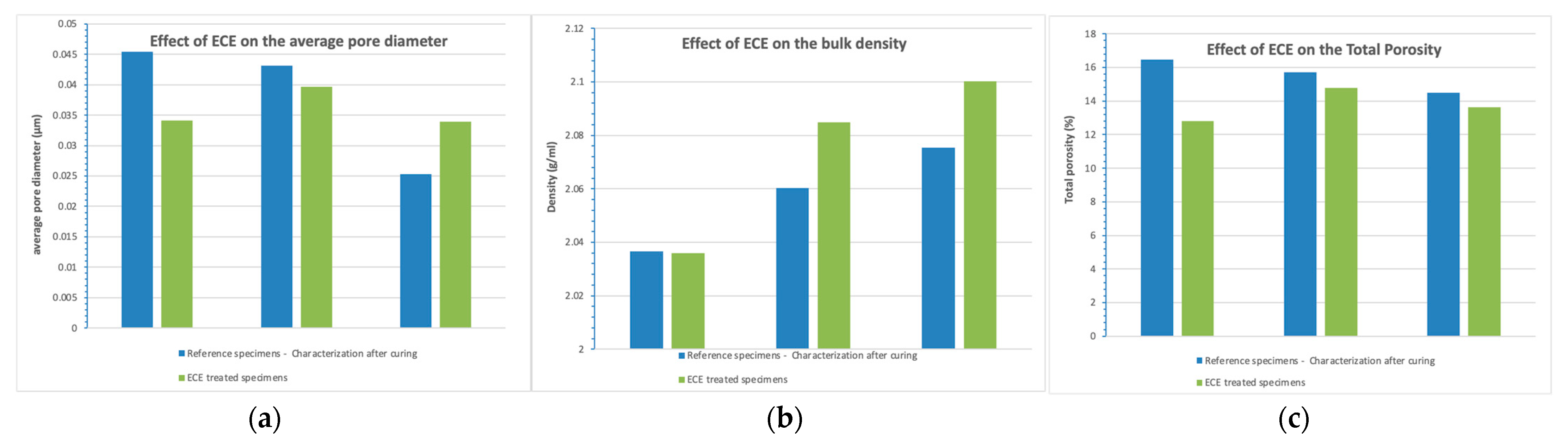
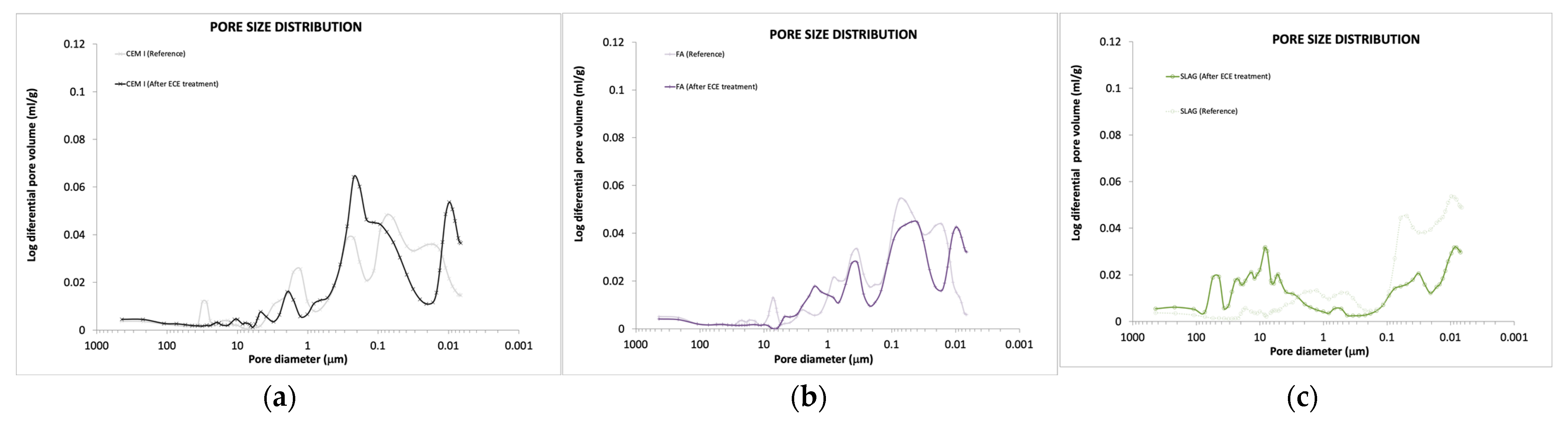
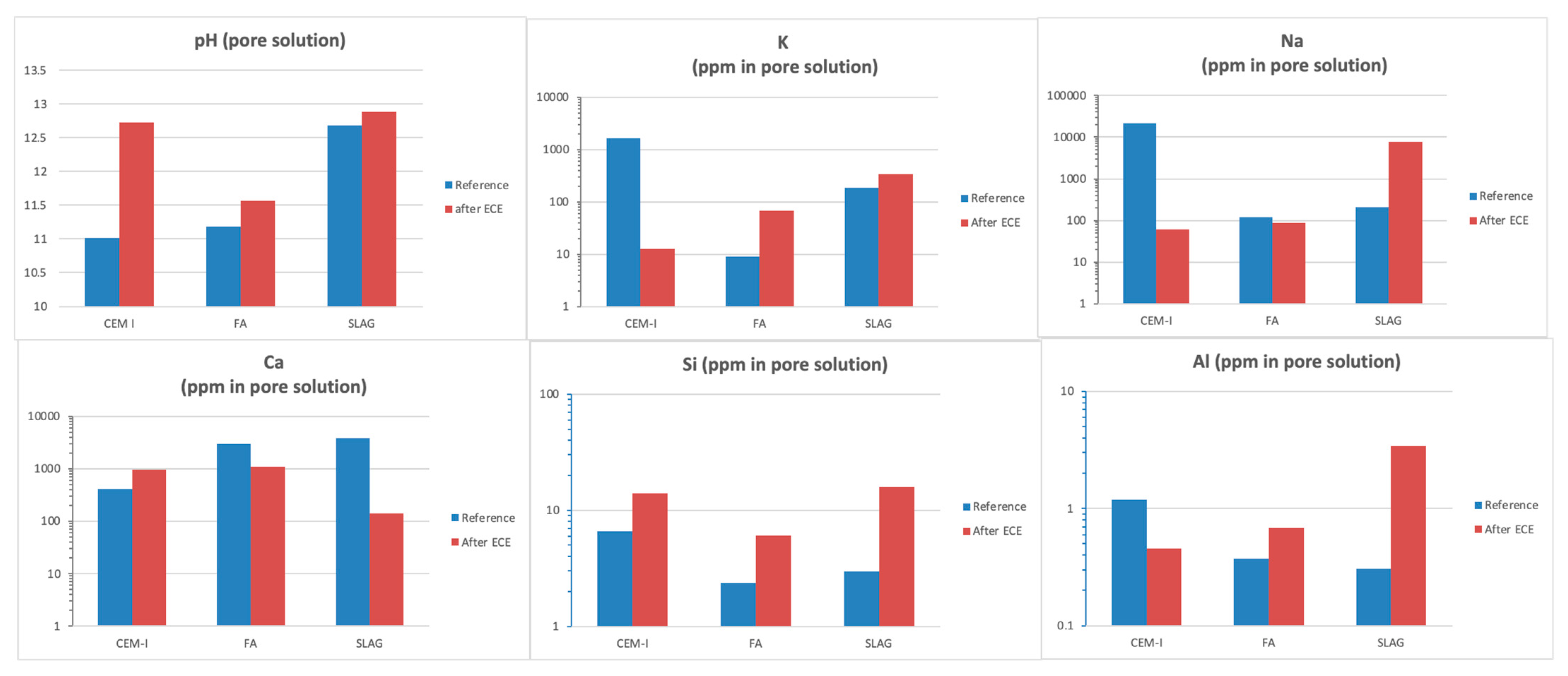
3.5.1. Pore Structure and Pore Solution
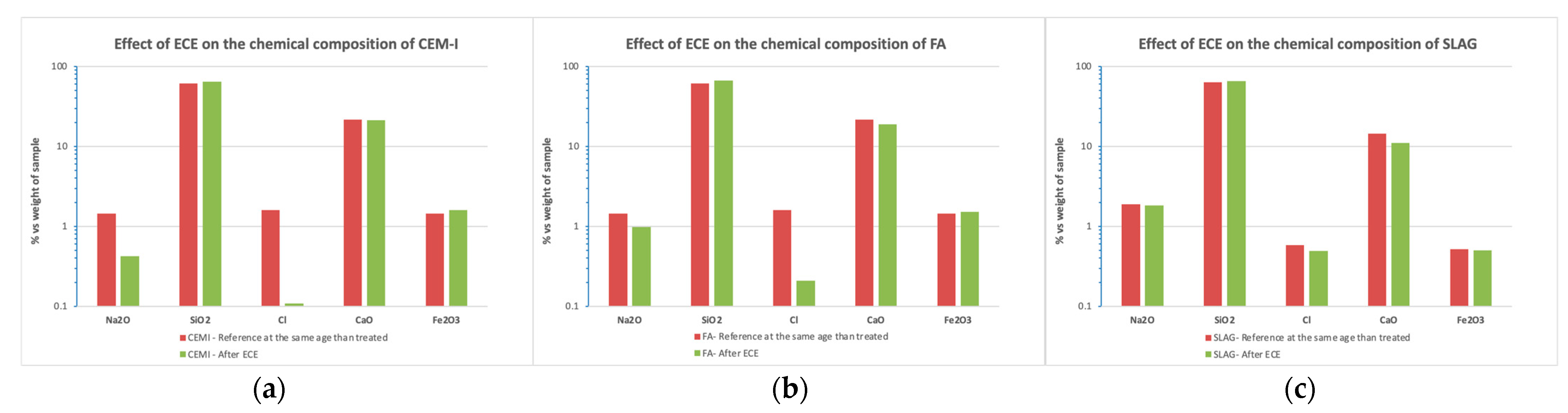
3.5.2. Bulk Chemical Composition
4. Conclusions
- Charge density normalized with resistivity values instead of charge density seems to be a better indicator to determine the end of the treatment, as the resistivity of the cementitious materials is one of the most important parameters to be considered to prior deduce if the material will have good behavior in an ECE treatment. Depolarized steel potential measured in periodical 24 h depolarizations also is shown as a good indicator of the treatment evolution. So, both parameters could be added to the ones already stablished to determine the end of the treatment.
- On the other hand, the resistance due to the charge transfer evaluated by the EIS during the treatment can provide important information about the treatment evolution. It is related to the double-layer capacity generated in the steel–mortar interface. The decrease (contraction of the semicircle) detected in both CEM I and FA can be related to the good progress of the treatment.
- Once the ECE has been stopped, corrosion rates determined by LPR or EIS provides reliable information about the steel passivation, and are in concordance with the destructive test applied (chloride profiles and visual inspection).
- All the parameters monitored indicate that in the case of FA, the passivation of the steels connected to treatment was reached in the same way as in plain CEM I specimens. A degree of protection is also detected in the nonconnected steel, which means that substitution of 8% in FA does not affect treatment efficiency and can also partially protect the metallic elements embedded in the same electrolyte but not connected to the treatment.
- On the other hand, in the case of using CEM III (slag), no steel passivation was reached.
- Notably, early age characterization and electrical resistivity values, which reflect the different characteristics of the mineral admixtures because of their hydration capacity, affect the ECE treatment. The slag mortar properties differ from the FA and CEM I and justify the low ionic mobility in this case.
- It is also noted that the different combination of chlorides (difference between total and free chlorides) affects the treatment efficiency. The addition of FA improves the combination of chloride with the cement phases; thus, total chloride remains high (as a result of the high degree combination), whereas free chloride content decreased with treatment. The chemical combination of chloride ions is frequently considered a beneficial effect in that it reduces the rate of chloride penetration in concrete.
- Changes in the pore structure and pore solution composition are detected. pH increases in all cases after treatment (more severely in the case of CEM I), whereas the ECE treatment reduces the porosity in all cases. Thus, the ECE treatment is able not only to reduce chloride but also to improve the mortar durability properties and its ability to passivate the steel.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmad, S. Reinforcement corrosion in concrete structures, its monitoring and service life prediction––A review. Cem. Concr. Compos. 2003, 25, 459–471. [Google Scholar] [CrossRef]
- Shi, X.; Xie, N.; Fortune, K.; Gong, J. Durability of steel reinforced concrete in chloride environments: An overview. Constr. Build. Mater. 2012, 30, 125–138. [Google Scholar] [CrossRef]
- Almusallam, A.A.; Al-Gahtani, A.S.; Aziz, A.R. Rasheeduzzafar Effect of reinforcement corrosion on bond strength. Constr. Build. Mater. 1996, 10, 123–129. [Google Scholar] [CrossRef]
- Castellote, M.; Andrade, C.; Alonso, C. Determination of the chloride diffusion coefficients in steady state by migration test based on conductivity measurements. In 2nd International Rilem Workshop on Testing and Modelling the Chloride Ingress into Concrete; Andrade, C., Kropp, J., Eds.; RILEM Publications SARL: Paris, France, 2000. [Google Scholar]
- Andrade, C.; Castellote, M.; Alonso, C.; Gonzalez, C. Non-steady-state chloride diffusion coefficients obtained from migration and natural diffusion tests. Part I: Comparison between several methods of calculation. Mater. Struct. 2000, 33, 21–28. [Google Scholar] [CrossRef]
- Martin-Perez, B.; Zibara, H.; Hooton, R.D.; Thomas, M. A study of the effect of chloride binding on service life predictions. Cem. Concr. Res. 2000, 30, 1215–1223. [Google Scholar] [CrossRef]
- Castellote, M.; Andrade, C.; Alonso, C. Changes in the chloride binding ability of concrete submitted to non-steady-state migration experiments in comparison with natural diffusion. In Second International RILEM Workshop on Testing and Modelling the Chloride Ingress into Concrete; RILEM Publications SARL: Paris, France, 2000; pp. 13–22. [Google Scholar]
- Buenfeld, N.R.; Glass, G.K.; Hassanein, A.M.; Zhang, J.-Z. Chloride Transport in Concrete Subjected to Electric Field. J. Mater. Civ. Eng. 1998, 10, 220–228. [Google Scholar] [CrossRef]
- Alonso, C.; Andrade, C.; Castellote, M.; Castro, P. Chloride threshold values to depassivate reinforcing bars embedded in a standardized OPC mortar. Cem. Concr. Res. 2000, 30, 1047–1055. [Google Scholar] [CrossRef]
- Angst, U.; Elsener, B.; Larsen, C.K.; Vennesland, Ø. Critical chloride content in reinforced concrete—A review. Cem. Concr. Res. 2009, 39, 1122–1138. [Google Scholar] [CrossRef]
- Bertolini, L.; Bolzoni, F.; Elsener, B.; Pedeferri, P.; Andrade, C. Realkalinisation and electrochemical removal of chloride in reinforced-concrete structures. Mater. De Constr. 1996, 46, 45–55. [Google Scholar] [CrossRef]
- Elsener, B.; Zimmermann, L.; Bürchler, D.; Böhni, H. Repair of Reinforced Concrete Structures by Electrochemical Techniques—Field Experience. In Corrosion of Reinforcement in Concrete—Monitoring, Prevention and Rehabilitation; CRC Press: Boca Raton, FL, USA, 2020; pp. 125–140. [Google Scholar]
- Meitz, J. Electrochemical Rehabilitation Methods for Reinforced Concrete Structures—A State of the Art Report; European Federation of Corrosion Publications, Ed.; IOM Communications Ltd.: London, UK, 1998. [Google Scholar]
- Martinez, I.; Andrade, C.; Castellote, M.; De Viedma, G.P. Advancements in non-destructive control of efficiency of electrochemical repair techniques. Corros. Eng. Sci. Technol. 2009, 44, 108–118. [Google Scholar] [CrossRef] [Green Version]
- Slater, J.E.; Lankard, D.R.; Moreland, P.J. Electrochemical removal of chlorides from concrete bridge decks. Mater. Perform. 1976, 15, 21–26. [Google Scholar]
- Invention Patent FI100101B. Inventors: John B Miller.; Oystein Vennesland.; Ole Arfinn Opsahl. Method for Removing Chlorides from Reinforced Concrete. 1989. Available online: https://patents.google.com/patent/FI100101B/en#citedBy (accessed on 25 May 2021).
- Bennett, J.E. Electrochemical chloride removal from concrete. A SHRP Contract Status Report. In Corrosion; NACE: Las Vegas, NV, USA, 1990. [Google Scholar]
- Elsener, B.; Molina, M.; Böhni, H. The electrochemical removal of chlorides from reinforced concrete. Corros. Sci. 1993, 35, 1563–1570. [Google Scholar] [CrossRef]
- Green, W.K.; Lyon, S.B.; Scantlebury, J.D. Electrochemical changes in chloride-contaminated reinforced-concrete following cathodic polarization. Corros. Sci. 1993, 35, 1627–1631. [Google Scholar] [CrossRef]
- Hansson, I.; Hansson, C. Electrochemical extraction of chlorides from concrete part I—A qualitative model of the process. Cem. Concr. Res. 1993, 23, 1141–1152. [Google Scholar] [CrossRef]
- Tritthart, J.; Pettersson, K.; Sorensen, B. Electrochemical removal of chloride from hardened cement paste. Cem. Concr. Res. 1993, 23, 1095–1104. [Google Scholar] [CrossRef]
- Cañón, A.; Garcés, P.; Climent, M.A.; Carmona, J.; Zornoza, E. Feasibility of electrochemical chloride extraction from structural reinforced concrete using a sprayed conductive graphite powder–cement paste as anode. Corros. Sci. 2013, 77, 128–134. [Google Scholar] [CrossRef]
- Zhu, J.-H.; Wei, L.; Wang, Z.; Liang, C.K.; Fang, Y.; Xing, F. Application of carbon-fiber-reinforced polymer anode in electrochemical chloride extraction of steel-reinforced concrete. Constr. Build. Mater. 2016, 120, 275–283. [Google Scholar] [CrossRef]
- Zhao, L.-C.; Chen, S.-S. Preparation of Graphene-Cement Paste Anode for Chloride Extraction from Marine Reinforced Concrete Structures. Int. J. Electrochem. Sci. 2016, 11, 9245–9253. [Google Scholar] [CrossRef]
- Climent, M.-Á.; Carmona, J.; Garcés, P. Graphite–Cement Paste: A New Coating of Reinforced Concrete Structural Elements for the Application of Electrochemical Anti-Corrosion Treatments. Coatings 2016, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Schneck, U. Non-Destructive Electrochemical Chloride Extraction on the Danube Bridge Pfaffenstein: Long-Term Experiences about a Structure- and Traffic-keeping Technology. Beton-Und Stahlbetonbau 2009, 104, 145–153. [Google Scholar] [CrossRef]
- Bouteiller, V.; Tong, Y.Y. Electrochemical chloride extraction of a beam polluted by chlorides after 40 years in the sea, Advances in Concrete Structural Durability. In Proceedings of the Icdcs2008, Beijing, China, 17–20 June 2008; Volumes 1 and 2, pp. 1278–1284. [Google Scholar]
- Orellan, J.; Escadeillas, G.; Arliguie, G. Electrochemical chloride extraction: Efficiency and side effects. Cem. Concr. Res. 2004, 34, 227–234. [Google Scholar] [CrossRef]
- Page, C.L.; Yu, S.W. Potential effects of electrochemical desalination of concrete on alkali–silica reaction. Mag. Concr. Res. 1995, 47, 23–31. [Google Scholar] [CrossRef]
- European Standard: CEN. Electrochemical Re-Alkalization and Chloride Extraction Treatments for Rein-Forced Concrete—Part 2: Chloride Extraction; European Standard: Pilsen, Czech Republic, 2011. [Google Scholar]
- Alonso, C.; Castellote, M.; Andrade, C. Dependence of chloride threshold with the electrical potential of reinforcements. In 2nd International Rilem Workshop on Testing and Modelling the Chloride Ingress into Concrete; Andrade, C., Kropp, J., Eds.; RILEM Publications SARL: Paris, France, 2000; pp. 415–425. [Google Scholar]
- Vennesland, M.A.; Climent, C.; Andrade, R.T.C. 178-TMC, Recommendation of RILEM TC 178-TMC: Testing and modelling chloride penetration in concrete Methods for obtaining dust samples by means of grinding concrete in order to determine the chloride concentration profile. Mater. Struct. 2013, 46, 337–344. [Google Scholar]
- Martinez, I.; Rozas, F.; Ramos-Cillan, S.; González, M.; Castellote, M. Chloride Electroremediation in reinforced structures: Preliminary electrochemical tests to detect the steel repassivation during the treatment. Electrochim. Acta 2015, 181, 288–300. [Google Scholar] [CrossRef]
- Juenger, M.C.G.; Siddique, R. Recent advances in understanding the role of supplementary cementitious materials in concrete. Cem. Concr. Res. 2015, 78, 71–80. [Google Scholar] [CrossRef]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary cementitious materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Park, J.-I.; Lee, K.-M.; Kwon, S.-O.; Bae, S.-H.; Jung, S.-H.; Yoo, S.-W. Diffusion Decay Coefficient for Chloride Ions of Concrete Containing Mineral Admixtures. Adv. Mater. Sci. Eng. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.; Dawson, A.; Thom, N.H. Macro/micro-pore structure characteristics and the chloride penetration of self-compacting concrete incorporating different types of filler and mineral admixture. Constr. Build. Mater. 2014, 72, 83–93. [Google Scholar] [CrossRef]
- Filho, J.H.; de Medeiros, M.H.F.; Pereira, E.; Helene, P.; Isaia, G. High-Volume Fly Ash Concrete with and without Hydrated Lime: Chloride Diffusion Coefficient from Accelerated Test. J. Mater. Civ. Eng. 2013, 25, 411–418. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.-M.; Ba, H.-J.; Chen, S.-J. Effect of fly ash and repeated loading on diffusion coefficient in chloride migration test. Constr. Build. Mater. 2011, 25, 2269–2274. [Google Scholar] [CrossRef]
- Yoo, J.; Park, S.; Oh, B. Effects of Cement and Mineral Admixture on Migration of Chloride Ions and Generation of Hydration Heat in Concrete; Daewoo Construction Technology Report; Daewoo Engineering & Construction Co., Ltd.: Seoul, Korea, 2007; Volume 29, pp. 106–115. [Google Scholar]
- Ismail, M.; Muhammad, B. Electrochemical chloride extraction effect on blended cements. Adv. Cem. Res. 2011, 23, 241–248. [Google Scholar] [CrossRef]
- Zhu, Z.; Chu, H.; Guo, M.-Z.; Shen, M.; Jiang, L.; Yu, L. Effect of silica fume and fly ash on the stability of bound chlorides in cement mortar during electrochemical chloride extraction. Constr. Build. Mater. 2020, 256, 119481. [Google Scholar] [CrossRef]
- Linderoth, O.; Johansson, P.; Wadsö, L. Development of pore structure, moisture sorption and transport properties in fly ash blended cement-based materials. Constr. Build. Mater. 2020, 261, 120007. [Google Scholar] [CrossRef]
- Polder, R.B. Test methods for on site measurement of resistivity of concrete—A RILEM TC-154 technical recommendation. Constr. Build. Mater. 2001, 15, 125–131. [Google Scholar] [CrossRef]
- Stern, M.; Geary, A.L. Electrochemical polarization: I. A theoretical analysis of the shape of polarization curves. J. Electrochem. Soc. 1957, 104, 56–63. [Google Scholar] [CrossRef]
- Andrade, C.; Gonzalez, J.A. Quantitative measurements of corrosion rate of reinforcing steels embedded in concrete using polarization resistance measurements. Mater. Corros. 1978, 29, 515–519. [Google Scholar] [CrossRef]
- Andrade, C.; Martínez, I. Calibration by gravimetric losses of electrochemical corrosion rate measurement using modulated confinement of the current. Mater. Struct. 2005, 38, 833–841. [Google Scholar] [CrossRef]
- Andrade, C.; Alonso, M.C. Values of corrosion rate of steel in concrete to predict service life of concrete structures. In Application of Accelerated Corrosion Tests to Service Life Prediction of Materials; Cragnolino, G., Sridhar, N., Eds.; ASTM: West Conshohocken, PA, USA, 1994; pp. 282–295. [Google Scholar]
- Christensen, B.J.; Mason, T.O.; Jennings, H.M. Impedance Spectroscopy and the Role of Admixtures in the Hydration of Portland Cement Pastes. In MRS Proceedings; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 1991; Volume 245. [Google Scholar]
- Novoa, X.R. Electrochemical aspects of the steelaEuroconcrete system. A review. J. Solid State Electrochem. 2016, 20, 2113–2125. [Google Scholar] [CrossRef]
- Martínez, I.; Andrade, C. Application of EIS to cathodically protected steel: Tests in sodium chloride solution and in chloride contaminated concrete. Corros. Sci. 2008, 50, 2948–2958. [Google Scholar] [CrossRef]
- Martínez, I.; González, M.; Rozas, F.; Castellote, M. New non-destructive passivity indicators for the control of electrochemical chloride extraction in concrete. In Proceedings of the Corrosion 2014, San Antonio, TA, USA, 9–13 March 2014. [Google Scholar]
- Castellote, M.; Andrade, C. Round-robin test on chloride analysis in concrete—Part II: Analysis of water soluble chloride content. Mater. Struct. 2001, 34, 589–596. [Google Scholar] [CrossRef]
- Castellote, M.; Andrade, C. Round-Robin test on chloride analysis in concrete—Part I: Analysis of total chloride content. Mater. Struct. 2001, 34, 532–549. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, Y.S.; Liu, S.F.; Zhang, Y.M. The influence of mineral admixtures on resistance to corrosion of steel bars in green high-performance concrete. Cem. Concr. Res. 2004, 34, 1781–1785. [Google Scholar] [CrossRef]
- Liang, W.Q.; He, Z.; Zhang, Y.C.; Chen, M.Z.; Yang, H.Q. Early-age electrical resistivity and reactive capacity of mineral admixtures in mortars. J. Wuhan Univ. Technology-Mater. Sci. Ed. 2006, 21, 158–162. [Google Scholar]
- Castellote, M.; De Viedma, P.G.; Andrade, C.; Martinez, I. Efficiency control of electrochemical repair techniques. In Concrete Repair, Rehabilitation and Retrofitting II; CRC Press: Boca Raton, FL, USA, 2008; pp. 49–56. [Google Scholar]
- ASTM. ASTM. ASTM C876-09-Standard test method for corrosion potentials of uncoated reinforcing steel in Concrete. In Book of Standards 03.2; ASTM: West Conshohocken, PA, USA, 2009. [Google Scholar]
- Abdelaziz, G.; Abdelalim, A.; Fawzy, Y. Evaluation of the short and long-term efficiencies of electro-chemical chloride extraction. Cem. Concr. Res. 2009, 39, 727–732. [Google Scholar] [CrossRef]
- Ramezanianpour, A.M.; Hooton, R.D. A study on hydration, compressive strength, and porosity of Portland-limestone cement mixes containing SCMs. Cem. Concr. Compos. 2014, 51, 1–13. [Google Scholar] [CrossRef]






| Components | OCP (CEM I) | Fly Ash (FA) | Blast-Furnace Slag |
|---|---|---|---|
| Cement * | 675 g | 621 g | 675 g |
| Aggregate | 2025 g | 2025 g | 2025 gr |
| Water | 371 g | 371 g | 371 gr |
| Sodium chloride | 56 g | 55.70 g | 56 gr |
| Fly ash (8% replacement) | — | 54 g | — |
| Mortar | Density, kg/dm3 | Occluded Air, % | ∅ Initial, cm | ∅ Final, cm |
|---|---|---|---|---|
| CEM I | 2.05 | 9.0 | 12.5 | 22.5 |
| FA | 2.12 | 6.1 | 13.0 | 23.0 |
| Slag | 2.08 | 7.2 | 14.6 | 23.0 |
| SiO2 | CaO | CO2 | Al2O3 | Na2O | Fe2O3 | MgO | SO3 | K2O | TiO2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| CEM I | 41.20 | 36.52 | 5.44 | 2.13 | 5.28 | 2.34 | 0.45 | 2.12 | 0.23 | 0.22 |
| FA | 68.22 | 18.08 | 5.30 | 1.88 | 1.33 | 1.69 | 0.28 | 1.29 | 0.18 | 0.13 |
| Slag | 67.91 | 14.41 | 4.62 | 3.25 | 1.92 | 0.65 | 2.37 | 2.20 | 0.27 | 0.18 |
| Mortar | Flexural Strength | Compressive Strength | ||||
|---|---|---|---|---|---|---|
| Average | St Dev | CoV(%) | Average | St Deb | CoV(%) | |
| CEM I | 7.29 | 1.01 | 13.92 | 35.69 | 3.47 | 9.71 |
| FA | 5.83 | 0.35 | 6.08 | 37.07 | 1.64 | 4.42 |
| Slag | 11.98 | --- | --- | 39.96 | 1.82 | 4.55 |
| Age (days) | |||
|---|---|---|---|
| ρ (KΩ·cm) | 1 | 28 | 100 |
| SLAG | 0.093 | 32.584 | 38.255 |
| SLAG (St Dev) | 0.005 | 3.839 | 7.273 |
| CEMI | 0.192 | 1.380 | 1.497 |
| CEMI (St Dev) | 0.019 | 0.058 | 0.185 |
| FA | 0.161 | 1.177 | 1.838 |
| FA (StDev) | 0.010 | 0.088 | 0.107 |
| C (F) | Rp (Ω) | Rs (Ω) | CPE | ||
|---|---|---|---|---|---|
| Slag | 0.00156 | 10,249 | 7.5925 | 0.99606 | |
| 24 h | CEM I | 0.00113 | 3343.2 | 9.0469 | 0.99645 |
| FA | 0.00097 | 3388.1 | 7.9897 | 0.99682 | |
| Slag | 0.00075 | 7801.2 | 1769.7 | 0.99604 | |
| 28 d | CEM I | 0.00176 | 9079.2 | 70.728 | 0.99391 |
| FA | 0.00133 | 12,082 | 63.868 | 0.99418 | |
| Slag | 0.00190 | 8392.6 | 2837.1 | 0.99675 | |
| 100 d | CEM I | 0.00511 | 3133.4 | 73.870 | 0.99396 |
| FA | 0.00211 | 7564.9 | 98.702 | 0.99754 |
| Mortar | Time (d) | CPE.Y0 (F) | Rp.R (Ω) | Rs.R (Ω) | CPE.N |
|---|---|---|---|---|---|
| CEM I | 20 | 0.0051512 | 1309.000 | 29.291 | 0.9952800 |
| 50 | 0.0017402 | 91.144 | 47.272 | 0.9977000 | |
| 0.0599460 | 199.010 | 119.820 | 0.9959300 | ||
| 85 | 0.0016220 | 95.399 | 51.982 | 0.9977100 | |
| 0.0647850 | 168.700 | 130.620 | 0.9974500 | ||
| 110 | 0.0032299 | 112.730 | 41.155 | 0.9973600 | |
| 0.0060538 | 208.480 | 40.247 | 0.9940000 | ||
| FA | 24 | 0.0067477 | 2358.700 | 90.951 | 0.9945700 |
| 44 | 0.0062760 | 2536.000 | 86.788 | 0.9938500 | |
| 64 | 0.0120370 | 1019.700 | 113.900 | 0.9934700 | |
| 94 | 0.0083477 | 571.710 | 106.550 | 0.9945100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, I.; Castellote, M. Preliminary Study of the Influence of Supplementary Cementitious Materials on the Application of Electro Remediation Processes. Materials 2021, 14, 6126. https://doi.org/10.3390/ma14206126
Martinez I, Castellote M. Preliminary Study of the Influence of Supplementary Cementitious Materials on the Application of Electro Remediation Processes. Materials. 2021; 14(20):6126. https://doi.org/10.3390/ma14206126
Chicago/Turabian StyleMartinez, Isabel, and Marta Castellote. 2021. "Preliminary Study of the Influence of Supplementary Cementitious Materials on the Application of Electro Remediation Processes" Materials 14, no. 20: 6126. https://doi.org/10.3390/ma14206126
APA StyleMartinez, I., & Castellote, M. (2021). Preliminary Study of the Influence of Supplementary Cementitious Materials on the Application of Electro Remediation Processes. Materials, 14(20), 6126. https://doi.org/10.3390/ma14206126







