Electrochemical Investigations of Steels in Seawater Sea Sand Concrete Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Test Solutions
2.2. Electrochemical Methods
2.3. Surface Characterization
3. Results and Discussion
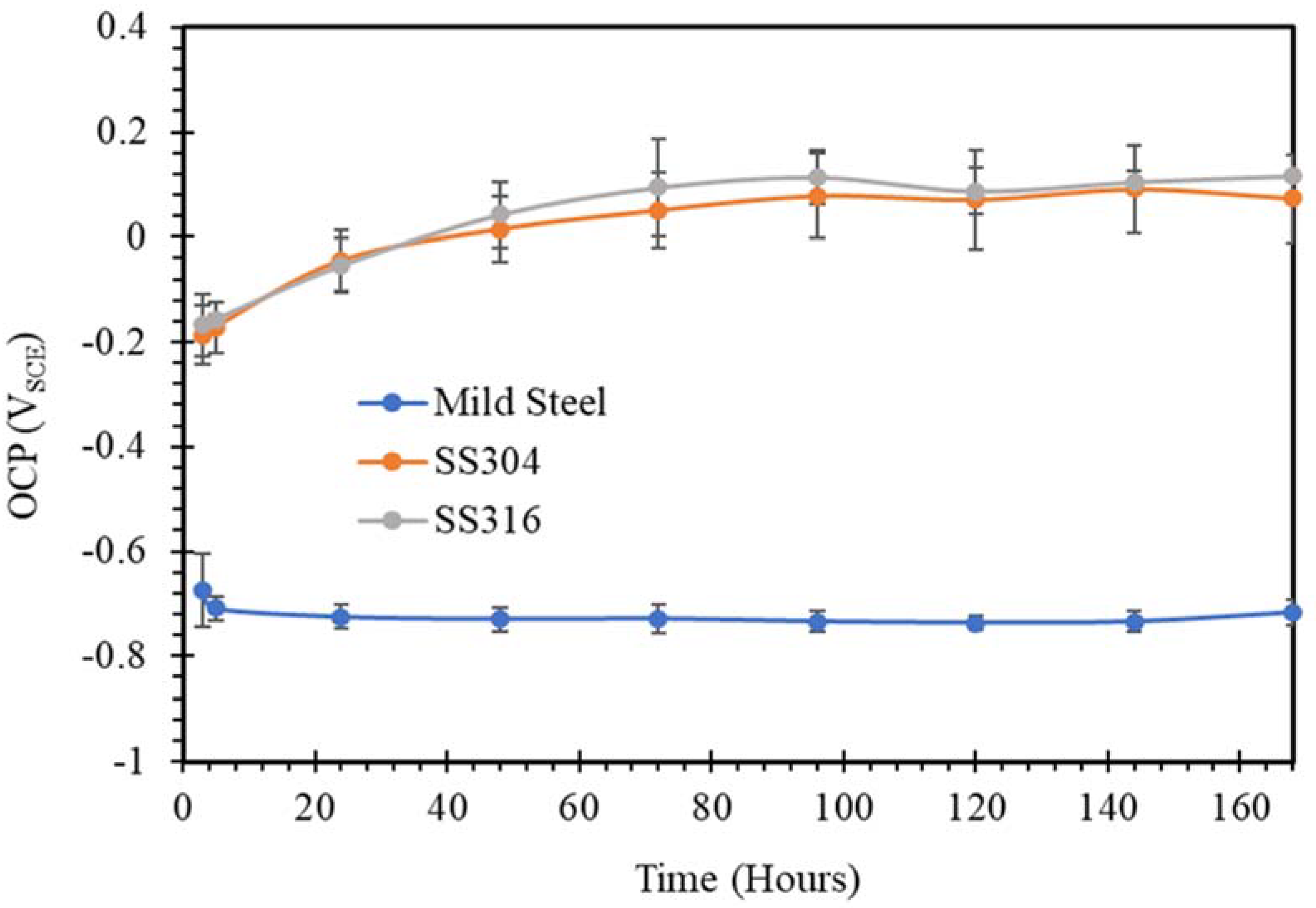
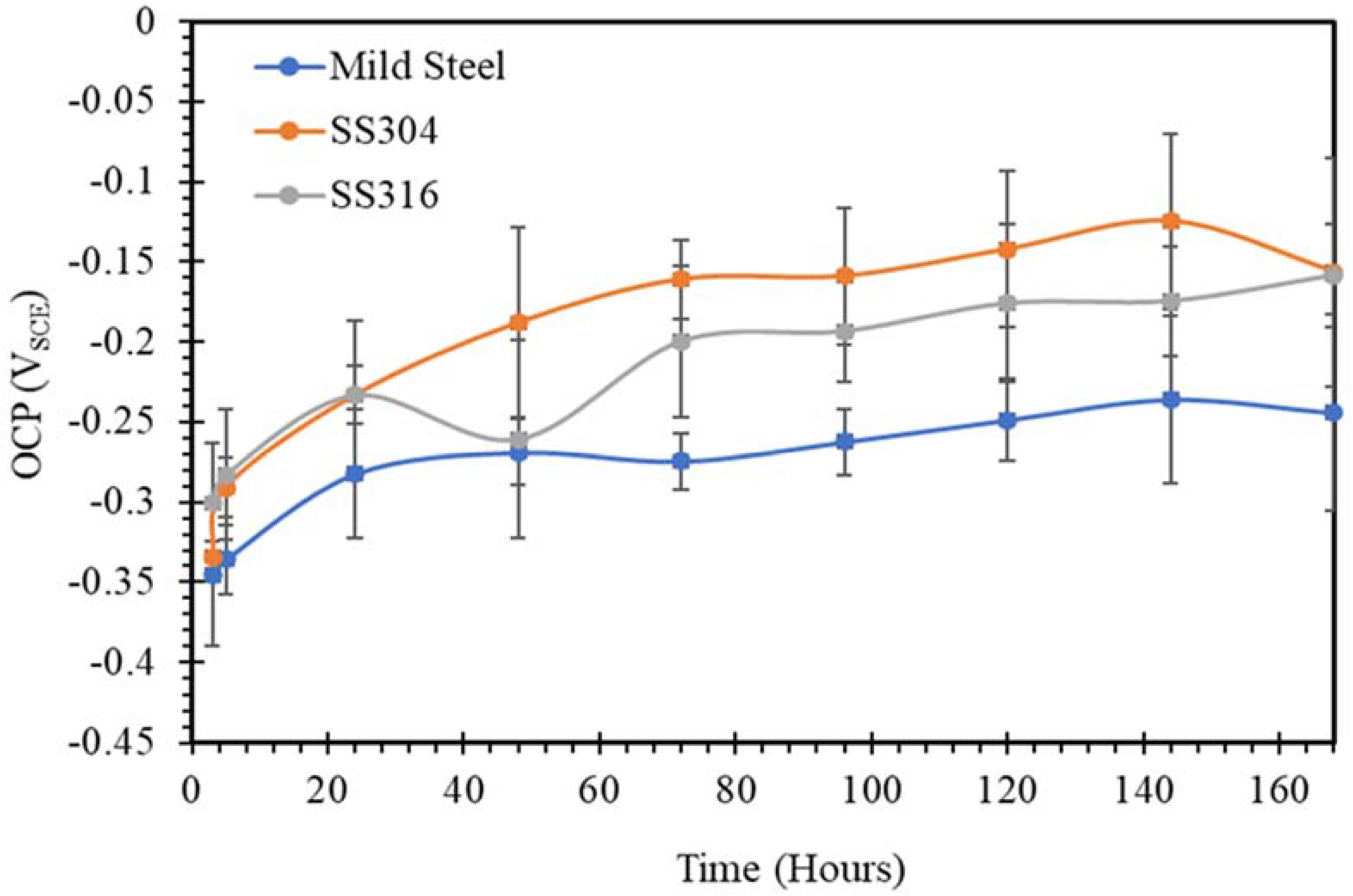
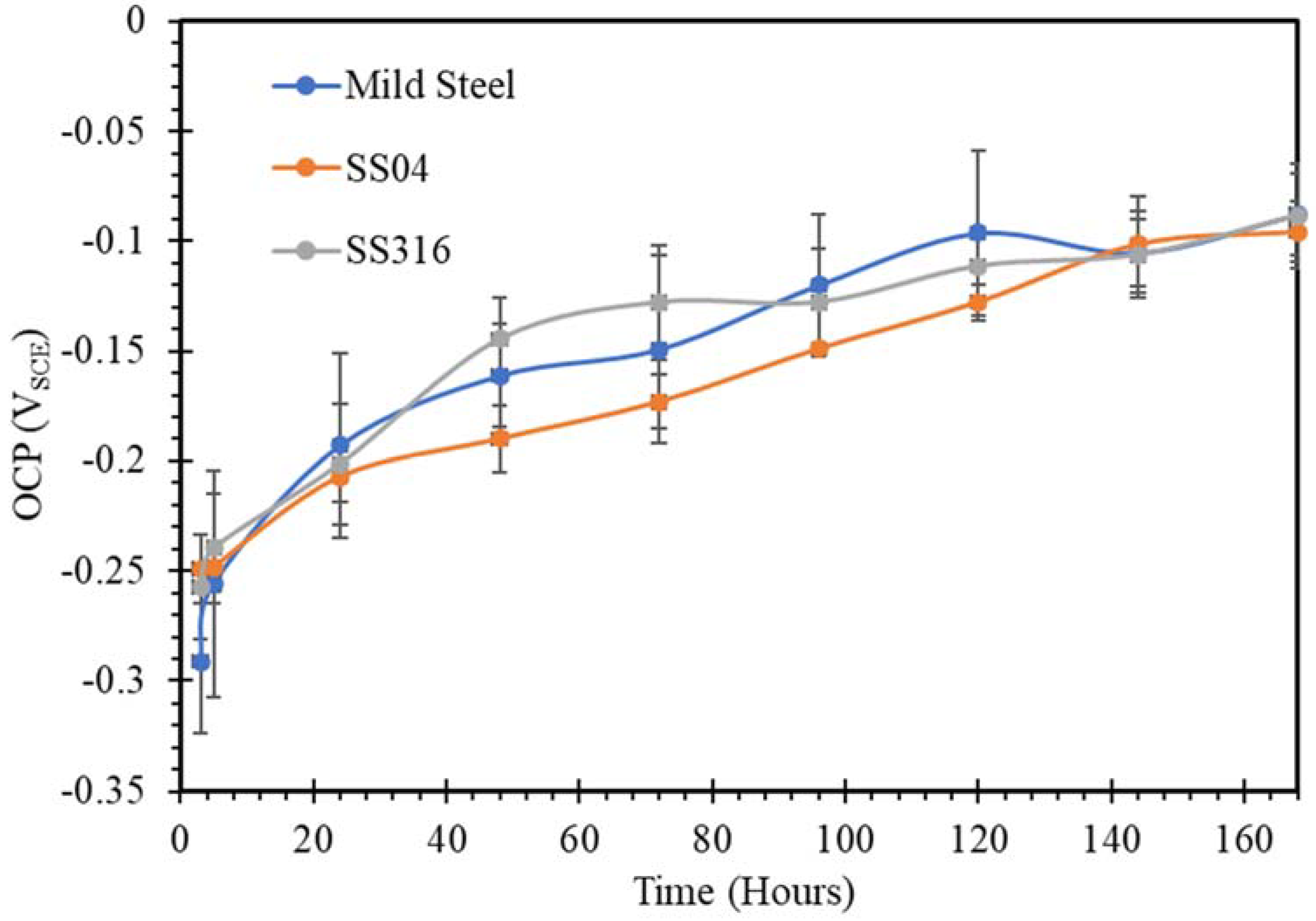
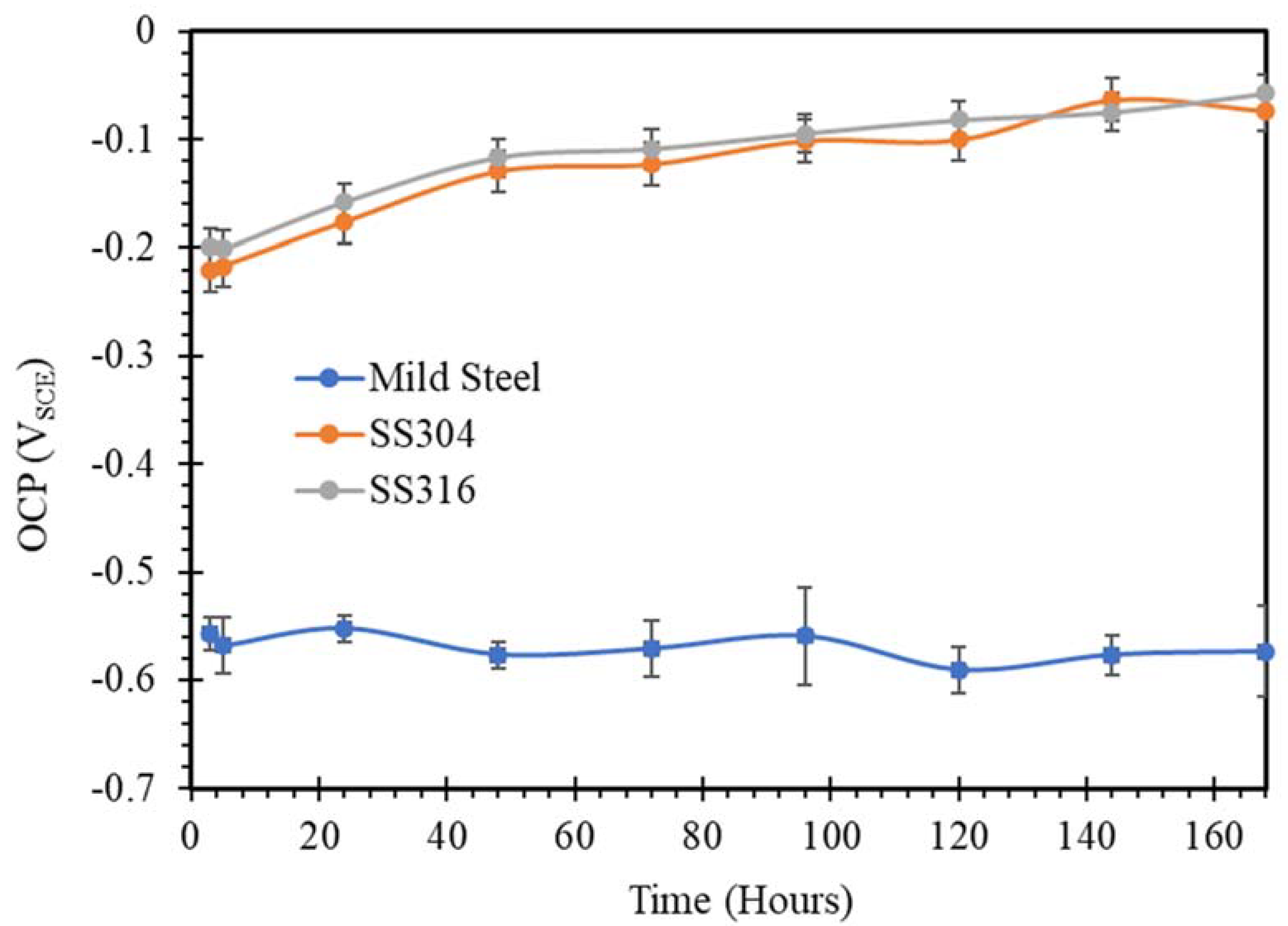
3.1. Open Circuit Potential (OCP)
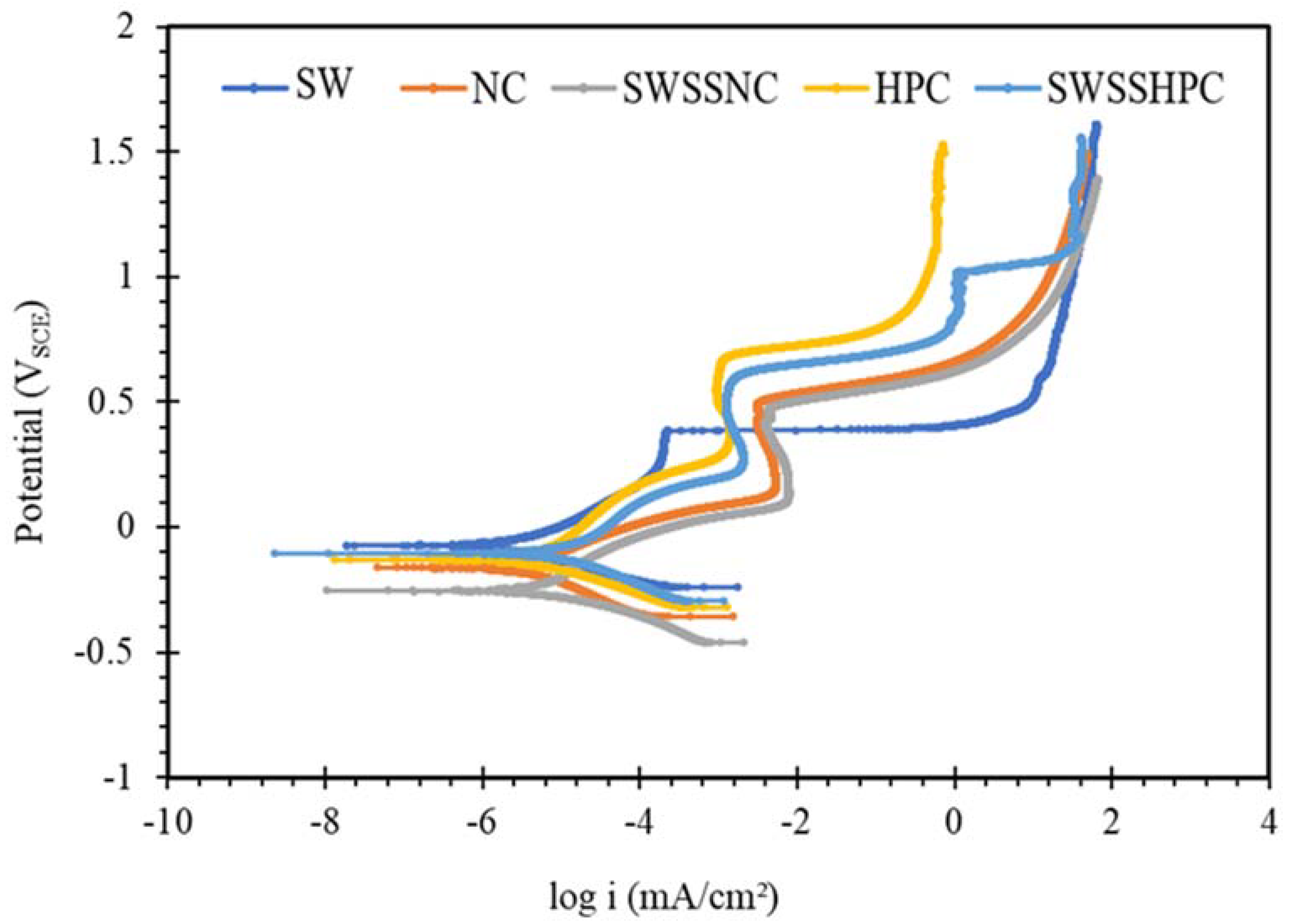
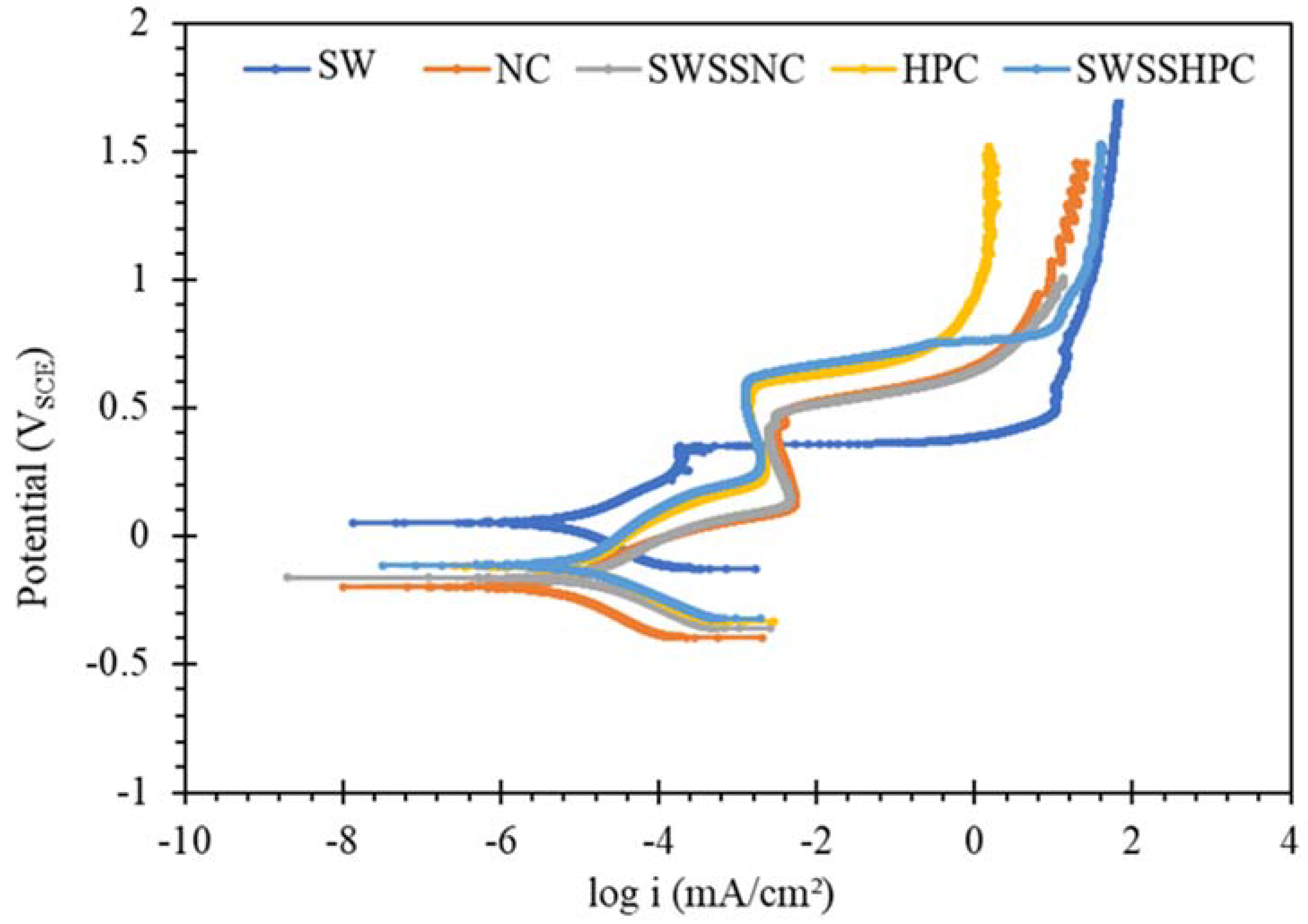
3.2. Potentiodynamic Polarization (PDP)
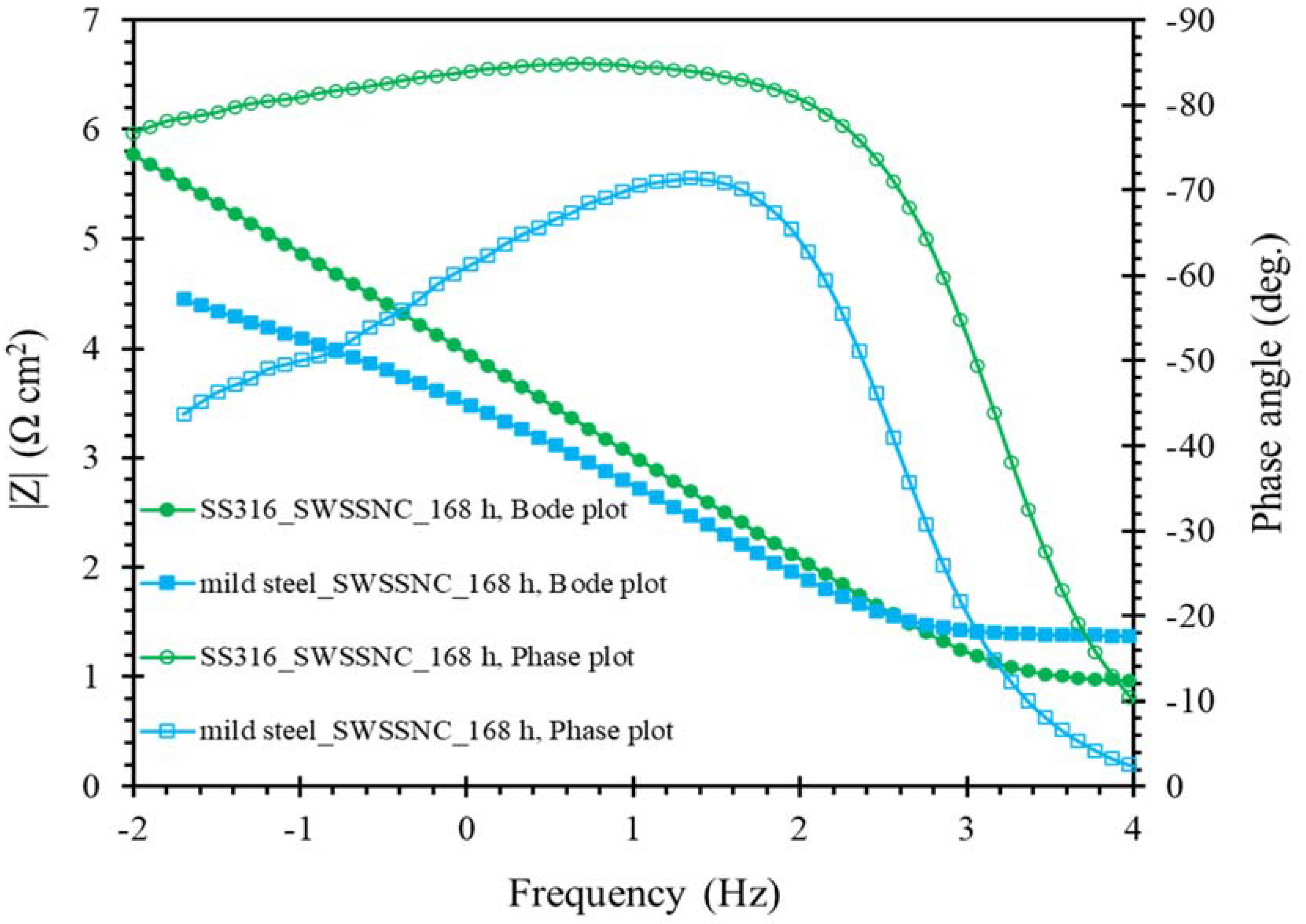
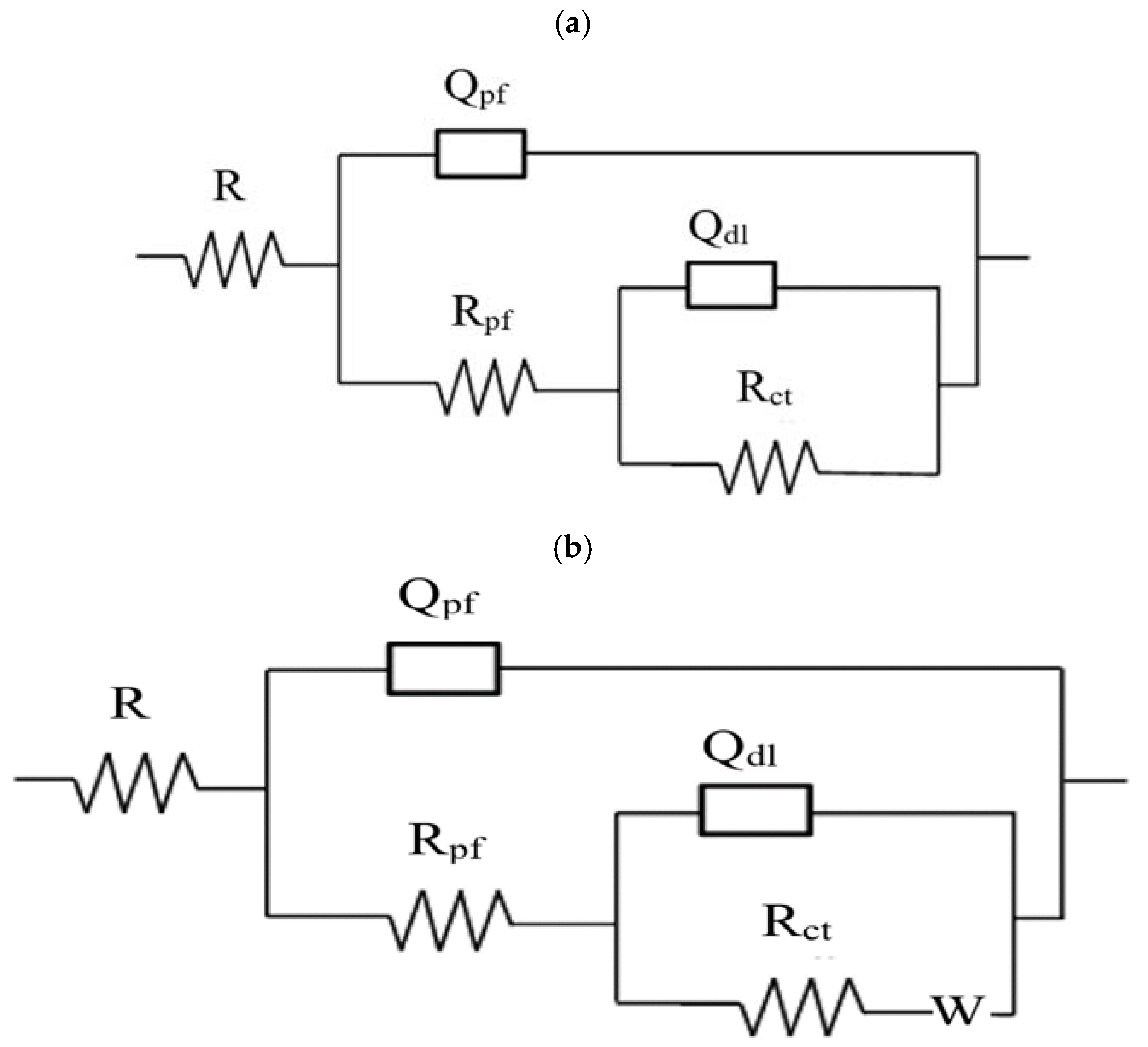
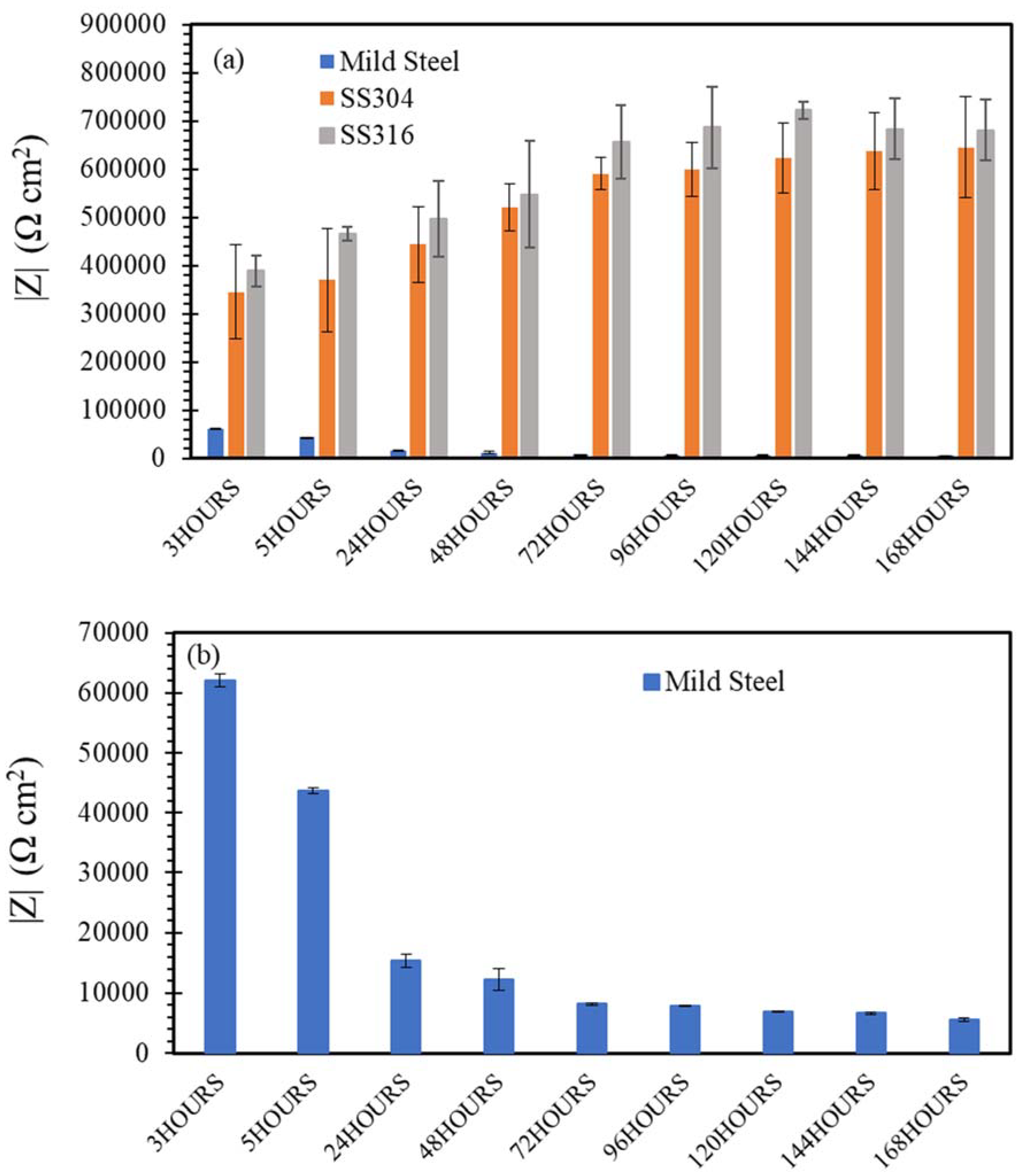
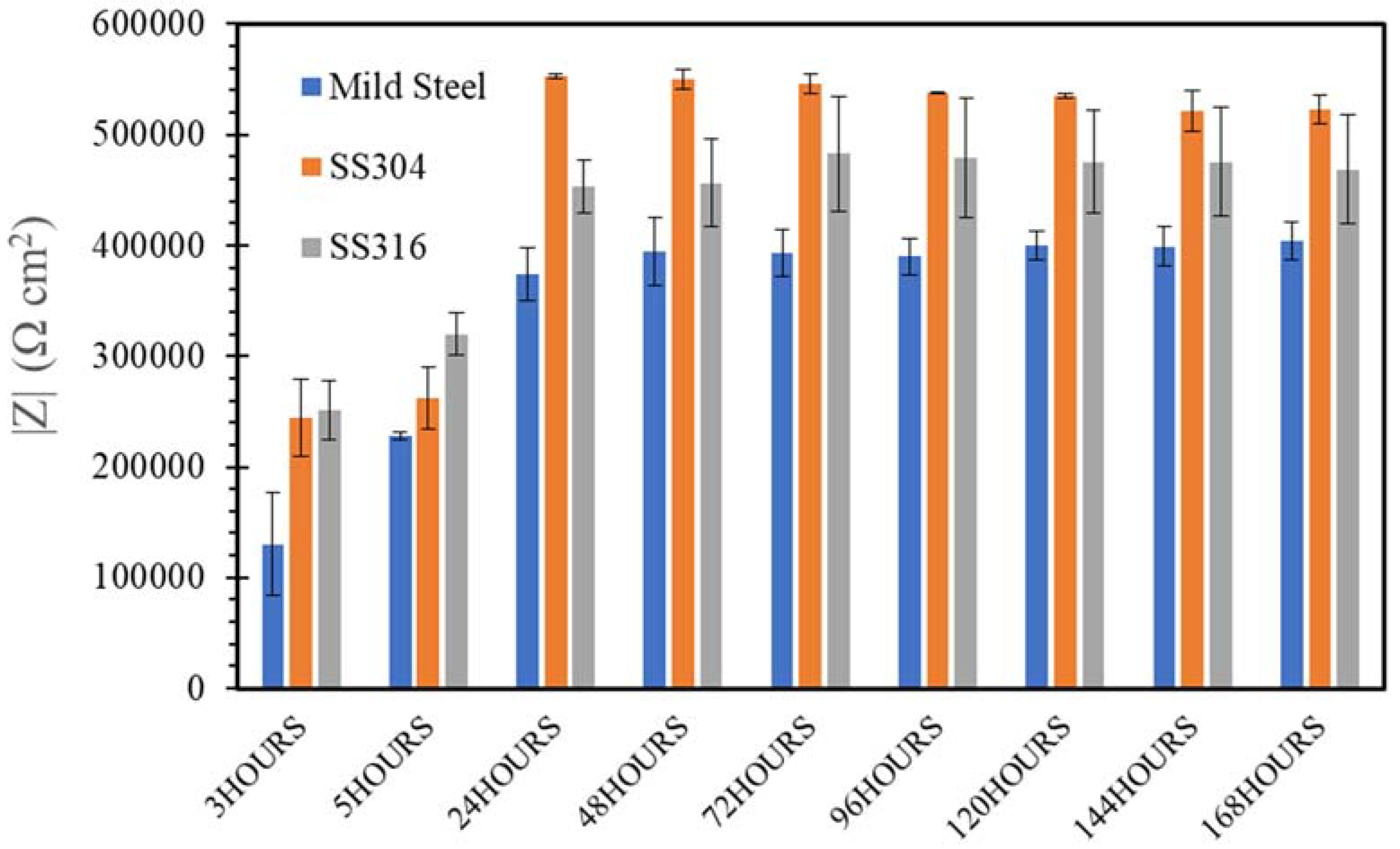
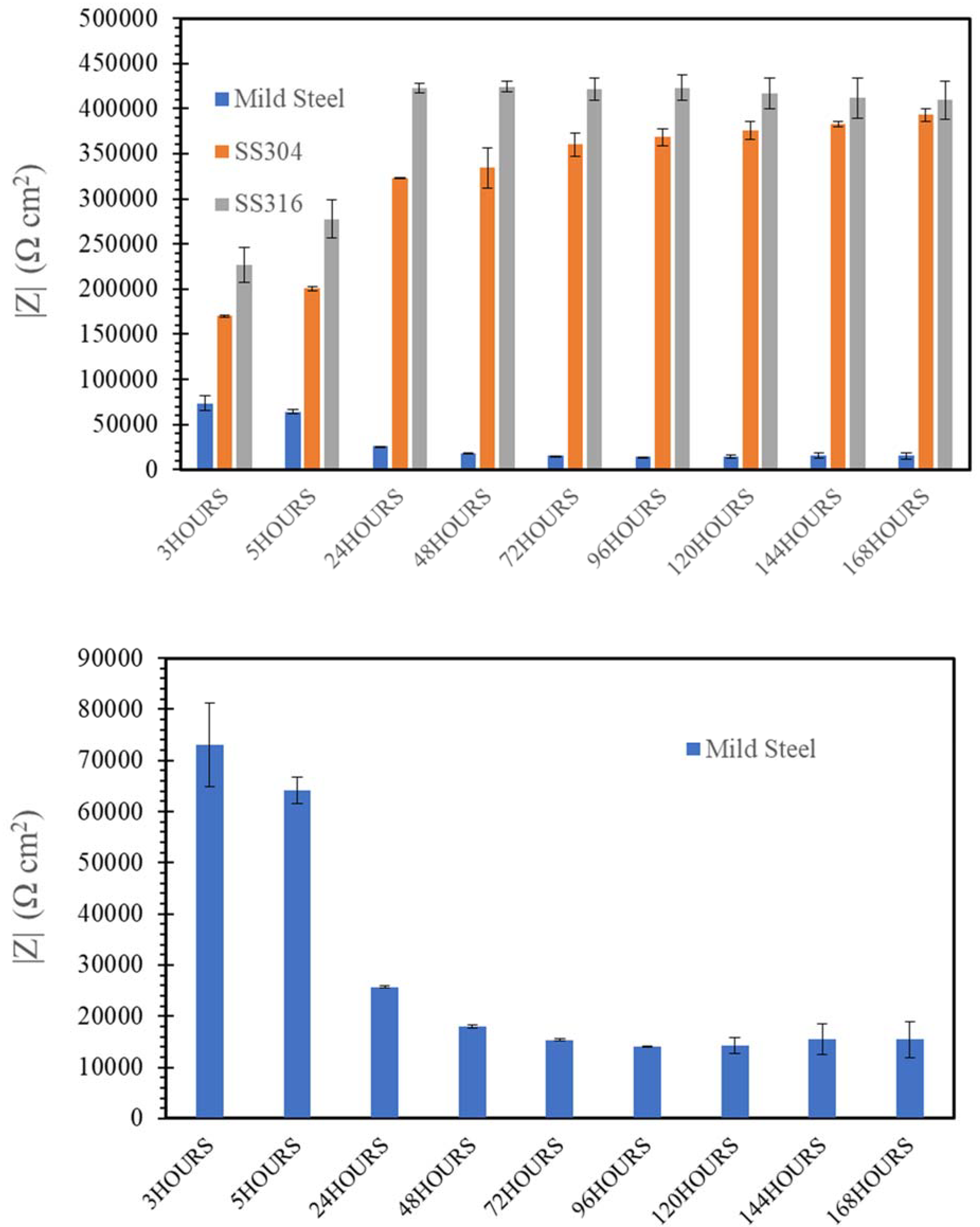
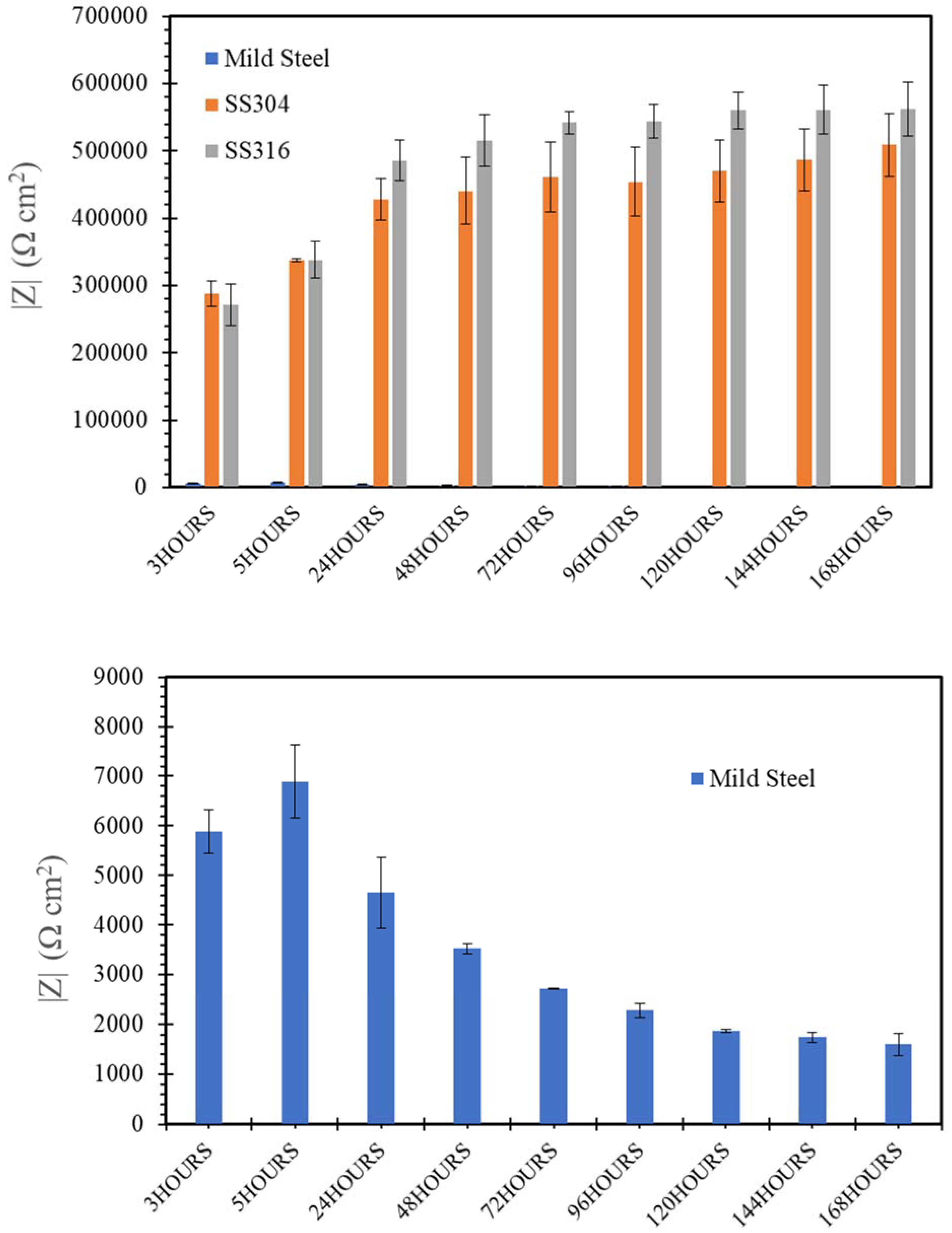
3.3. Electrochemical Impedance Spectroscopy (EIS)
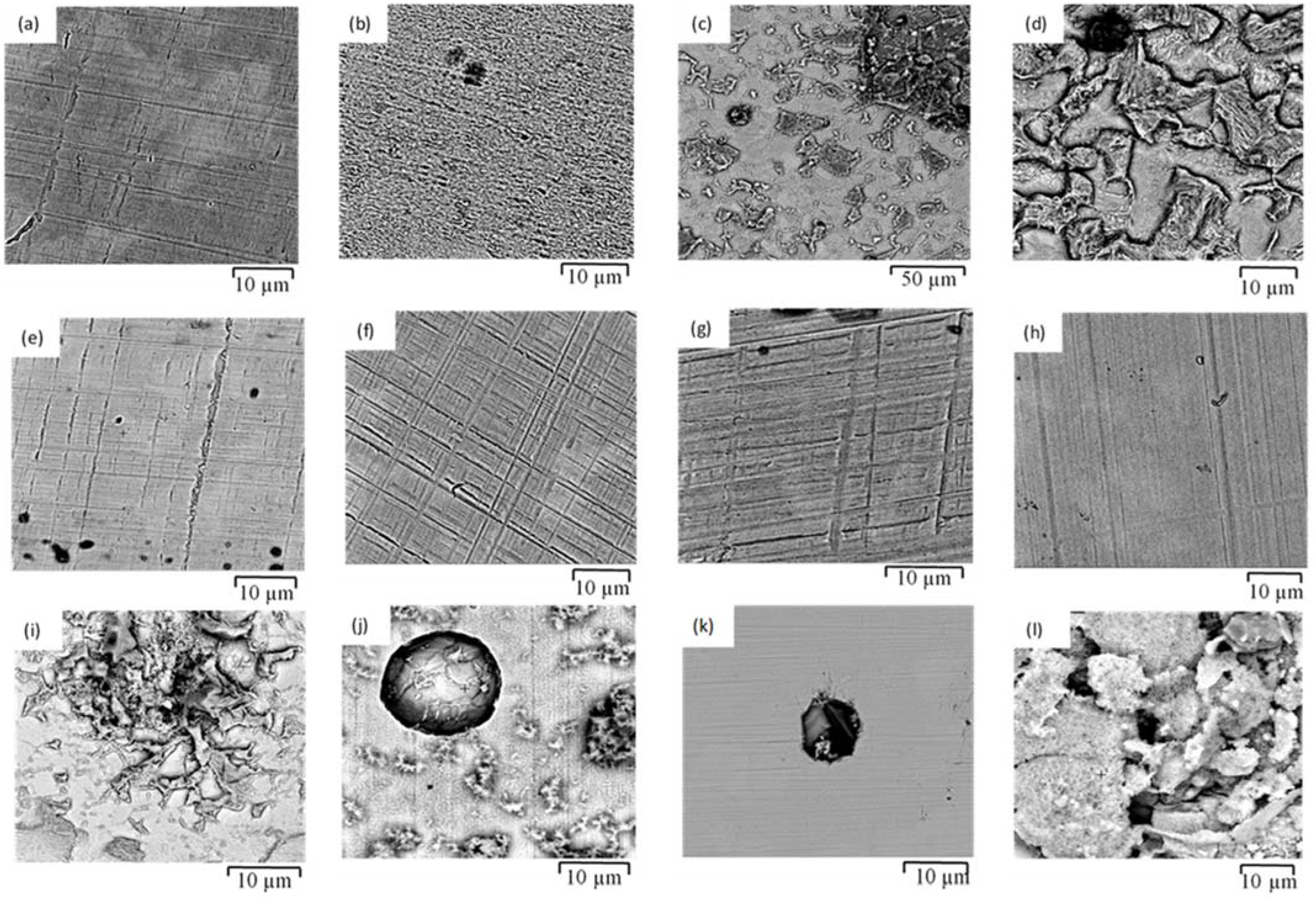
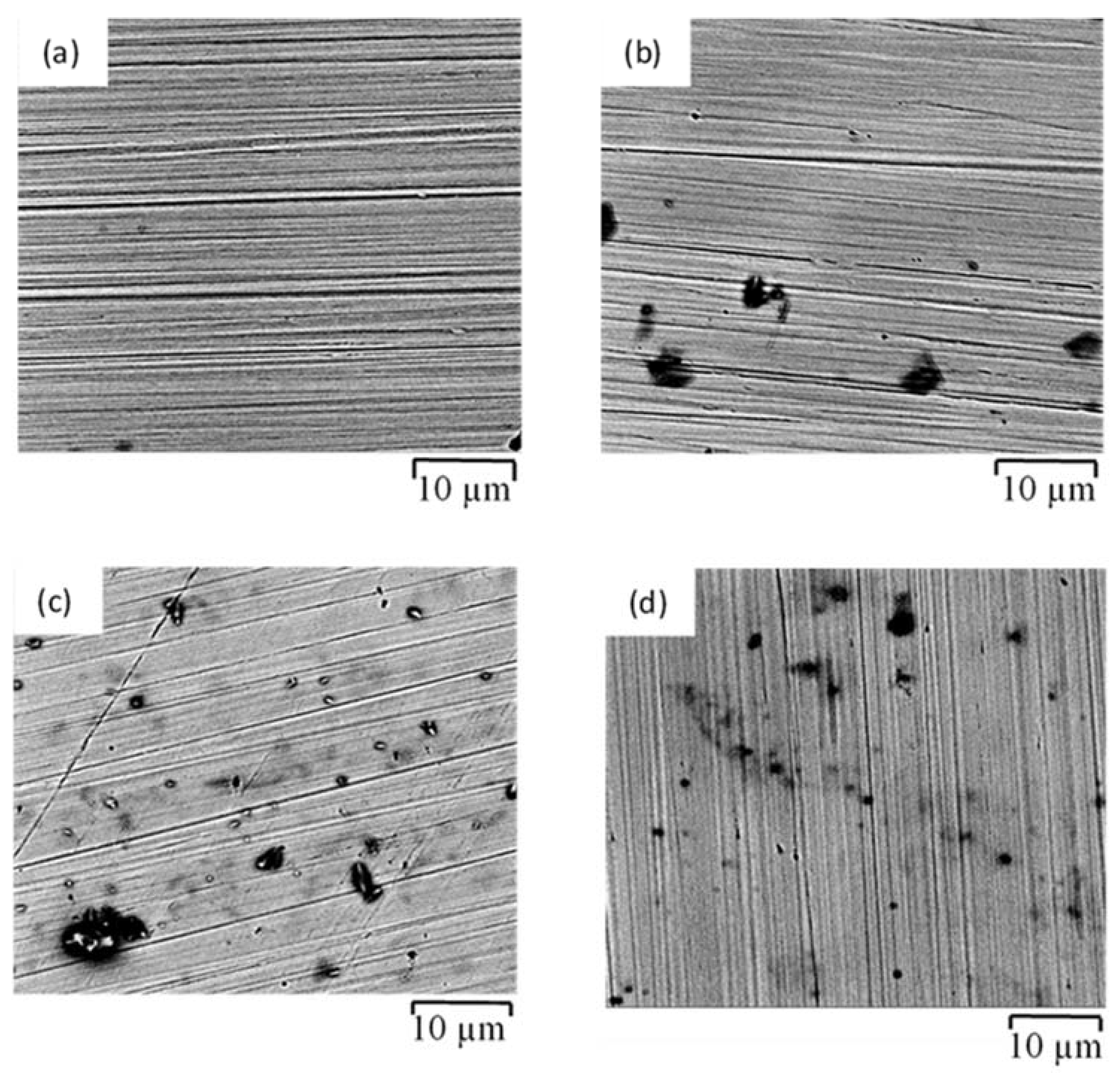
3.4. Corrosion Morphology
4. Conclusions
- SW has an inimical effect on corrosion resistance of mild steel, whereas it has little impact on stainless steels (SS316 and SS304).
- The corrosion resistances of mild steel are similar in the initial stages of immersions in the simulated solutions of SWSSHPC, SWSSNC and SW because chloride content of each solution was able to disrupt the protective layer of iron oxide/hydroxide. However, as time progressed, the corrosion resistance slowly started to improve in the SWSSNC solution that had high alkali content, as also demonstrated through SEM imaging, whereas corrosion resistance was compromised considerably in SWSSHPC and SW.
- The corrosion resistance of SS is compromised in the highly alkaline environment as suggested by electrochemical results. Post-corrosion morphologies support this finding, as the dark spots started to develop on steel surfaces due to localised attack in 168 h of exposure to NC, and in 72 h to SWSSNC and SWSSHPC solutions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Li, Y.L.; Zhao, X.L.; Singh, R.K.R.; Al-Saadi, S. Experimental study on seawater and sea sand concrete filled GFRP and stainless steel tubular stub columns. Thin-Walled Struct. 2016, 106, 390–406. [Google Scholar] [CrossRef]
- Teng, J.G. Performance enhancement of structures through the use of fiber reinforced polymer (FRP) composites. In Proceedings of the 23rd Australasian Conference on the Mechanics of Structures and Materials (ACMSM23), Lismore, NSW, Australia, 9 December 2014. [Google Scholar]
- Teng, J.; Yu, T.; Dai, J.; Chen, G. Proceedings of The International Workshop on Seawater Sea-Sand Concrete (SSC) Structures Reinforced with FRP Composites. In International Workshop on Seawater Sea-Sand Concrete (SSC) Structures Reinforced with FRP Composites 2016; The Hong Kong Polytechnic University: Hong Kong, China, 2016. [Google Scholar]
- Wang, Z.; Zhao, X.-L.; Xian, G.; Wu, G.; Raman, R.S.; Al-Saadi, S.; Haque, A. Long-term durability of basalt-and glass-fibre reinforced polymer (BFRP/GFRP) bars in seawater and sea sand concrete environment. Constr. Build. Mater. 2017, 139, 467–489. [Google Scholar] [CrossRef]
- Li, Y.-L.; Zhao, X.-L.; Raman, R.S.; Al-Saadi, S. Thermal and mechanical properties of alkali-activated slag paste, mortar and concrete utilising seawater and sea sand. Constr. Build. Mater. 2018, 159, 704–724. [Google Scholar] [CrossRef]
- Xiao, J.; Qiang, C.; Nanni, A.; Zhang, K. Use of sea-sand and seawater in concrete construction: Current status and future opportunities. Constr. Build. Mater. 2017, 155, 1101–1111. [Google Scholar] [CrossRef]
- Katano, K.; Takeda, N.; Ishizeki, Y.; Iriya, K. Properties and application of concrete made with sea water and un-washed sea sand. In Proceedings of the Third International Conference on Sustainable Construction Materials and Technologies, Kyoto, Japan, 18–22 August 2013. [Google Scholar]
- Mohammed, T.U.; Hamada, H.; Yamaji, T. Performance of seawater-mixed concrete in the tidal environment. Cem. Concr. Res. 2004, 34, 593–601. [Google Scholar] [CrossRef]
- Nishida, T.; Otsuki, N.; Ohara, H.; Garba-Say, Z.M.; Nagata, T. Some Considerations for Applicability of Seawater as Mixing Water in Concrete. J. Mater. Civ. Eng. 2015, 27, B4014004. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, S.; Islam, S. Suitability of sea water for mixing structural concrete exposed to a marine environment. Cem. Concr. Compos. 1995, 17, 177–185. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Allouche, E.N. Impact of alkali silica reaction on fly ash-based geopolymer concrete. J. Mater. Civ. Eng. 2013, 25, 131–139. [Google Scholar] [CrossRef]
- Japan Concrete Institute Technical Committee. Report on the Use of Seawater in Concrete Japan; Japan Concrete Institute: Tokyo, Japan, 2015. [Google Scholar]
- Swamy, R.N.; AlL-Asali, M.M. Effect of Alkali-Silica Reaction on the Structural Behavior of Reinforced Concrete Beams. ACI Struct. J. 1989, 86, 451–459. [Google Scholar]
- Dong, Z.; Wu, G.; Xu, Y. Experimental study on the bond durability between steel-FRP composite bars (SFCBs) and sea sand concrete in ocean environment. Constr. Build. Mater. 2016, 115, 277–284. [Google Scholar] [CrossRef]
- Guo, F.; Al-Saadi, S.; Raman, R.S.; Zhao, X. Durability of fiber reinforced polymer (FRP) in simulated seawater sea sand concrete (SWSSC) environment. Corros. Sci. 2018, 141, 1–13. [Google Scholar] [CrossRef]
- Verbruggen, H.; Terryn, H.; De Graeve, I. Inhibitor evaluation in different simulated concrete pore solution for the protection of steel rebars. Constr. Build. Mater. 2016, 124, 887–896. [Google Scholar] [CrossRef]
- Li, C.Q.; Zheng, J.J.; Lawanwisut, W.; Melchers, R.E. Concrete Delamination Caused by Steel Reinforcement Corrosion. J. Mater. Civ. Eng. 2007, 19, 591–600. [Google Scholar] [CrossRef]
- Vacek, V.; Kolisko, J.; Pokorný, P.; Kostelecká, M. Steel Reinforcement Corrosion—Its Impact on Features of Steel PSC Strand. Key Eng. Mater. 2020, 868, 57–64. [Google Scholar] [CrossRef]
- Addari, D.; Elsener, B.; Rossi, A. Electrochemistry and surface chemistry of stainless steels in alkaline media simulating concrete pore solutions. Electrochim. Acta 2008, 53, 8078–8086. [Google Scholar] [CrossRef]
- Abreu, C.; Cristóbal, M.; Losada, R.; Nóvoa, X.; Pena, G.; Pérez, M. The effect of Ni in the electrochemical properties of oxide layers grown on stainless steels. Electrochim. Acta 2006, 51, 2991–3000. [Google Scholar] [CrossRef]
- Stellwag, B. The mechanism of oxide film formation on austenitic stainless steels in high temperature water. Corros. Sci. 1998, 40, 337–370. [Google Scholar] [CrossRef]
- Alhozaimy, A.; Hussain, R.R.; Al-Zaid, R.; Negheimish, A.A. Investigation of severe corrosion observed at intersection points of steel rebar mesh in reinforced concrete construction. Constr. Build. Mater. 2012, 37, 67–81. [Google Scholar] [CrossRef]
- Seethammaraju, S.; Rangarajan, M. Corrosion of stainless steels in acidic, neutral and alkaline saline media: Electrochemical and microscopic analysis. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012188. [Google Scholar]
- Montemor, M.; Simões, A.; Ferreira, M.; Belo, M.D.C. The role of Mo in the chemical composition and semiconductive behaviour of oxide films formed on stainless steels. Corros. Sci. 1999, 41, 17–34. [Google Scholar] [CrossRef]
- Wu, X.; Jing, H.; Zheng, Y.; Yao, Z.; Ke, W. Resistance of Mo-bearing stainless steels and Mo-bearing stainless-steel coating to naphthenic acid corrosion and erosion-corrosion. Corros. Sci. 2004, 46, 1013–1032. [Google Scholar] [CrossRef]
- Cramer, S.D.; Covino, B.S.; Bullard, S.J.; Holcomb, G.R.; Russell, J.H.; Nelson, F.J.; Laylor, H.M.; Soltesz, S.M. Corrosion prevention and remediation strategies for reinforced concrete coastal bridges. Cem. Concr. Compos. 2002, 24, 101–117. [Google Scholar] [CrossRef]
- Jones, D.A. The technology and evaluation of corrosion. In Principles and Prevention of Corrosion, 2nd ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 1996. [Google Scholar]
- Xie, Y.; Zhang, J.; Aldemir, T.; Denning, R. Multi-state Markov modeling of pitting corrosion in stainless steel exposed to chloride-containing environment. Reliab. Eng. Syst. Saf. 2018, 172, 239–248. [Google Scholar] [CrossRef]
- Fontana, M.G. Corrosion Engineering; Tata McGraw-Hill Education: New York, NY, USA, 2005. [Google Scholar]
- Elfström, B.-O. The effect of chloride ions on passive layers on stainless steels. Mater. Sci. Eng. 1980, 42, 173–180. [Google Scholar] [CrossRef]
- Leek, D.; Poole, A. The breakdown of the passive film on high yield mild steel by chloride ions. Presented at the Third International Symposium on “Corrosion of Reinforcement in Concrete Construction”, Wishaw, UK, 21–24 May 1990. [Google Scholar]
- Albani, O.A.; Gassa, L.M.; Zerbino, J.O.; Vilche, J.R.; Arvia, A.J. Comparative study of the passivity and the breakdown of passivity of polycrystalline iron in different alkaline solutions. Electrochim. Acta 1990, 35, 1437–1444. [Google Scholar] [CrossRef]
- Ghods, P.; Isgor, O.; Brown, J.; Bensebaa, F.; Kingston, D. XPS depth profiling study on the passive oxide film of carbon steel in saturated calcium hydroxide solution and the effect of chloride on the film properties. Appl. Surf. Sci. 2011, 257, 4669–4677. [Google Scholar] [CrossRef]
- Chen, Y.; Davalos, J.F.; Ray, I.; Kim, H.-Y. Accelerated aging tests for evaluations of durability performance of FRP reinforcing bars for concrete structures. Compos. Struct. 2007, 78, 101–111. [Google Scholar] [CrossRef]
- Guo, F.; Khoo, W.; Al-Saadi, S.H.M.; Li, Y.; Singh, R.R.K.; Zhao, X.L. Preliminary Study on Durability of FRP and Stainless Steel in Seawater and Sea Sand Concrete (SWSSC) Environment. In Proceedings of the ASCCS International Conference on Steel-Concrete Composite and Hybrid Structures 2015, Beijing, China, 3–5 December 2015; Tsinghua University Press: Beijing, China, 2015; pp. 1–8. [Google Scholar]
- Wang, Z.; Zhao, X.; Xian, G.; Wu, G.; Raman, R.S.; Al-Saadi, S. Tensile properties of basalt-fibre reinforced polymer (BFRP) bars within seawater and sea sand concrete environment. In Proceedings of the 7th International Conference on Fibre-Reinforced Polymer (FRP) Composites in Civil Engineering (CICE 2016), Hong Kong, China, 14–16 December 2016. [Google Scholar]
- During, E.D. Corrosion Atlas: A Collection of Illustrated Case Histories; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Luo, H.; Su, H.; Dong, C.; Li, X. Passivation and electrochemical behavior of 316L stainless steel in chlorinated simulated concrete pore solution. Appl. Surf. Sci. 2017, 400, 38–48. [Google Scholar] [CrossRef]
- Mundra, S.; Criado, M.; Bernal, S.A.; Provis, J.L. Chloride-induced corrosion of steel rebars in simulated pore solutions of alkali-activated concretes. Cem. Concr. Res. 2017, 100, 385–397. [Google Scholar] [CrossRef]
- Ghods, P.; Burkan Isgor, O.; Bensebaa, F.; Kingston, D. Angle-resolved XPS study of carbon steel passivity and chloride-induced depassivation in simulated concrete pore solution. Corros. Sci. 2012, 58, 159–167. [Google Scholar] [CrossRef]
- Li, L.; Sagüés, A.A. Chloride Corrosion Threshold of Reinforcing Steel in Alkaline Solutions Open-Circuit Immersion Tests. Corrosion 2001, 57, 19–28. [Google Scholar] [CrossRef]
- Ha, H.-Y.; Jang, M.-H.; Lee, T.-H. Influences of Mn in solid solution on the pitting corrosion behaviour of Fe-23 wt.% Cr-based alloys. Electrochim. Acta 2016, 191, 864–875. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.; Li, X.; Lu, T.J. Corrosion behavior of low-Cr steel rebars in alkaline solutions with different pH in the presence of chlorides. J. Electroanal. Chem. 2017, 803, 40–50. [Google Scholar] [CrossRef]



| Steel Type | Chemical Composition (wt.%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | Mn | Si | S | P | Cr | Ni | Cu | Mo | Co | N | Al | |
| SS316 | 0.027 | 1.800 | 0.360 | 0.026 | 0.038 | 16.800 | 10.060 | 0.450 | 2.030 | 0.190 | 0.075 | |
| SS304 | 0.021 | 1.580 | 0.290 | 0.021 | 0.038 | 18.300 | 8.0300 | 0.520 | 0.360 | 0.170 | 0.078 | |
| Mild steel | 0.140 | 1.220 | 0.200 | 0.025 | 0.022 | 0.030 | 0.010 | 0.008 | 0.002 | - | - | 0.005 |
| Simulated Solution | Quantities (g/L) | pH | |||
|---|---|---|---|---|---|
| NaOH | KOH | Ca(OH)2 | NaCl | ||
| NC a | 2.4 | 19.6 | 2 | 13.4 | |
| SWSSNC b | 2.4 | 19.6 | 2 | 35 | 13.4 |
| HPC a | 0.6 | 1.4 | 0.037 | 12.7 | |
| SWSSHPC b | 0.6 | 1.4 | 0.037 | 35 | 12.7 |
| SW | 35 | 7.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Al-Saadi, S.; Zhao, X.-L.; Raman, R.K.S. Electrochemical Investigations of Steels in Seawater Sea Sand Concrete Environments. Materials 2021, 14, 5713. https://doi.org/10.3390/ma14195713
Yu X, Al-Saadi S, Zhao X-L, Raman RKS. Electrochemical Investigations of Steels in Seawater Sea Sand Concrete Environments. Materials. 2021; 14(19):5713. https://doi.org/10.3390/ma14195713
Chicago/Turabian StyleYu, Xiang, Saad Al-Saadi, Xiao-Ling Zhao, and R. K. Singh Raman. 2021. "Electrochemical Investigations of Steels in Seawater Sea Sand Concrete Environments" Materials 14, no. 19: 5713. https://doi.org/10.3390/ma14195713
APA StyleYu, X., Al-Saadi, S., Zhao, X.-L., & Raman, R. K. S. (2021). Electrochemical Investigations of Steels in Seawater Sea Sand Concrete Environments. Materials, 14(19), 5713. https://doi.org/10.3390/ma14195713








