Assessment of Disinfection Potential of Q-Switch Nd: YAG Laser on Contaminated Titanium Implant Surfaces
Abstract
:1. Introduction
2. Materials and Methods
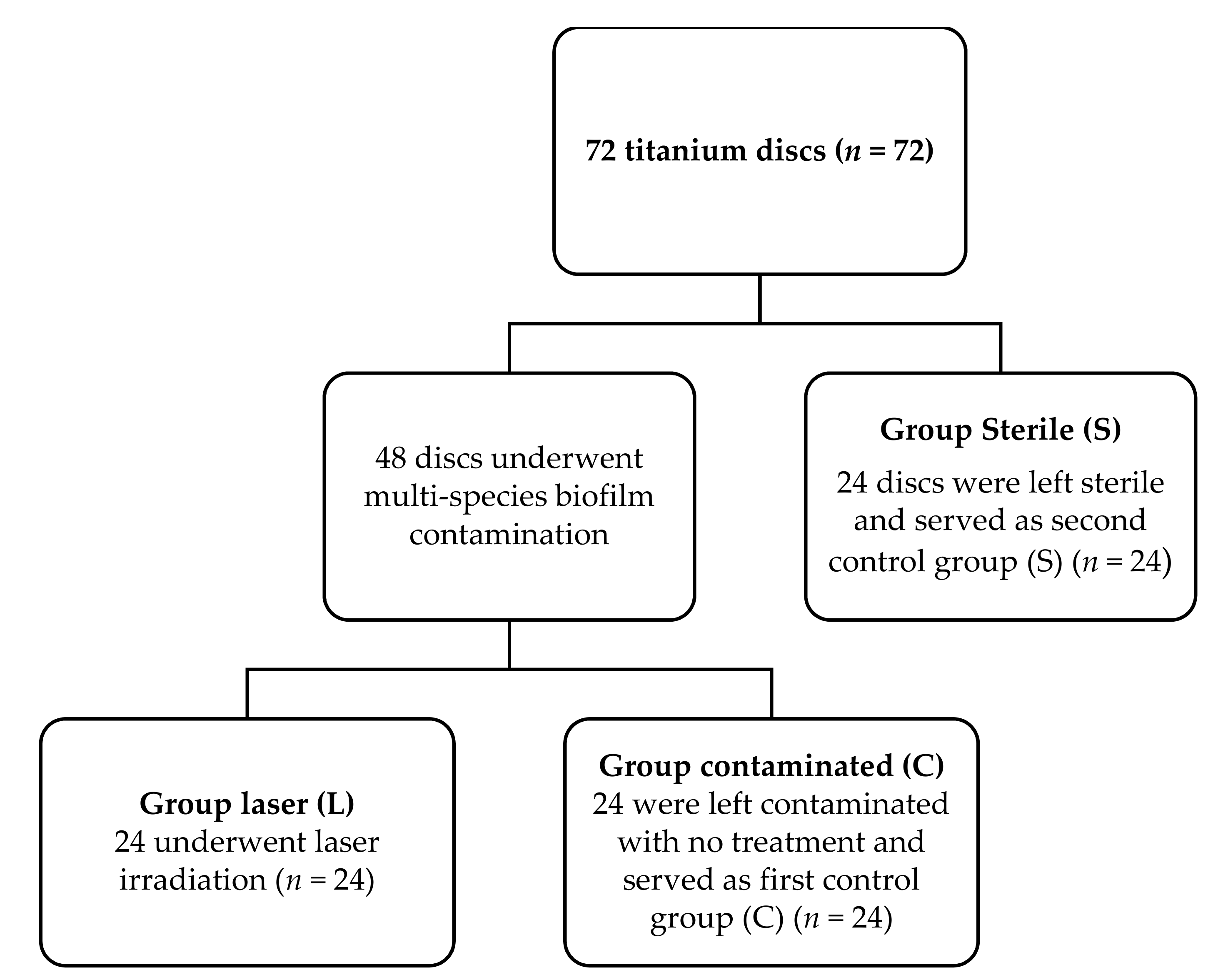
2.1. Study Design
2.1.1. Group L
2.1.2. Group C
2.1.3. Group S
2.2. Contamination of the Titanium Discs with Multi-Species Biofilm
2.2.1. Bacterial Straining, Media and Growing Settings
- All included multi-species were grown on an agar blood (Oxoid, Ltd., Basingstoke, UK) added by means of 1 µg/mL menadione, 5 µg/mL hemin (Sigma-Aldrich Co, St.-Louis, MO, USA), and 5% of horse blood that is sterile (E&O Laboratories Ltd., Bonnybridge, UK).
- Actinomycetemcomitans, S. cristatus, S. mitis, S. gordonii, S. oralis, S. mutans, S. parasanguinis, S. salivarius, S. sobrinus, and S. sanguinis were grown at 37 °C in a 5% carbon dioxide (CO2) environment. As for the Actinomyces naeslundii, Actinomyces viscosus, F. nucleatum, P. gingivalis, P. intermedia, and V. parvula, they were grown at 37 °C in anaerobic conditions (80% diazote, ten percent dihydrogen, and 10% CO2).
- Blood agar plates were used to prepare and collect the single species planktonic cultures. Afterwards, the prepared species were injected in 10 mm brain–heart infusion broth (BHI) (Difco Laboratories, Detroit, MI, USA) and then incubated under identical conditions as the blood agar plates, depending on bacterial species. Spectrophotometry (OD600, Gene Quant Spectrophotomoeter, Biochrom Ltd., Cambridge, UK) was used to assess the optical densities at 600 nm.
- Multi-species biofilms were grown in modified BHI broth, consisting of 37 g/L BHI added with 2.5 g/L mucin from porcine stomach type-III (Sigma-Aldrich Co, St.-Louis, MO, USA), 1.0 g/L yeast extract (Oxoid, Basingstoke, UK), 0.13 gram per liter cysteine HCl (Merck-Calbiochem, San Diego, CA, USA), 2.0 g/L sodium bicarbonate (Merck, Darmstadt, Germany), and 3.65 g/L 0.25% glutamic acid (Merck-Calbiochem, San Diego, CA, USA). Multi-species biofilms were grown at 37 °C with microaerophilic circumstances (6% O2, 7% CO2, 7% H2, and 80% N2).
2.2.2. Bioreactor-Derived Multi-Species Biofilms and Community
2.3. Laser Protocol and Irradiation Parameters
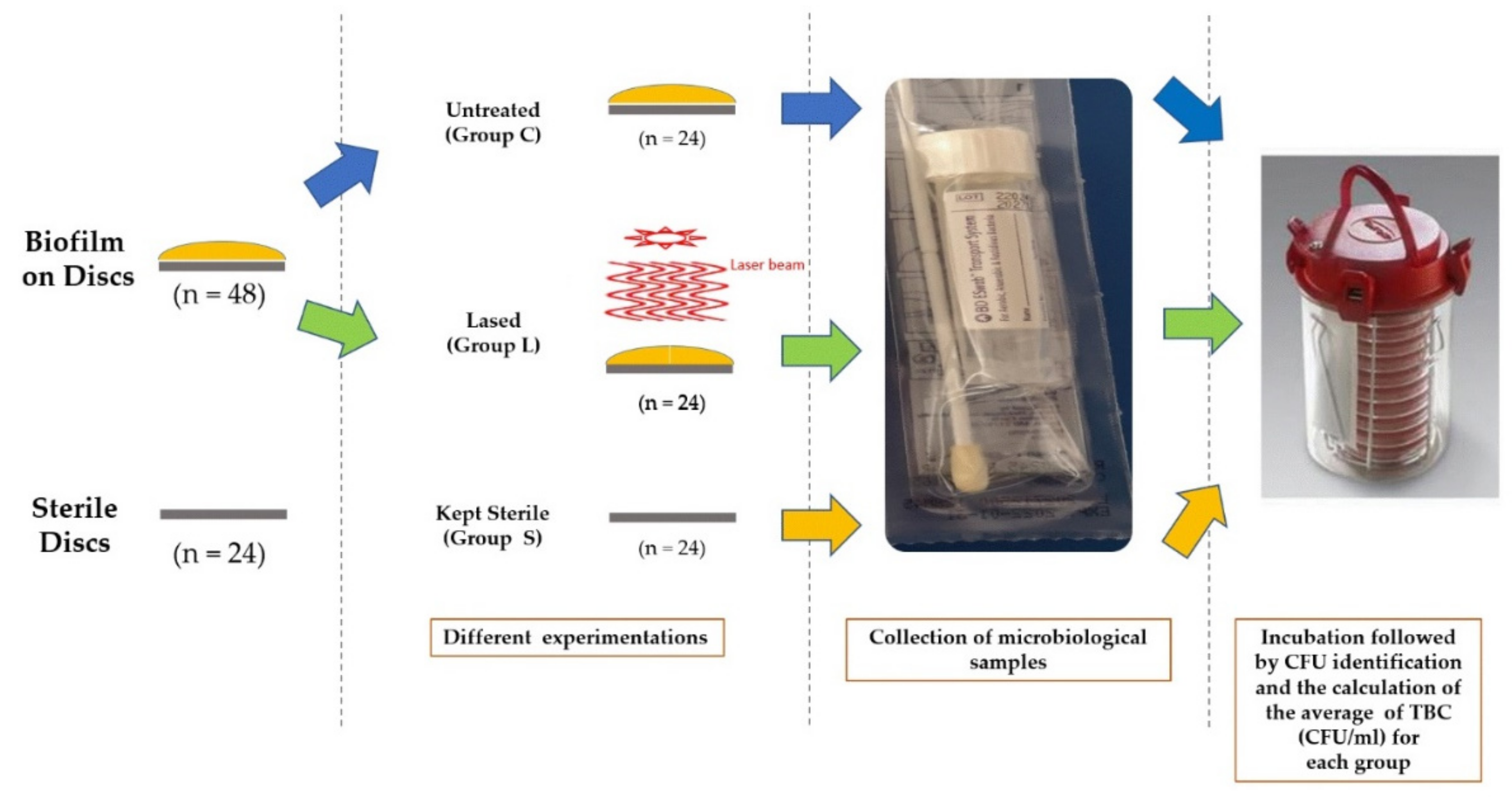
2.4. Microbiological Assessment
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Berglundh, T.; Jepsen, S.; Stadlinger, B.; Terheyden, H. Peri-implantitis and its prevention. Clin. Oral Implants Res. 2019, 30, 150–155. [Google Scholar] [CrossRef]
- Araujo, M.G.; Lindhe, J. Peri-implant health. J. Clin. Periodontol. 2018, 45, S230–S236. [Google Scholar] [CrossRef] [Green Version]
- Berglundh, T.; Wennström, J.L.; Lindhe, J. Long-term outcome of surgical treatment of peri-implantitis. A 2–11-year retrospective study. Clin. Oral Implants Res. 2018, 29, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Salvi, G.E.; Lang, N.P. Diagnostic parameters for monitoring peri-implant conditions. Int. J. Oral Maxillofac. Implants 2004, 116–127. [Google Scholar]
- Lang, M.S.; Miyamoto, T.; Nunn, M.E. Validity of fractal analysis of implants in individuals with healthy and diseased peri-implant mucosa. Clin. Oral Implants Res. 2020, 31, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Kotsovilis, S.; Karoussis, I.K.; Trianti, M.; Fourmousis, I. Therapy of peri-implantitis: A systematic review. J. Clin. Periodontol. 2008, 35, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Ortiz, G.A.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Clin. Periodontol. 2018, 45, S286–S291. [Google Scholar] [CrossRef] [Green Version]
- Smeets, R.; Henningsen, A.; Jung, O.; Heiland, M.; Hammächer, C.; Stein, J.M. Definition, etiology, prevention and treatment of peri-implantitis—A review. Head Face Med. 2014, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- French, D.; Grandin, H.M.; Ofec, R. Retrospective cohort study of 4591 dental implants: Analysis of risk indicators for bone loss and prevalence of peri-implant mucositis and peri-implantitis. J. Periodontol. 2019, 90, 691–700. [Google Scholar] [CrossRef] [Green Version]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincón, M.V.; Gómez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and Microbial Biofilm Profiles of Peri-Implantitis: A Systematic Review. J. Periodontol. 2017, 88, 1066–1089. [Google Scholar] [CrossRef]
- Fu, J.; Wang, H. Breaking the wave of peri-implantitis. Periodontology 2000 2020, 84, 145–160. [Google Scholar] [CrossRef]
- Passi, D.; Singh, M.; Dutta, S.R.; Sharma, S.; Atri, M.; Ahlawat, J.; Jain, A. Newer proposed classification of periimplant defects: A critical update. J. Oral Biol. Craniofacial Res. 2017, 7, 58–61. [Google Scholar] [CrossRef] [Green Version]
- Salvi, G.E.; Persson, G.R.; Heitz-Mayfield, L.J.A.; Frei, M.; Lang, N.P. Adjunctive local antibiotic therapy in the treatment of peri-implantitis II: Clinical and radiographic outcomes. Clin. Oral Implants Res. 2007, 18, 281–285. [Google Scholar] [CrossRef]
- Liu, S.; Limiñana-Cañal, J.; Yu, J. Does chlorhexidine improve outcomes in non-surgical management of peri-implant mucositis or peri-implantitis? A systematic review and meta-analysis. Med. Oral Patol. Oral Cirugía Bucal 2020, 25, e608–e615. [Google Scholar] [CrossRef]
- Verdugo, F.; Laksmana, T.; Uribarri, A. Systemic antibiotics and the risk of superinfection in peri-implantitis. Arch. Oral Biol. 2015, 64, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Galofré, M.; Palao, D.; Vicario, M.; Nart, J.; Violant, D. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. J. Periodontal Res. 2018, 53, 378–390. [Google Scholar] [CrossRef]
- Tada, H.; Masaki, C.; Tsuka, S.; Mukaibo, T.; Kondo, Y.; Hosokawa, R. The effects of Lactobacillus reuteri probiotics combined with azithromycin on peri-implantitis: A randomized placebo-controlled study. J. Prosthodont. Res. 2018, 62, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, E.C.; Zhou, Y.; Chen, C.C.; Tamerler, C.; Snead, M.L. Mitigation of Peri-implantitis by Rational Design of Bifunctional Peptides with Antimicrobial Properties. ACS Biomater. Sci. Eng. 2019, 6, 2682–2695. [Google Scholar] [CrossRef]
- Mattar, H.; Bahgat, M.; Ezzat, A.; El-Din, B.B.; Keraa, K.; El Taftazany, I. Management of peri-implantitis using a diode laser (810 nm) vs conventional treatment: A systematic review. Lasers Med. Sci. 2020, 36, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ashnagar, S.; Gianfilippo, R.D.; Arnett, M.; Kinney, J.; Wang, H. Laser-assisted Regenerative Surgical Therapy for Peri-implantitis: A Randomized Controlled Clinical Trial. J. Periodontol 2021, 92, 378–388. [Google Scholar] [CrossRef]
- Namour, M.; El Mobadder, M.; Magnin, D.; Peremans, A.; Verspecht, T.; Teughels, W.; Lamard, L.; Nammour, S.; Rompen, E.; Mobadder, E. Q-Switch Nd: YAG Laser-Assisted Decontamination of Implant Surface. Dent. J. 2019, 7, 99. [Google Scholar] [CrossRef] [Green Version]
- Namour, M.; Verspecht, T.; El Mobadder, M.; Teughels, W.; Peremans, A.; Nammour, S.; Rompen, E. Q-Switch Nd:YAG Laser-Assisted Elimination of Multi-Species Biofilm on Titanium Surfaces. Materials 2020, 13, 1573. [Google Scholar] [CrossRef] [Green Version]
- Slomka, V.; Herrero, E.R.; Boon, N.; Bernaerts, K.; Trivedi, H.M.; Daep, C.; Quirynen, M.; Teughels, W. Oral prebiotics and the influence of environmental conditions in vitro. J. Periodontol. 2018, 89, 708–717. [Google Scholar] [CrossRef]
- Marquez, F.; Quintana, E.; Roca, I.; Salgado, J. Physical-mechanical effects of Nd: YAG laser on the surface of sound dental enamel. Biomaterials 1993, 14, 313–316. [Google Scholar] [CrossRef]
- Parker, S.P. Laser–tissue interaction. In Lasers in Dentistry—Current Concepts; Springer: Berlin/Heidelberg, Germany, 2017; pp. 29–55. [Google Scholar]
- Lin, G.-H.; Del Amo, F.S.L.; Wang, H.-L. Laser therapy for treatment of peri-implant mucositis and peri-implantitis: An American Academy of Periodontology best evidence review. J. Periodontol. 2018, 89, 766–782. [Google Scholar] [PubMed]
- Kotsakis, G.; Konstantinidis, I.; Karoussis, I.K.; Ma, X.; Chu, H. Systematic Review and Meta-Analysis of the Effect of Various Laser Wavelengths in the Treatment of Peri-Implantitis. J. Periodontol. 2014, 85, 1203–1213. [Google Scholar] [CrossRef] [PubMed]
- Misischia, W.P.; Xenoudi, P.; Yukna, R.A.; Schurr, M.J. Bacterial reduction effect of four different dental lasers on titanium surfaces in vitro. Lasers Med. Sci. 2021, 36, 1759–1767. [Google Scholar] [CrossRef]
- Geminiani, A.; Caton, J.G.; Romanos, G.E. Temperature change during non-contact diode laser irradiation of implant surfaces. Lasers Med. Sci. 2011, 27, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Monzavi, A.; Shahabi, S.; Fekrazad, R.; Behruzi, R.; Chiniforush, N. Implant Surface Temperature Changes during Er:YAG Laser Irradiation with Different Cooling Systems. J. Dent. 2014, 11, 210. [Google Scholar]
- Kushima, S.S.; Nagasawa, M.; Shibli, J.A.; Brugnera, A., Jr.; Rodrigues, J.A.; Cassoni, A. Evaluation of Temperature and Roughness Alteration of Diode Laser Irradiation of Zirconia and Titanium for Peri-Implantitis Treatment. Photomed. Laser Surg. 2016, 34, 194–199. [Google Scholar] [CrossRef]
- Wakim, R.N.; Namour, M.; Nguyen, H.V.; Peremans, A.; Zeinoun, T.; Vanheusden, A.; Rompen, E.; Namour, S. Decontamination of Dental Implant Surfaces by the Er:YAG Laser Beam: A Comparative in Vitro Study of Various Protocols. Dent. J. 2018, 6, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshmi, M.R.; Penmetsa, G.S.; Ramesh, K.S.V.; Bypalli, V. Photobiomodulation in Management of Periodontitis and Periimplantitis-A Review. Eur. J. Mol. Clin. Med. 2021, 9, 3109–3118. [Google Scholar]
- Chala, M.; Anagnostaki, E.; Mylona, V.; Chalas, A.; Parker, S.; Lynch, E. Adjunctive use of lasers in peri-implant mucositis and peri-implantitis treatment: A systematic review. Dent. J. 2020, 8, 68. [Google Scholar] [CrossRef]
- Al-Askar, M.H.; Abdullatif, F.A.; Alshihri, A.A.; Ahmed, A.; Divakar, D.D.; Almoharib, H.; Alzoman, H. Comparison of photobiomodulation and photodynamic therapy as adjuncts to mechanical debridement for the treatment of peri-implantitis. Technol. Health Care 2021, 1–10, preprint. [Google Scholar] [CrossRef]
- Arbildo-Vega, H.I.; Panda, S.; Bal, A.; Mohanty, R.; Rendón-Alvarado, A.; Das, A.C.; Cruzado-Oliva, F.H.; Infantes-Ruíz, E.D.; Manfredi, B.; Vásquez-Rodrigo, H.; et al. Clinical effectiveness of Lactobacillus reuteri in the treatment of peri-implant diseases: A systematic review and meta-analysis. J. Biol. Regul. Homeost. Agents 2021, 35, 79–88. [Google Scholar] [CrossRef]
- Gao, J.; Yu, S.; Zhu, X.; Yan, Y.; Zhang, Y.; Pei, D. Does Probiotic Lactobacillus Have an Adjunctive Effect in the Nonsurgical Treatment of Peri-Implant Diseases? A Systematic Review and Meta-analysis. J. Évid. Based Dent. Pr. 2020, 20, 101398. [Google Scholar] [CrossRef] [PubMed]
- Machtei, E.E.; Romanos, G.; Kang, P.; Travan, S.; Schmidt, S.; Papathanasiou, E.; Tatarakis, N.; Tandlich, M.; Liberman, L.H.; Horwitz, J.; et al. Repeated delivery of chlorhexidine chips for the treatment of peri-implantitis: A multicenter, randomized, comparative clinical trial. J. Periodontol. 2021, 92, 11–20. [Google Scholar] [CrossRef] [PubMed]


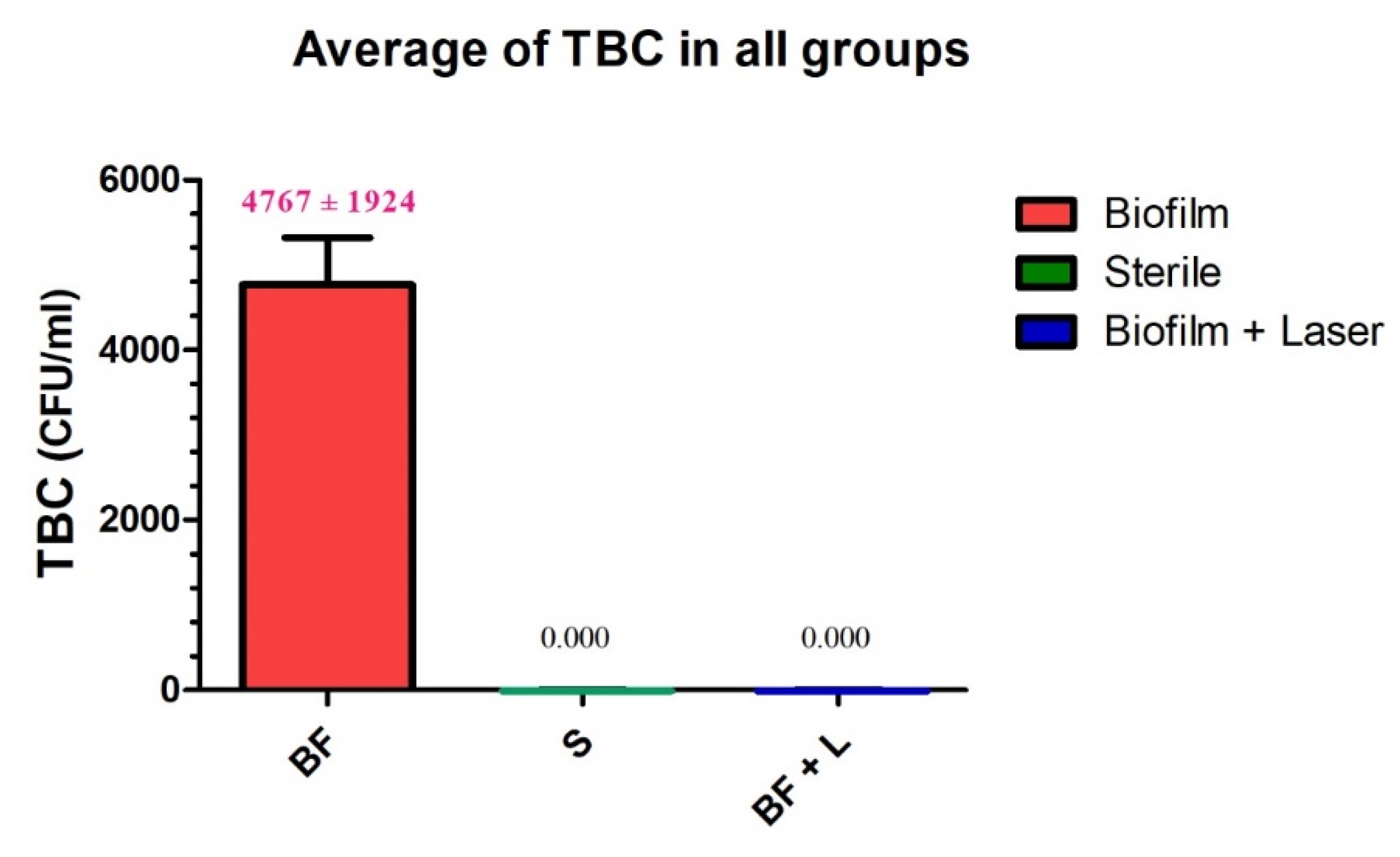
| Group L | Group C | Group S | |
|---|---|---|---|
| sample size | 24 | 24 | 24 |
| mean value | 0.000 A | 4767 B | 0.000 A |
| std deviation | 0.000 | 1924 | 0.000 |
| std. error | 0.000 | 555.3 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Namour, M.; El Mobadder, M.; Mulongo, B.; Fagnart, O.; Harb, A.; Peremans, A.; Verspecht, T.; Teughels, W.; Nammour, S.; Rompen, E. Assessment of Disinfection Potential of Q-Switch Nd: YAG Laser on Contaminated Titanium Implant Surfaces. Materials 2021, 14, 6078. https://doi.org/10.3390/ma14206078
Namour M, El Mobadder M, Mulongo B, Fagnart O, Harb A, Peremans A, Verspecht T, Teughels W, Nammour S, Rompen E. Assessment of Disinfection Potential of Q-Switch Nd: YAG Laser on Contaminated Titanium Implant Surfaces. Materials. 2021; 14(20):6078. https://doi.org/10.3390/ma14206078
Chicago/Turabian StyleNamour, Melanie, Marwan El Mobadder, Baudouin Mulongo, Olivier Fagnart, Assaf Harb, André Peremans, Tim Verspecht, Wim Teughels, Samir Nammour, and Eric Rompen. 2021. "Assessment of Disinfection Potential of Q-Switch Nd: YAG Laser on Contaminated Titanium Implant Surfaces" Materials 14, no. 20: 6078. https://doi.org/10.3390/ma14206078
APA StyleNamour, M., El Mobadder, M., Mulongo, B., Fagnart, O., Harb, A., Peremans, A., Verspecht, T., Teughels, W., Nammour, S., & Rompen, E. (2021). Assessment of Disinfection Potential of Q-Switch Nd: YAG Laser on Contaminated Titanium Implant Surfaces. Materials, 14(20), 6078. https://doi.org/10.3390/ma14206078








