Investigation of MXenes Oxidation Process during SPS Method Annealing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Annealing Process
2.3. Powders Characterization
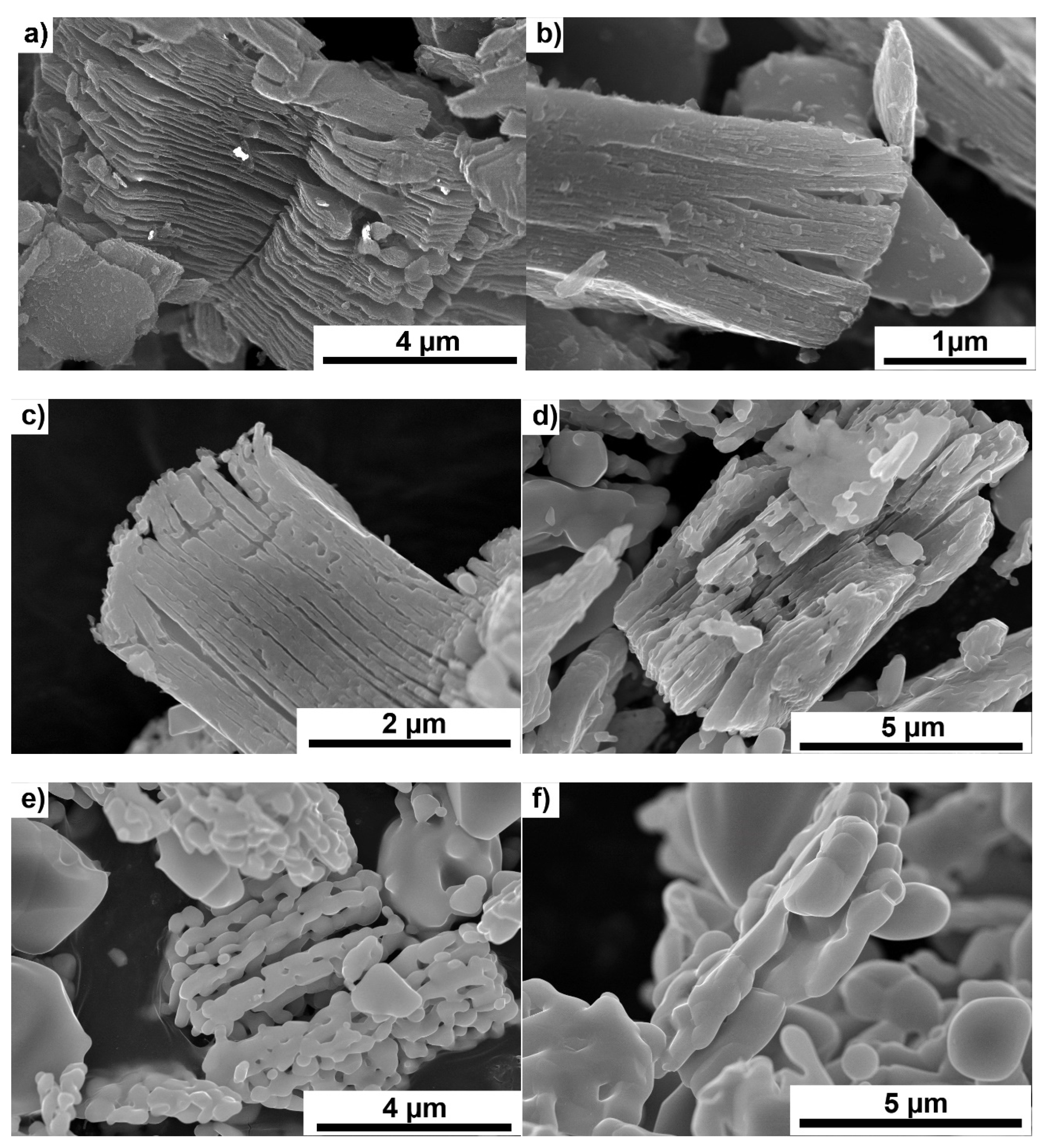
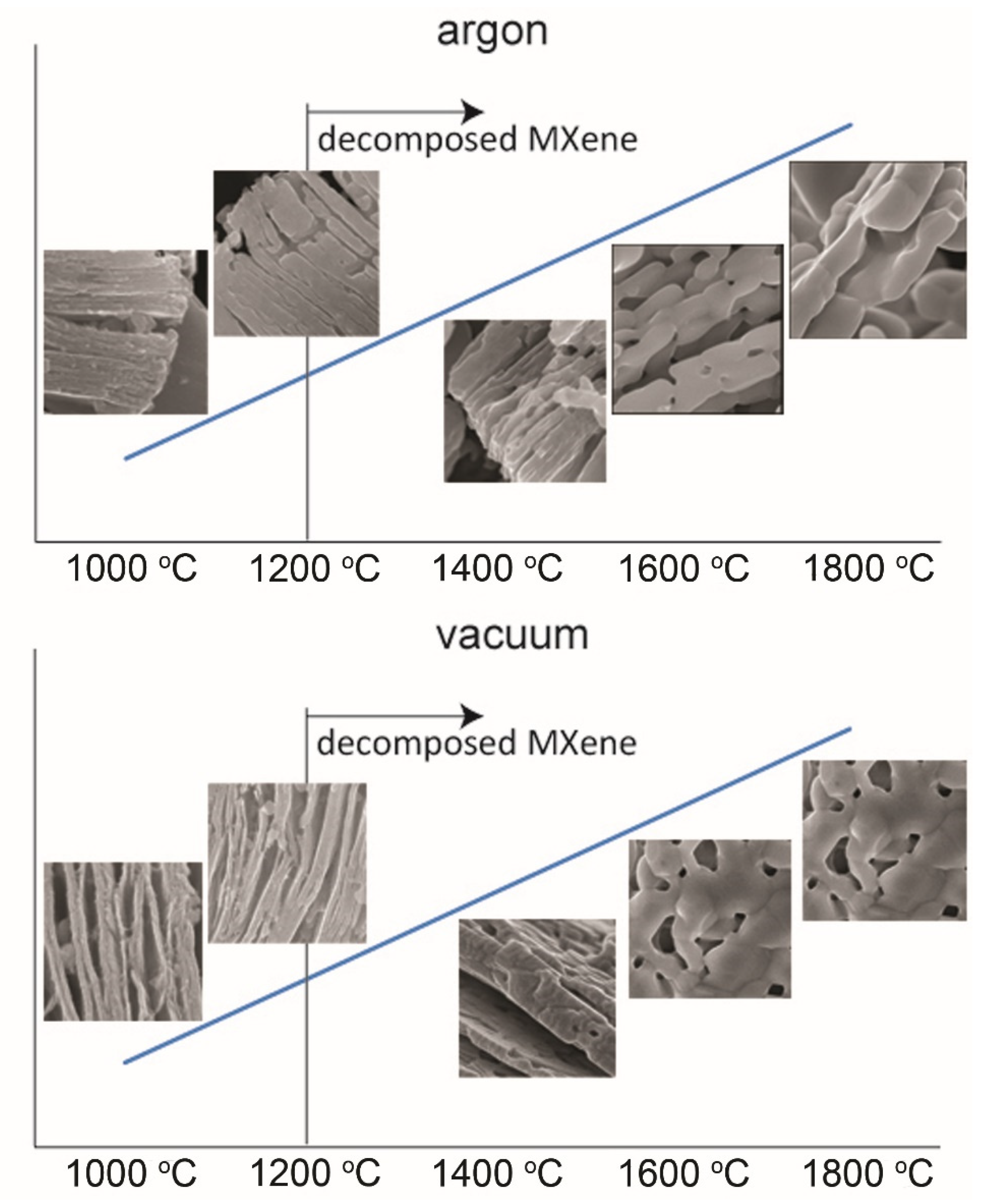
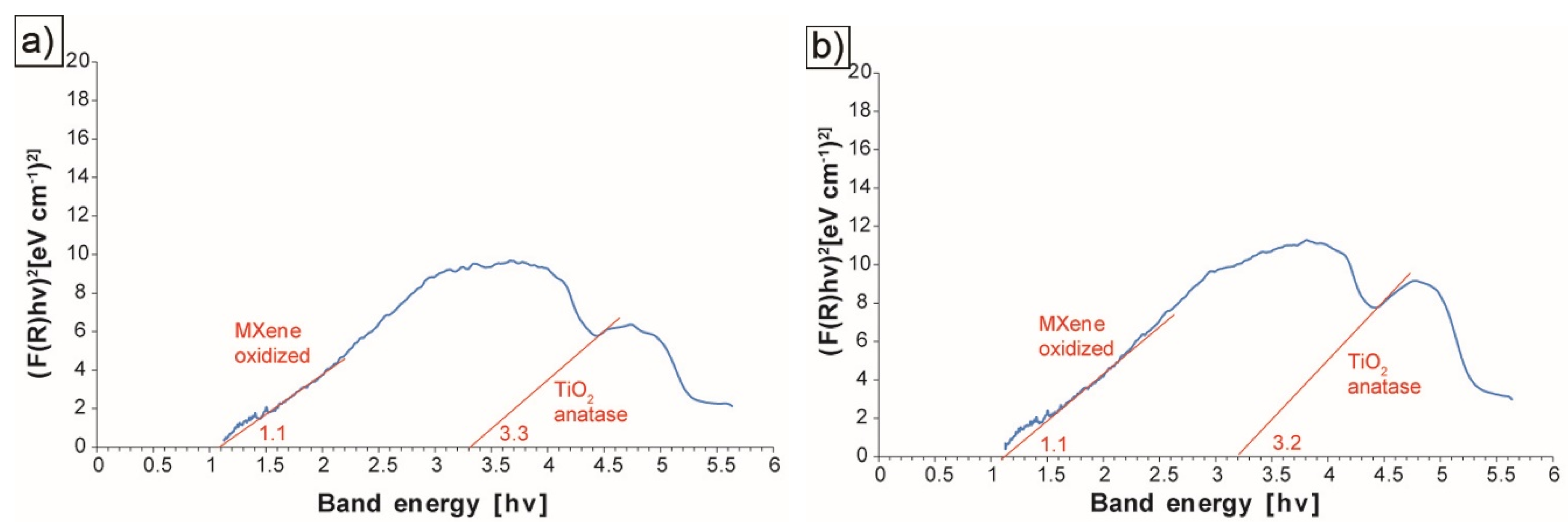
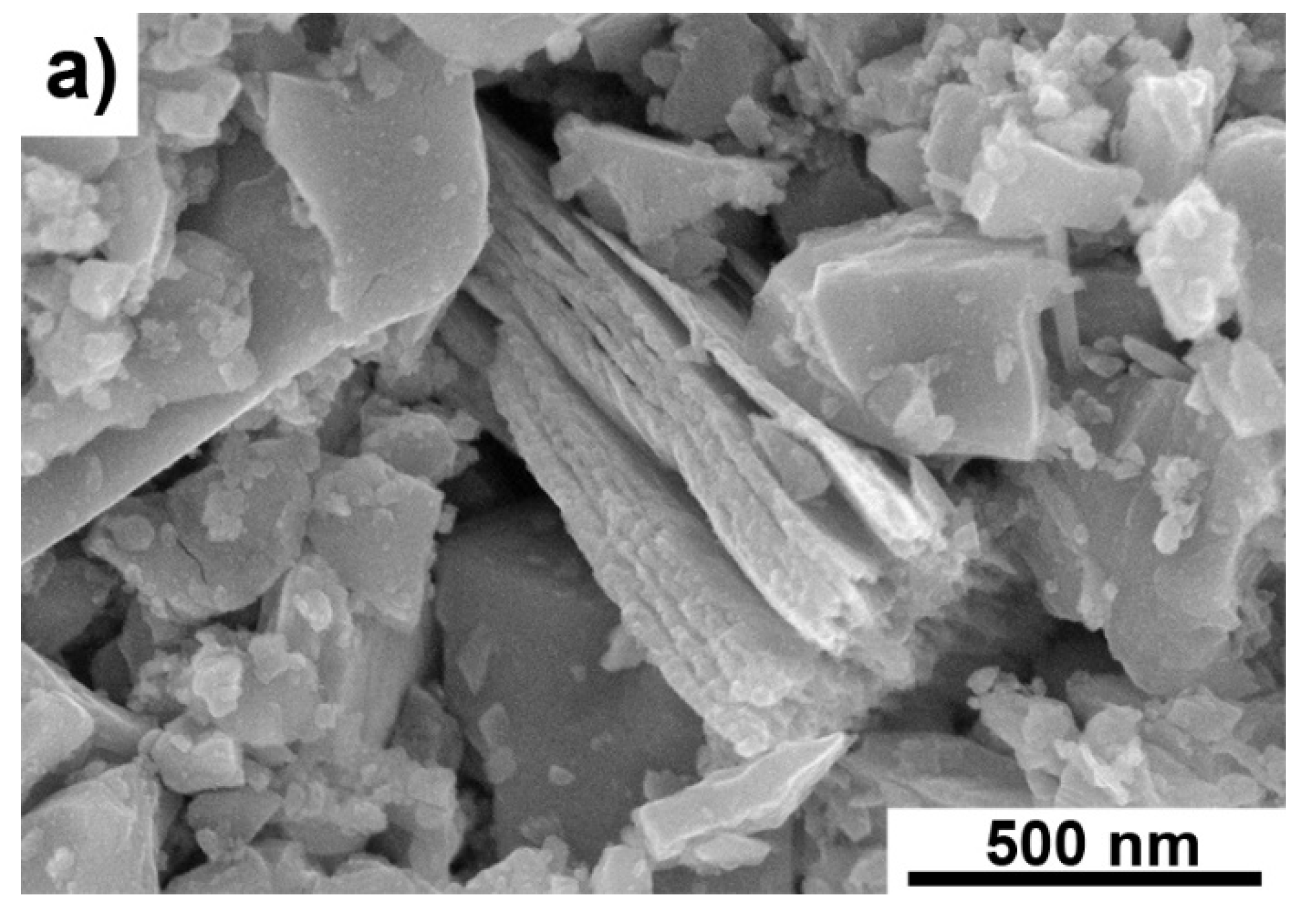
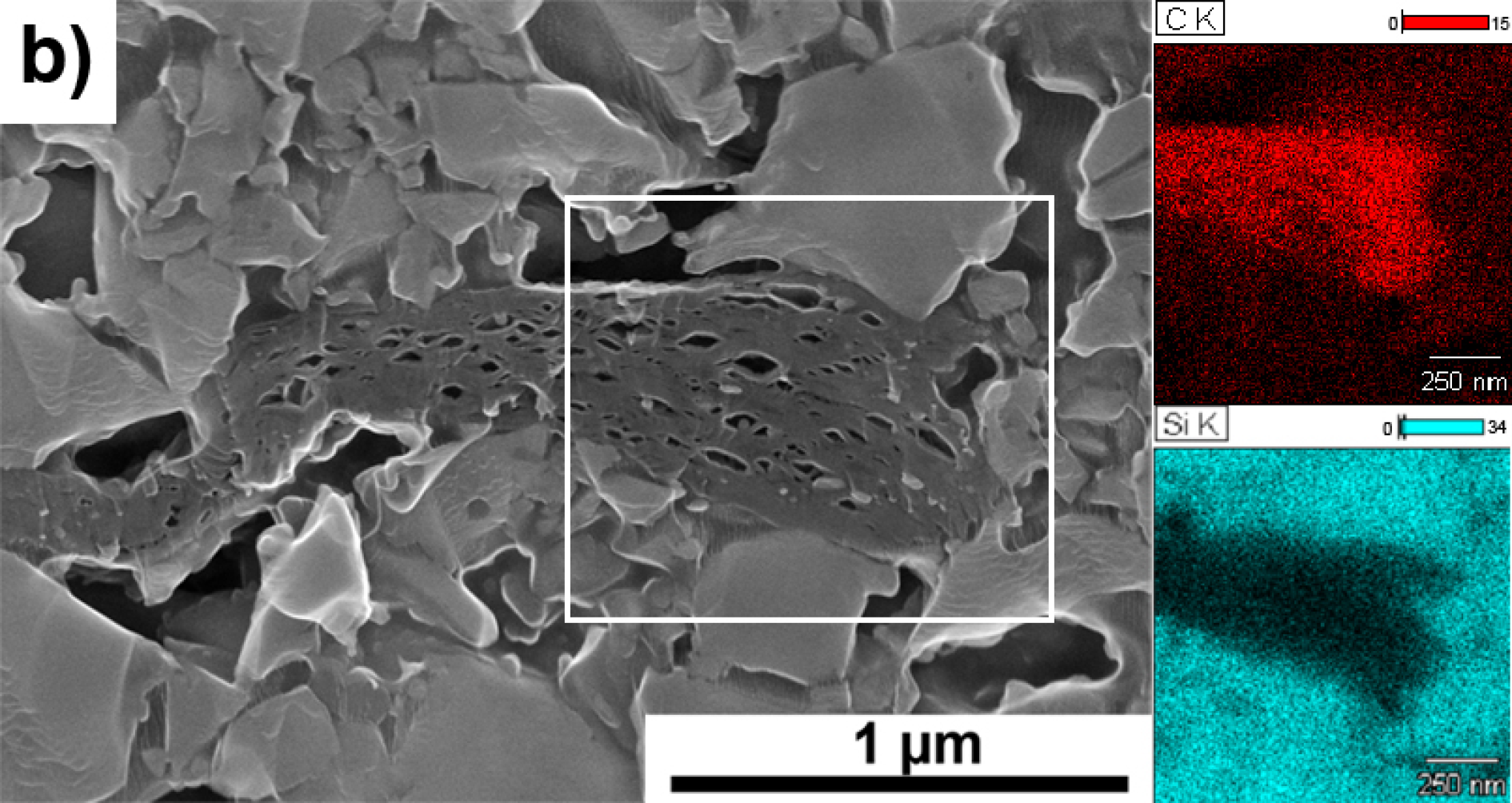
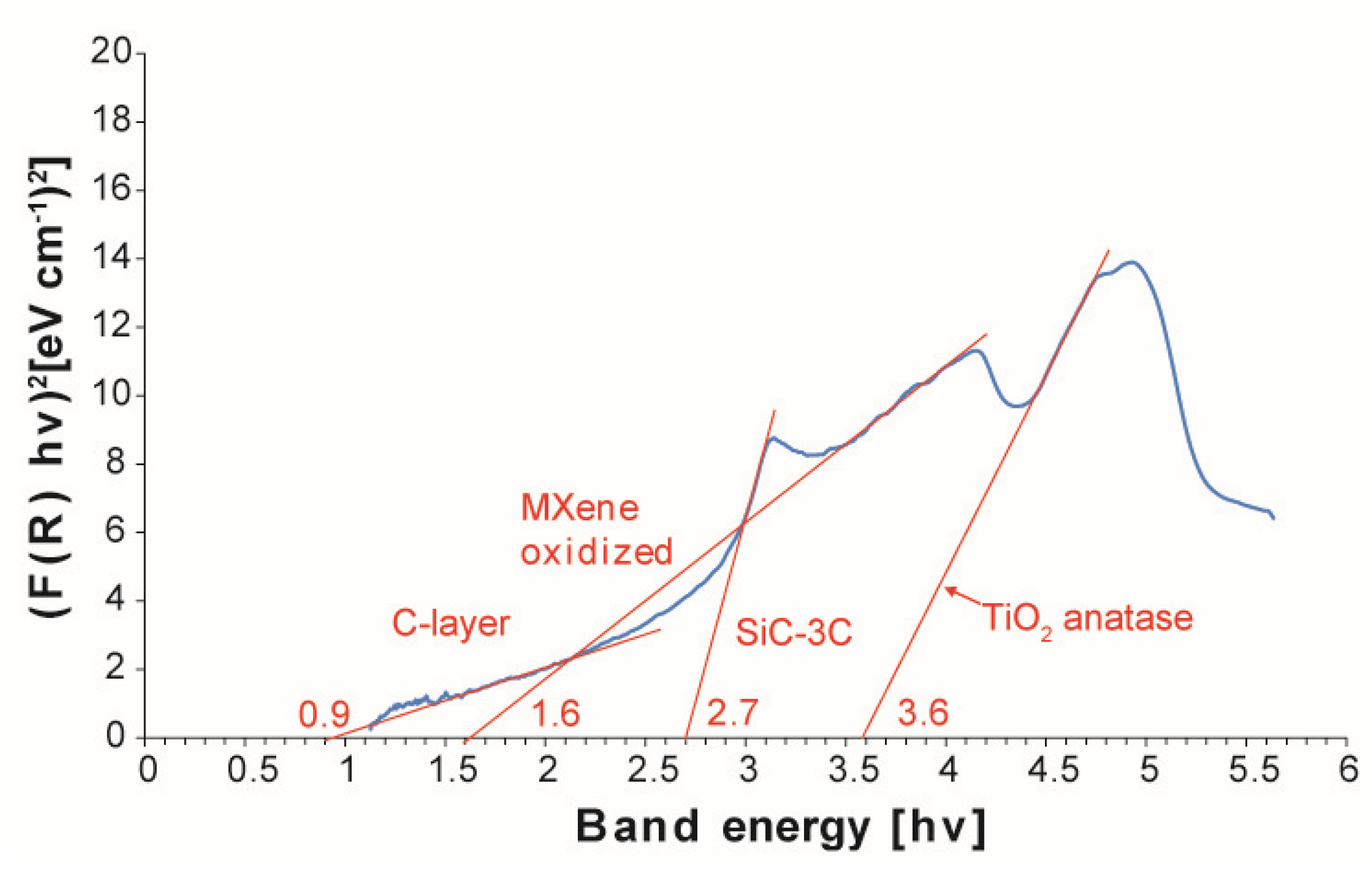
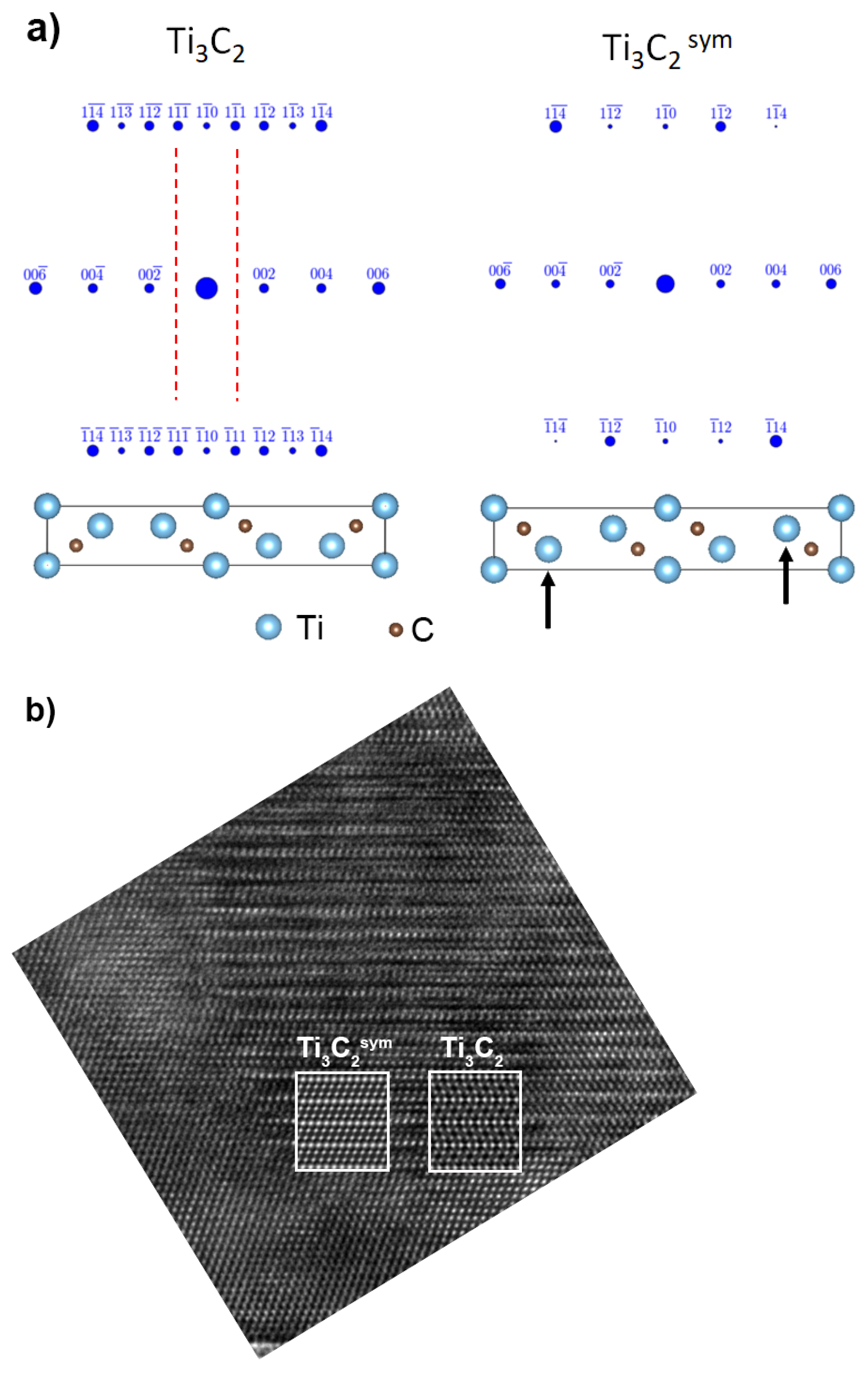
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Maters. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowotny, V.H. Strukturchemie einiger Verbindungen der Übergangsmetalle mit den elementen C, Si, Ge, Sn. Prog. Solid State Chem. 1971, 5, 27–70. [Google Scholar] [CrossRef]
- Jeitschko, W.; Nowotny, H.; Benesovsky, F. Die H-Phasen: Ti2CdC, Ti2GaC, Ti2GaN, Ti2InN, Zr2InN und Nb2GaC. Monatshefte Für Chemie 1964, 95, 178–179. [Google Scholar] [CrossRef]
- Jeitschko, W.; Nowotny, H.; Benesovsky, F. Kohlenstoffhaltige ternäre Verbindungen (H-Phase). Monatshefte Für Chemie 1963, 94, 672–676. [Google Scholar] [CrossRef]
- Naguib, M.; Unocic, R.R.; Armstrong, B.L.; Nanda, J. Large-scale delamination of multi-layers transition metal carbides and carbonitrides “mXenes”. Dalton Trans. 2015, 44, 9353–9358. [Google Scholar] [CrossRef]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes promising anode materials for Li ion batteries? Computational studies on electronic properties and Li storage capability of Ti3C2 and Ti3C2X2 (X = F, OH) monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef]
- Sun, D.; Wang, M.; Li, Z.; Fan, G.; Fan, L.Z.; Zhou, A. Two-dimensional Ti3C2 as anode material for Li-ion batteries. Electrochem. Commun. 2014, 47, 80–83. [Google Scholar] [CrossRef]
- Naguib, M.; Come, J.; Dyatkin, B.; Presser, V.; Taberna, P.L.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. MXene: A promising transition metal carbide anode for lithium-ion batteries. Electrochem. Commun. 2012, 16, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Sun, D.; Wu, Q.; Wang, H.; Wang, L.; Liu, B.; Zhou, A.; He, J. MXene: A new family of promising hydrogen storage medium. J. Phys. Chem. A 2013, 117, 14253–14260. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, J.; Zhang, Q.; Xiang, J.; Liu, B.; Zhou, A.; Liu, R.; Tian, Y. Unique lead adsorption behavior of activated hydroxyl group in two-dimensional titanium carbide. J. Am. Chem. Soc. 2014, 136, 4113–4116. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, L.; Li, Z.; Zhou, A.; Hu, Q.; Cao, X. Preparation of MXene-Cu2O nanocomposite and effect on thermal decomposition of ammonium perchlorate. Solid State Sci. 2014, 35, 62–65. [Google Scholar] [CrossRef]
- Lotfi, R.; Naguib, M.; Yilmaz, D.E.; Nanda, J.; Van Duin, A.C. A comparative study on the oxidation of two-dimensional Ti3C2 MXene structures in different environments. J. Mater. Chem. A 2018, 6, 12733–12743. [Google Scholar] [CrossRef]
- Naguib, M.; Mashtalir, O.; Lukatskaya, M.R.; Dyatkin, B.; Zhang, C.; Presser, V.; Gogotsi, Y.; Barsoum, M.W. One-step synthesis of nanocrystalline transition metal oxides on thin sheets of disordered graphitic carbon by oxidation of MXenes. Chem. Commun. 2014, 50, 7420–7423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Wang, L.; Sun, D.; Zhang, Y.; Liu, B.; Hu, Q.; Zhou, A. Synthesis and thermal stability of two-dimensional carbide MXene Ti3C2. Materi. Sci. Eng. B Solid-State Mater. Adv. Technol. 2015, 191, 33–40. [Google Scholar] [CrossRef]
- Petrus, M.; Woźniak, J.; Cygan, T.; Lachowski, A.; Rozmysłowska-Wojciechowska, A.; Wojciechowski, T.; Ziemkowska, W.; Chlubny, L.; Jastrzębska, A.; Adamczyk-Cieślak, B.; et al. Silicon carbide nanocomposites reinforced with disordered graphitic carbon formed in situ through oxidation of Ti3C2 MXene during sintering. Arch. Civ. Mech. Eng. 2021, 21, 1–12. [Google Scholar] [CrossRef]
- Petrus, M.; Wo, J.; Cygan, T.; Lachowski, A.; Moszczy, D.; Adamczyk-Cie, B.; Rozmysłowska-Wojciechowska, A.; Wojciechowski, T.; Ziemkowska, W.; Jastrz, A.; et al. Influence of Ti3C2Tx MXene and Surface-Modified Ti3C2Tx MXene Addition on Microstructure and Mechanical Properties of Silicon Carbide Composites Sintered via Spark Plasma Sintering Method. Materials 2021, 14, 3558. [Google Scholar] [CrossRef] [PubMed]
- Cygan, T.; Wozniak, J.; Petrus, M.; Lachowski, A.; Pawlak, W.; Adamczyk-Cieślak, B.; Jastrzębska, A.; Rozmysłowska-Wojciechowska, A.; Wojciechowski, T.; Ziemkowska, W.; et al. Microstructure and mechanical properties of alumina composites with addition of structurally modified 2d Ti3C2 (Mxene) phase. Materials 2021, 14, 829. [Google Scholar] [CrossRef]
- Wozniak, J.; Petrus, M.; Cygan, T.; Lachowski, A.; Adamczyk-Cieślak, B.; Moszczyńska, D.; Jastrzębska, A.; Wojciechowski, T.; Ziemkowska, W.; Olszyna, A. Influence of MXene (Ti3C2) Phase Addition on the Microstructure and Mechanical Properties of Silicon Nitride Ceramics. Materials 2020, 13, 5221. [Google Scholar] [CrossRef] [PubMed]
- Abdullahi, S.S.; Güner, S.; Ibrahim, Y.; Musa, M.; Adamu, B.I.; Abdulhamid, M. Simple Method for the Determination of Band Gap of a Nanopowdered Sample Usıng Kubelka Munk Theory. J. Niger. Assoc. Math. Phys. 2016, 35, 241–246. [Google Scholar]
- Zhang, J.; Xi, J.; Ji, Z. Mo + N Codoped TiO2 sheets with dominant {001} facets for enhancing visible-light photocatalytic activity. J. Mater. Chem. 2012, 22, 17700–17708. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 011002. [Google Scholar] [CrossRef] [Green Version]
- Gu, X.-F.; Furuhara, T.; Zhang, W.-Z. PTCLab: Free and open-source software for calculating phase transformation crystallography. J. Appl. Crystallogr. 2016, 49, 1099–1106. [Google Scholar] [CrossRef]
- Koch, C. Determination of Core Structure Periodicity and Point Defect Density along Dislocations; Arizona State University: Tempe, Arizona, 2002. [Google Scholar]
- Pierre, P.S. A Note on the Melting Point of Titanium Dioxide. J. Am. Ceram. Soc. 1952, 35, 188. [Google Scholar] [CrossRef]
- Ghosh, T.B.; Dhabal, S.; Datta, A.K. On crystallite size dependence of phase stability of nanocrystalline TiO2. J. Appl. Phys. 2003, 94, 4577. [Google Scholar] [CrossRef]
- Hanaor, D.; Sorrell, C.; Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wang, X.; Li, Z.; Zhou, Y. Al stabilized TiC twinning platelets. J. Mater. Res. 2014, 29, 1113–1121. [Google Scholar] [CrossRef]
- Emmerlich, J.; Music, D.; Eklund, P.; Wilhelmsson, O.; Jansson, U.; Schneider, J.M.; Högberg, H.; Hultman, L. Thermal stability of Ti3SiC2 thin films. Acta Mater. 2007, 55, 1479–1488. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wozniak, J.; Petrus, M.; Cygan, T.; Lachowski, A.; Kostecki, M.; Jastrzębska, A.; Wojciechowska, A.; Wojciechowski, T.; Olszyna, A. Investigation of MXenes Oxidation Process during SPS Method Annealing. Materials 2021, 14, 6011. https://doi.org/10.3390/ma14206011
Wozniak J, Petrus M, Cygan T, Lachowski A, Kostecki M, Jastrzębska A, Wojciechowska A, Wojciechowski T, Olszyna A. Investigation of MXenes Oxidation Process during SPS Method Annealing. Materials. 2021; 14(20):6011. https://doi.org/10.3390/ma14206011
Chicago/Turabian StyleWozniak, Jaroslaw, Mateusz Petrus, Tomasz Cygan, Artur Lachowski, Marek Kostecki, Agnieszka Jastrzębska, Anita Wojciechowska, Tomasz Wojciechowski, and Andrzej Olszyna. 2021. "Investigation of MXenes Oxidation Process during SPS Method Annealing" Materials 14, no. 20: 6011. https://doi.org/10.3390/ma14206011
APA StyleWozniak, J., Petrus, M., Cygan, T., Lachowski, A., Kostecki, M., Jastrzębska, A., Wojciechowska, A., Wojciechowski, T., & Olszyna, A. (2021). Investigation of MXenes Oxidation Process during SPS Method Annealing. Materials, 14(20), 6011. https://doi.org/10.3390/ma14206011








