Synthesis of Metal Matrix Composites Based on CrxNiy-TiN for Additive Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Method of Obtaining SHS Powders Used in Additive Manufacturing
2.2. Obtaining Samples of Composite Materials Using Additive Manufacturing
2.3. Research Methods
3. Results and Discussion
3.1. Investigation of Combustion in the CrN-TiNi Mixture Samplers
3.2. Investigation of the Phase Composition and Structure of SH Synthesis Products Obtained from a CrN-TiNi Mixture
3.3. Investigation of the Phase Composition and Structure of Materials Obtained by Direct Laser Deposition from CrNi-TiN SHS Composites
3.4. Investigation of the Mechanical Properties of Materials Obtained by Direct Laser Deposition from CrNi-TiN SHS Composites
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Gong, X.; Chou, K. Review on powder-bed laser additive manufacturing of Inconel 718 parts. Proc. Inst. Mech. Eng. Part B J. Eng. Manuf. 2017, 231, 1890–1903. [Google Scholar] [CrossRef]
- Pinkerton, A.J.; Li, L. Modelling the geometry of a moving laser melt pool and deposition track via energy and mass balances. J. Phys. D Appl. Phys. 2004, 37, 1885–1895. [Google Scholar] [CrossRef]
- Gu, D.; Meiners, W.; Hagedorn, Y.-C.; Wissenbach, K.; Poprawe, R. Bulk-form TiCx/Ti nanocomposites with controlled nanostructure prepared by a new method: Selective laser melting. J. Phys. D Appl. Phys. 2010, 43, 295402. [Google Scholar] [CrossRef]
- Kruth, J.P.; Leu, M.C.; Nakagawa, T. Progress in additive manufacturing and rapid prototyping. CIRP Ann. 1998, 47, 525–540. [Google Scholar] [CrossRef]
- Thijs, L.; Verhaeghe, F.; Craeghs, T.; Van Humbeeck, J.; Kruth, J.P. A study of the microstructural evolution during selective laser melting of Ti–6Al–4V. Acta Mater. 2010, 58, 3303–3312. [Google Scholar] [CrossRef]
- Giannatsis, J.; Dedoussis, V. Additive fabrication technologies applied to medicine and health care: A review. Int. J. Adv. Manuf. Technol. 2009, 40, 116–127. [Google Scholar] [CrossRef]
- Sungail, C.; Abid, A. Spherical tantalum feed powder for metal additive manufacturing. Metal. Powder Rep. 2018, 73, 316–318. [Google Scholar] [CrossRef]
- Philips, N.R.; Carl, M.; Cunningham, N.J. New opportunities in refractory alloys. Metall. Mater. Trans. A 2020, 51, 3299–3310. [Google Scholar] [CrossRef]
- Rai, S.K.; Kumar, A.; Shankar, V.; Jayakumar, T.; Rao, K.B.S.; Raj, B. Characterization of microstructures in Inconel 625 using X-ray diffraction peak broadening and lattice parameter measurements. Scr. Mater. 2004, 51, 59–63. [Google Scholar] [CrossRef]
- Hu, H.X.; Zheng, Y.; Qin, C.P. Comparison of Inconel 625 and Inconel 600 in resistance to cavitation erosion and jet impingement erosion. Nucl. Eng. Des. 2010, 240, 2721–2730. [Google Scholar] [CrossRef]
- Prokhorov, A.M. Nichrome, 3rd ed.; Soviet Encyclopedia: Moscow, Russia, 1978. [Google Scholar]
- Gonzalez, J.A.; Mireles, J.S.; Stafford, W.; Perez, M.A.; Terrazas, C.A.; Wicker, R.B. Characterization of Inconel 625 fabricated using powder-bed-based additive manufacturing technologies. J. Mater. Process. Technol. 2019, 264, 200–210. [Google Scholar] [CrossRef]
- ASTM F3056-14. Standard Specification for Additive Manufacturing Nickel Alloy (UNS N06625) With Powder Bed Fusion; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Qi, H.; Azer, M.; Ritter, A. Studies of standard heat treatment effects on microstructure and mechanical properties of laser net shape manufactured Inconel 718. Met. Mater. Trans. A 2009, 40, 2410–2422. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Lin, X.; Huang, W. Study on microstructure and mechanical properties of laser rapid forming Inconel 718. Mat. Sci. Eng. A Struct. 2008, 478, 119–124. [Google Scholar] [CrossRef]
- Chlebus, E.; Gruber, K.; Kuźnicka, B.; Kurzac, J.; Kurzynowski, T. Effect of heat treatment on microstructure and mechanical properties of Inconel 718 processed by selective laser melting. Mat. Sci. Eng. A Struct. 2015, 639, 647–655. [Google Scholar] [CrossRef]
- Wilson, J.M.; Shin, Y.C. Microstructure and wear properties of laser-deposited functionally graded Inconel 690 reinforced with TiC. Surf. Coat. Technol. 2012, 207, 517–522. [Google Scholar] [CrossRef]
- Wanga, R.; Wanga, W.; Zhua, G.; Pana, W.; Zhoua, W.; Wanga, D.; Lia, F.; Huanga, H.; Jiaa, Y.; Dua, D.; et al. Microstructure and mechanical properties of the TiN particles reinforced IN718C composite. J. Alloys Compd. 2018, 762, 237–245. [Google Scholar] [CrossRef]
- Hua, W.; Huangab, Z.; Yua, Q.; Wanga, Y.; Jiaoa, Y.; Leia, C.; Caia, L.; Zhaia, H.; Zhou, Y. Ti2AlC triggered in-situ ultrafine TiC/Inconel 718 composites: Microstructure and enhanced properties. J. Mater. Sci. Technol. 2020, 51, 70–78. [Google Scholar] [CrossRef]
- Zhang, B.; Bi, G.; Nai, M.; Sun, C.N.; Wei, J. Microhardness and microstructure evolution of TiB2 reinforced Inconel 625/TiB2 composite produced by selective laser melting. Opt. Laser Technol. 2016, 80, 186–195. [Google Scholar] [CrossRef]
- Ezugwu, E.O.; Wang, Z.M.; Machado, A.R. The machinability of nickel-based alloys: A review. J. Mater. Process. Technol. 1999, 86, 1–16. [Google Scholar] [CrossRef]
- Fattahi, Z.; Sajjadi, S.A.; Babakhani, A.; Saba, F. Ni–Cr matrix composites reinforced with nano-and micron-sized surface-modified zirconia: Synthesis, microstructure and mechanical properties. J. Alloys Compd. 2020, 817, 152755. [Google Scholar] [CrossRef]
- Cooper, D.E.; Blundell, N.; Maggs, S.; Gibbons, G.J. Additive layer manufacture of Inconel 625 metal matrix composites, reinforcement material evaluation. J. Mater. Process. Technol. 2013, 213, 2191–2200. [Google Scholar] [CrossRef]
- Merzhanov, A.G.; Rogachev, A.S. Structural macrokinetics of SHS process. Pure Appl. Chem. 1992, 64, 941–953. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, K.; Obara, Y.; Kudoh, M. Fabrication of TiB2 particle dispersed FeAl-based composites by self-propagating high-temperature synthesis. ISIJ Int. 2006, 46, 871–874. [Google Scholar] [CrossRef] [Green Version]
- Amosov, A.P.; Borovinskaya, I.P.; Merzhanov, A.G. Powder Technology of Self-Propagating High-Temperature Synthesis of Materials; Mashinostroenie: Moscow, Russia, 2007. [Google Scholar]
- Promakhov, V.; Matveev, A.; Schulz, N.; Grigoriev, M.; Olisov, A.; Vorozhtsov, A.; Zhukov, A.; Klimenko, V. High-temperature synthesis of metal–matrix composites (Ni-Ti)-TiB2. Appl. Sci. 2021, 11, 2426. [Google Scholar] [CrossRef]
- Zhukov, I.A.; Kozulin, A.A.; Khrustalyov, A.P.; Matveev, A.E.; Platov, V.V.; Vorozhtsov, A.B.; Zhukova, T.V.; Promakhov, V.V. The impact of particle reinforcement with Al2O3, TiB2, and TiC and severe plastic deformation treatment on the combination of strength and electrical conductivity of pure aluminum. Metals 2019, 9, 65. [Google Scholar] [CrossRef] [Green Version]
- Zhukov, I.; Promakhov, V.; Dubkova, Y.; Matveev, A.; Ziatdinov, M.; Zhukov, A. Al-Ti-B4C materials obtained by high-temperature synthesis and used as a master-alloy for aluminum. MATEC Web Conf. EDP Sci. 2018, 243, 00010. [Google Scholar] [CrossRef]
- Matveev, A.; Zhukov, I.; Ziatdinov, M.; Zhukov, A. Planetary milling and self-propagating high-temperature synthesis of Al-TiB2 composites. Materials 2020, 13, 1050. [Google Scholar] [CrossRef] [Green Version]
- Ladd, M.; Palmer, R. Structure Determination by X-Ray crystallography. Analysis by X-Rays and Neutrons; Springer: New York, NY, USA, 2013; 784p. [Google Scholar] [CrossRef]
- Rietveld, H.M. Line profiles of neutron powder-diffraction peaks for structure refinement. Acta Crystallogr. 1967, 22, 151–152. [Google Scholar] [CrossRef]
- Mossino, P. Some aspects in self-propagating high-temperature synthesis. Ceram. Int. 2004, 30, 311–332. [Google Scholar] [CrossRef]
- Shekari, M.; Adeli, M.; Khobzi, A.; Kobashi, M.; Kanetake, N. Induction-activated self-propagating, high-temperature synthesis of nickel aluminide. Adv. Powder Technol. 2017, 28, 2974–2979. [Google Scholar] [CrossRef]
- Samsonov, G.V. Nitrides; Naukova Dumka: Kiev, Ukraine, 1969. [Google Scholar]
- Newport, A.; Carmalt, C.J.; Parkin, I.P.; O’Neill, S.A. The dual source APCVD of titanium nitride thin films from reaction of hexamethyldisilazane and titanium tetrachloride. J. Mater. Chem. 2002, 12, 1906–1909. [Google Scholar] [CrossRef]
- Huang, K.; Marthinsen, K.; Zhao, Q.; Logé, R.E. The double-edge effect of second-phase particles on the recrystallization behaviour and associated mechanical properties of metallic materials. Prog. Mater. Sci. 2018, 92, 284–359. [Google Scholar] [CrossRef]
- Sadovnikov, S.I.; Gusev, A.I. The effect of temperature on the particle sizes and the recrystallization of silver sulfide nanopowders. Phys. Solid State 2018, 60, 1308–1315. [Google Scholar] [CrossRef]
- Hu, Y.L.; Lin, X.; Yu, X.B.; Xu, J.J.; Lei, M.; Huang, W.D. Effect of Ti addition on cracking and microhardness of Inconel 625 during the laser solid forming processing. J. Alloys Compd. 2017, 711, 267–277. [Google Scholar] [CrossRef]
- Kaynak, Y.; Tascioglu, E. Finish machining-induced surface roughness, microhardness and XRD analysis of selective laser melted Inconel 718 alloy. Procedia CIRP 2018, 71, 500–504. [Google Scholar] [CrossRef]
- Yang, H.; Yang, J.; Huang, W.; Wang, Z.; Zeng, X. The printability, microstructure, crystallographic features and microhardness of selective laser melted Inconel 718 thin wall. Mater. Des. 2018, 156, 407–418. [Google Scholar] [CrossRef]
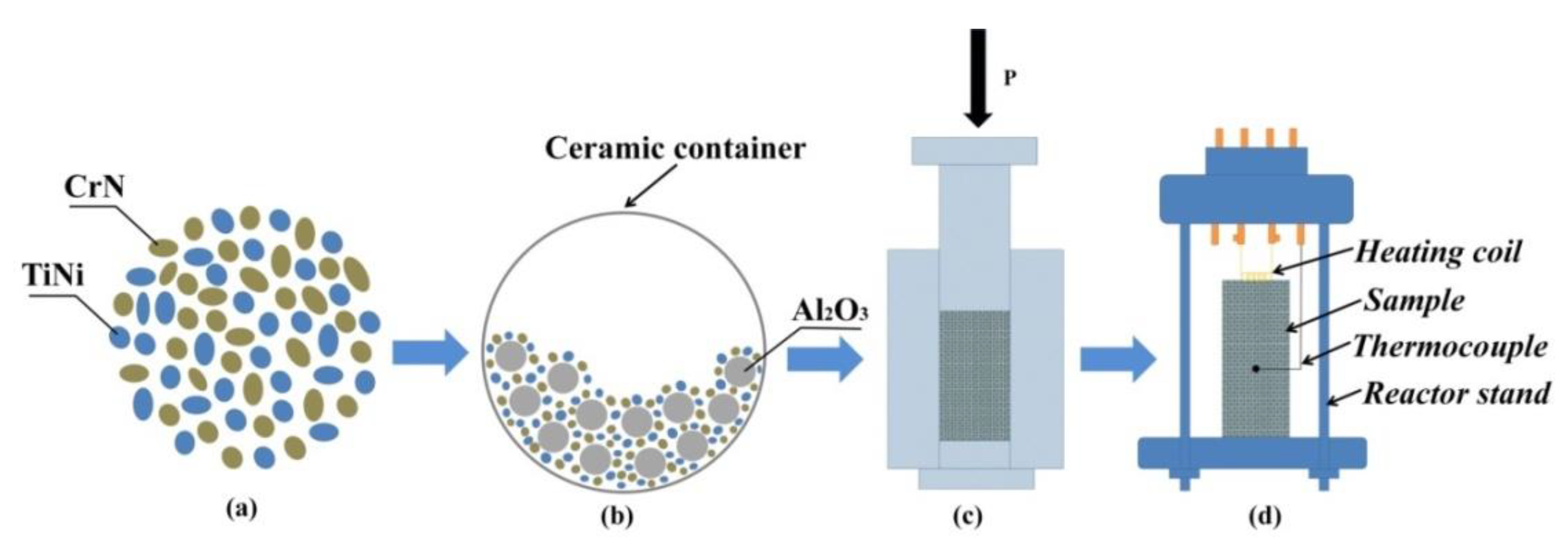
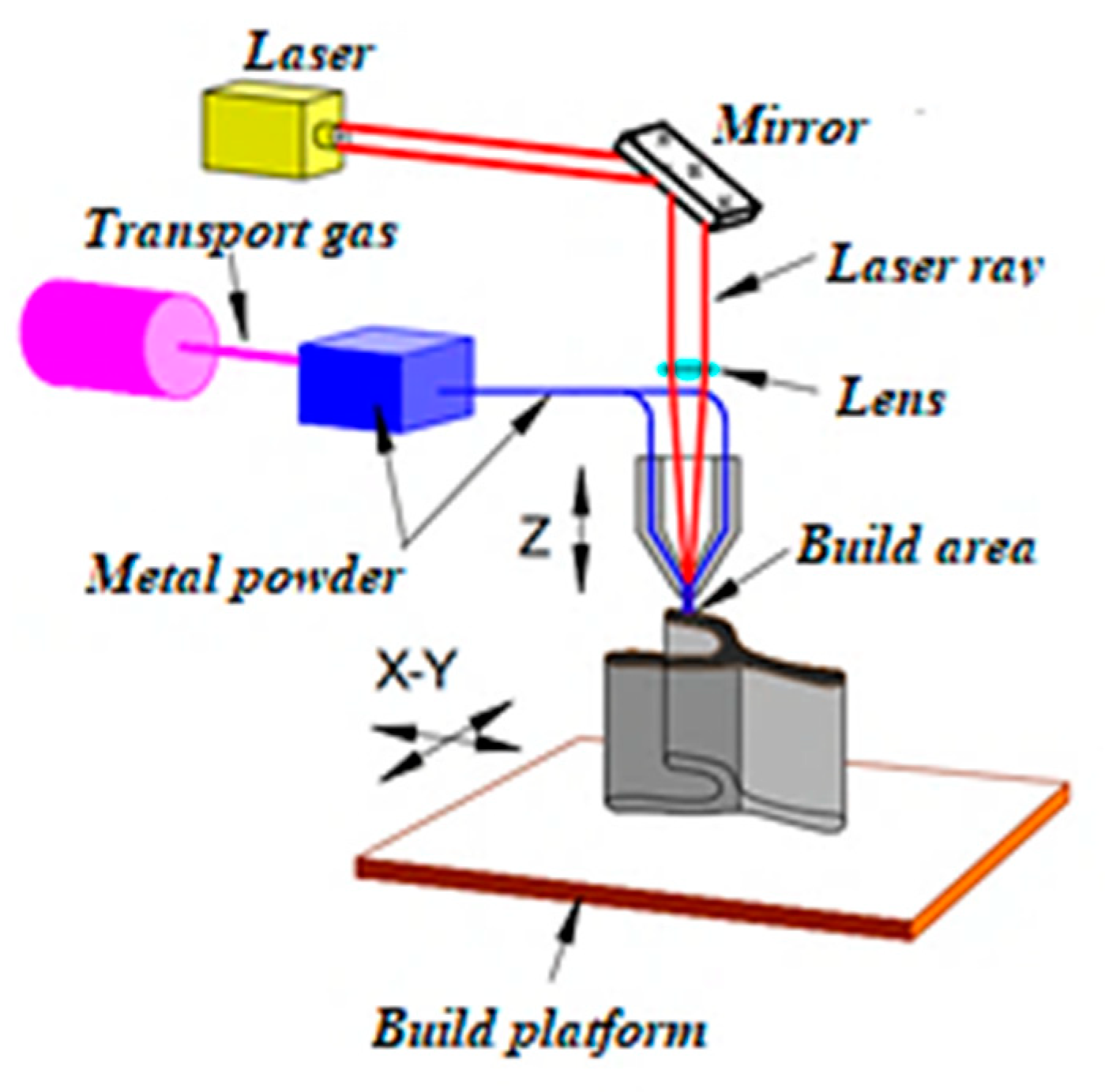
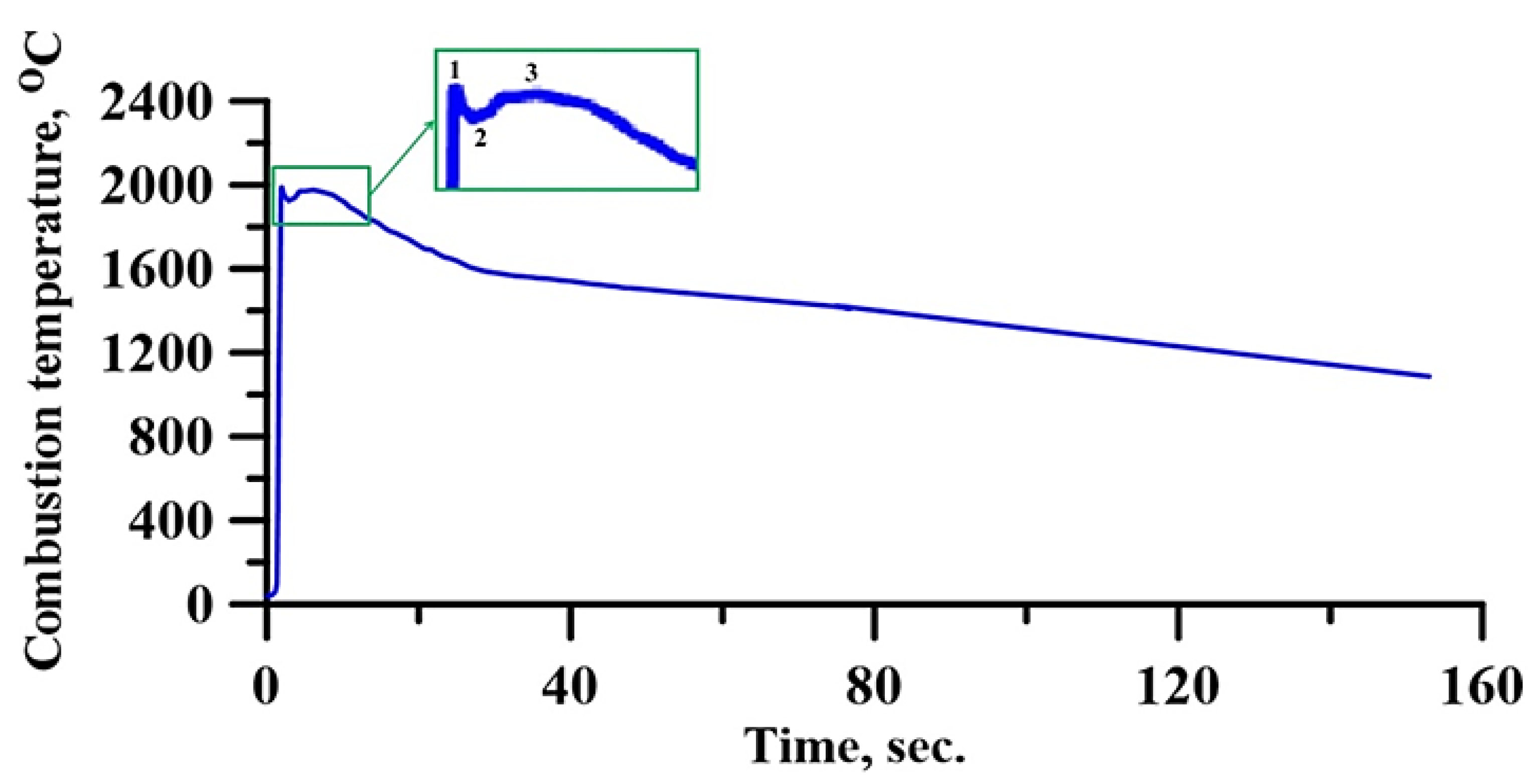
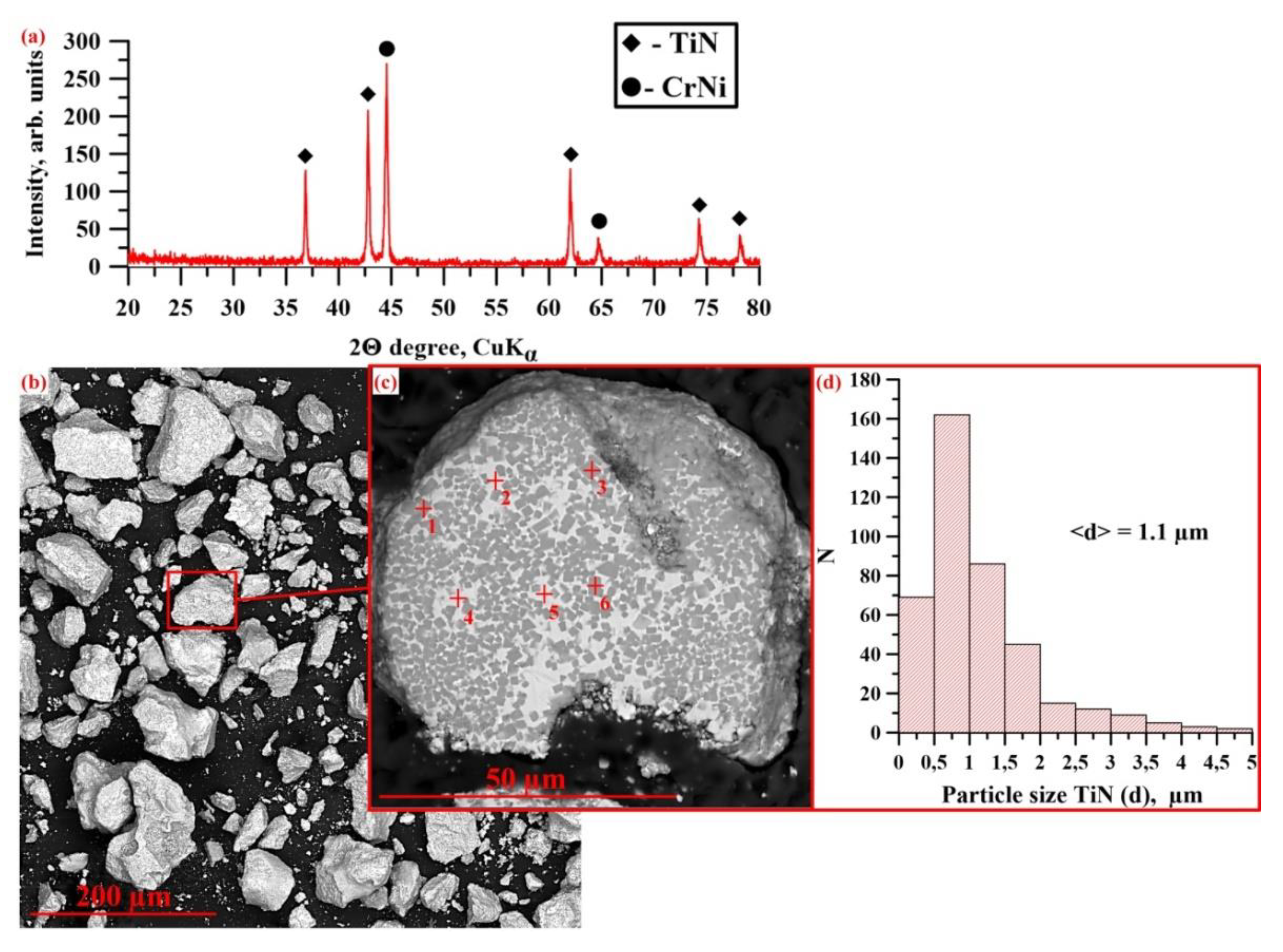
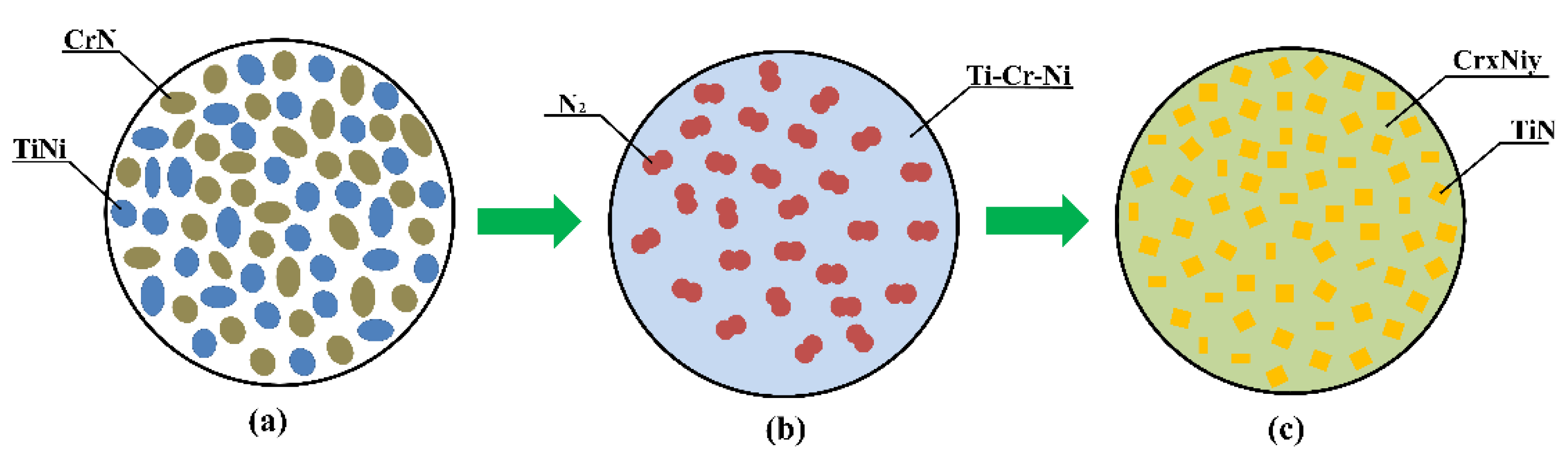
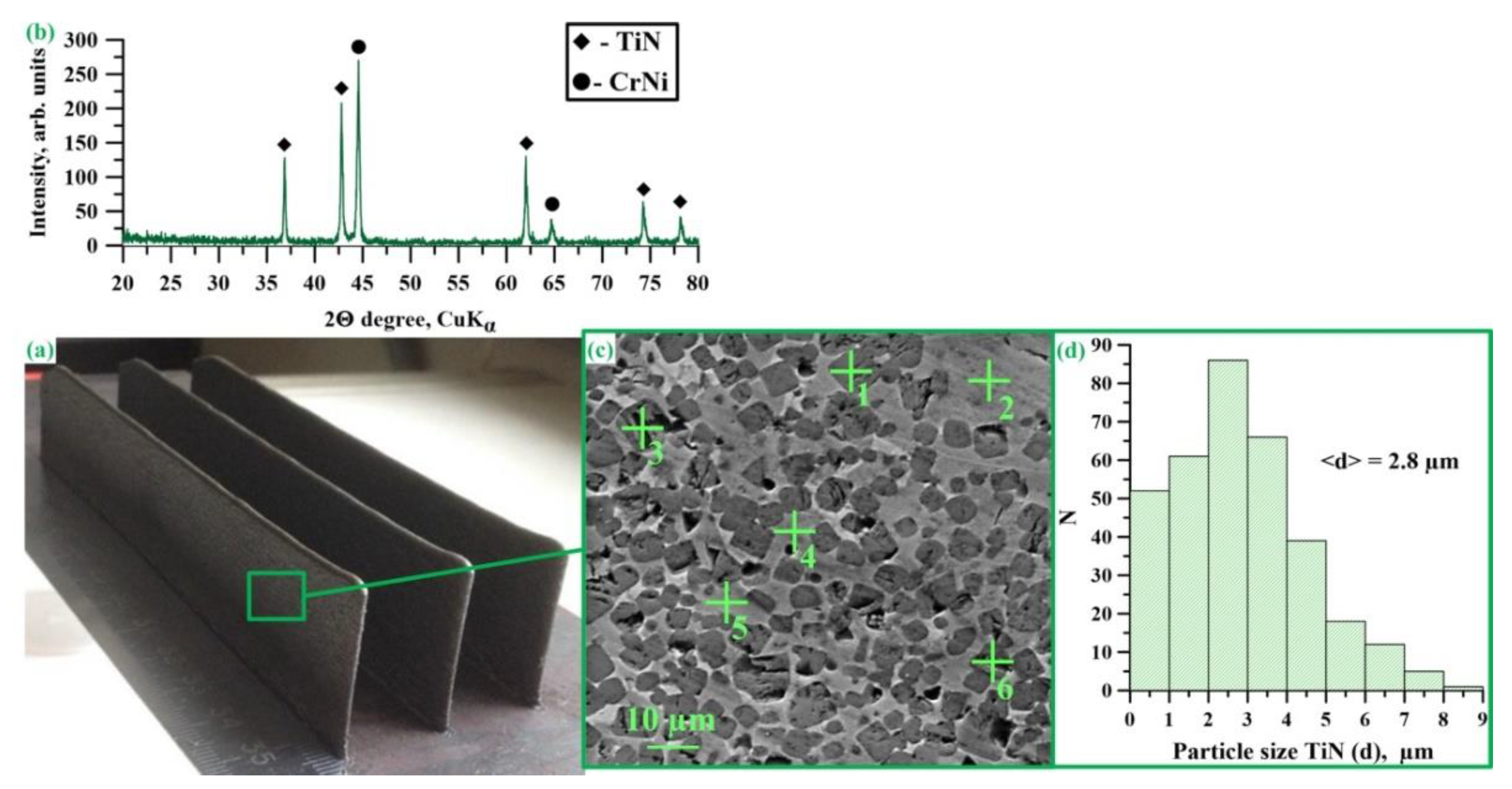
| Radiation Power, W | Process Speed, mm/s | Layer Pitch, mm | Beam Diameter, mm | Powder Flow Rate, % |
|---|---|---|---|---|
| 1300 | 25 | 0.6 | 2 | 40 |
| Sample of the Initial Mixture | Phases Discovered in the Synthesis Products | Phase Content, Mass % | Lattice Parameters, Ǻ | CSR Size, nm |
|---|---|---|---|---|
| CrN-TiNi | TiN_225 | 70 | a = 4.2374 | 45 |
| CrNi_229 | 30 | a = 2.8849 | 43 |
| Point No. (+) | Detected Elements, at. % | |||
|---|---|---|---|---|
| Ti | N | Cr | Ni | |
| 1 | 63.6 | 17.5 | 16.4 | 2.6 |
| 2 | 28.5 | 9.2 | 48.5 | 17.3 |
| 3 | 48.9 | 13.3 | 33.3 | 4.6 |
| 4 | 27.7 | 11.2 | 27.3 | 33.8 |
| 5 | 25.7 | 10.2 | 29.5 | 34.6 |
| 6 * | 32.4 | 14.9 | 47.1 | 5.6 |
| Sample | Detected Phases | Phase Content, Mass % | Lattice Parameters, Ǻ | CSR Size, nm |
|---|---|---|---|---|
| CrxNiy-TiN | TiN_225 | 70 | a = 4.2425 | 85 |
| CrNi_229 | 30 | a = 2.8877 | 55 |
| Point No. (+) | Detected Elements, at. % | |||
|---|---|---|---|---|
| Ti | N | Cr | Ni | |
| 1 | 68.5 | 19.3 | 9.3 | 2.9 |
| 2 | 8.6 | 2.8 | 59.3 | 29.3 |
| 3 | 63.6 | 21.2 | 11.4 | 3.8 |
| 4 | 15.7 | 5.2 | 52.3 | 26.8 |
| 5 | 17.2 | 5.7 | 51.4 | 25.7 |
| 6 * | 62.8 | 20.9 | 10.8 | 5.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveev, A.; Promakhov, V.; Schultz, N.; Vorozhtsov, A. Synthesis of Metal Matrix Composites Based on CrxNiy-TiN for Additive Technology. Materials 2021, 14, 5914. https://doi.org/10.3390/ma14205914
Matveev A, Promakhov V, Schultz N, Vorozhtsov A. Synthesis of Metal Matrix Composites Based on CrxNiy-TiN for Additive Technology. Materials. 2021; 14(20):5914. https://doi.org/10.3390/ma14205914
Chicago/Turabian StyleMatveev, Alexey, Vladimir Promakhov, Nikita Schultz, and Alexander Vorozhtsov. 2021. "Synthesis of Metal Matrix Composites Based on CrxNiy-TiN for Additive Technology" Materials 14, no. 20: 5914. https://doi.org/10.3390/ma14205914
APA StyleMatveev, A., Promakhov, V., Schultz, N., & Vorozhtsov, A. (2021). Synthesis of Metal Matrix Composites Based on CrxNiy-TiN for Additive Technology. Materials, 14(20), 5914. https://doi.org/10.3390/ma14205914





