1. Introduction
Asphalt is an excellent engineering material, widely used in road pavement, waterproof coating, and other fields [
1,
2]. Due to the nature of asphalt itself, its further application is affected by some performance defects, such as aging at high temperature and cracking at low temperature. By incorporating modifiers, modifying asphalt is the main way to solve its limited application. At present, the most widely used asphalt modifiers are polymer asphalt modifiers, mainly including styrene–butadiene–styrene (SBS), styrene–butadiene rubber (SBR), ethylene–vinyl acetate (EVA), and so on [
3]. SBS is the most commonly used polymer asphalt modifier [
4,
5], the polystyrene end-blocks impact the strength to the polymer while the polybutadiene mid-blocks give the material its better elasticity [
6]. SBS modified asphalt has good thermal stability [
7], but it is very susceptible to ultraviolet (UV) radiation [
8,
9]. In addition, SBS modified asphalt also has good crack resistance [
7], can improve the adhesion between asphalt and aggregates [
10], and effectively improve the performance of asphalt.
Since SBS modified asphalt is the most widely used modified asphalt, the mechanism of interaction between SBS and asphalt has naturally been a hot research topic in the field of asphalt science. Airey [
11] modified two base asphalts (A and B) with SBS and found that the extent of polymer modification has differed depending on the nature of the base asphalt and subsequently the compatibility of the asphalt-polymer system. The asphalt A showed a greater degree of polymer modification compared to the asphalt B, which is related to the larger proportion of the aromatic hydrocarbon present in the asphalt A. The content of the aromatic hydrocarbon in asphalt played an important role in compatibility between the asphalt and polymer [
12]. By building models and simulating calculations, we can further observe and analyze at the microscopic molecular level, which is helpful to better understand the macroscopic experimental phenomena. Some researchers [
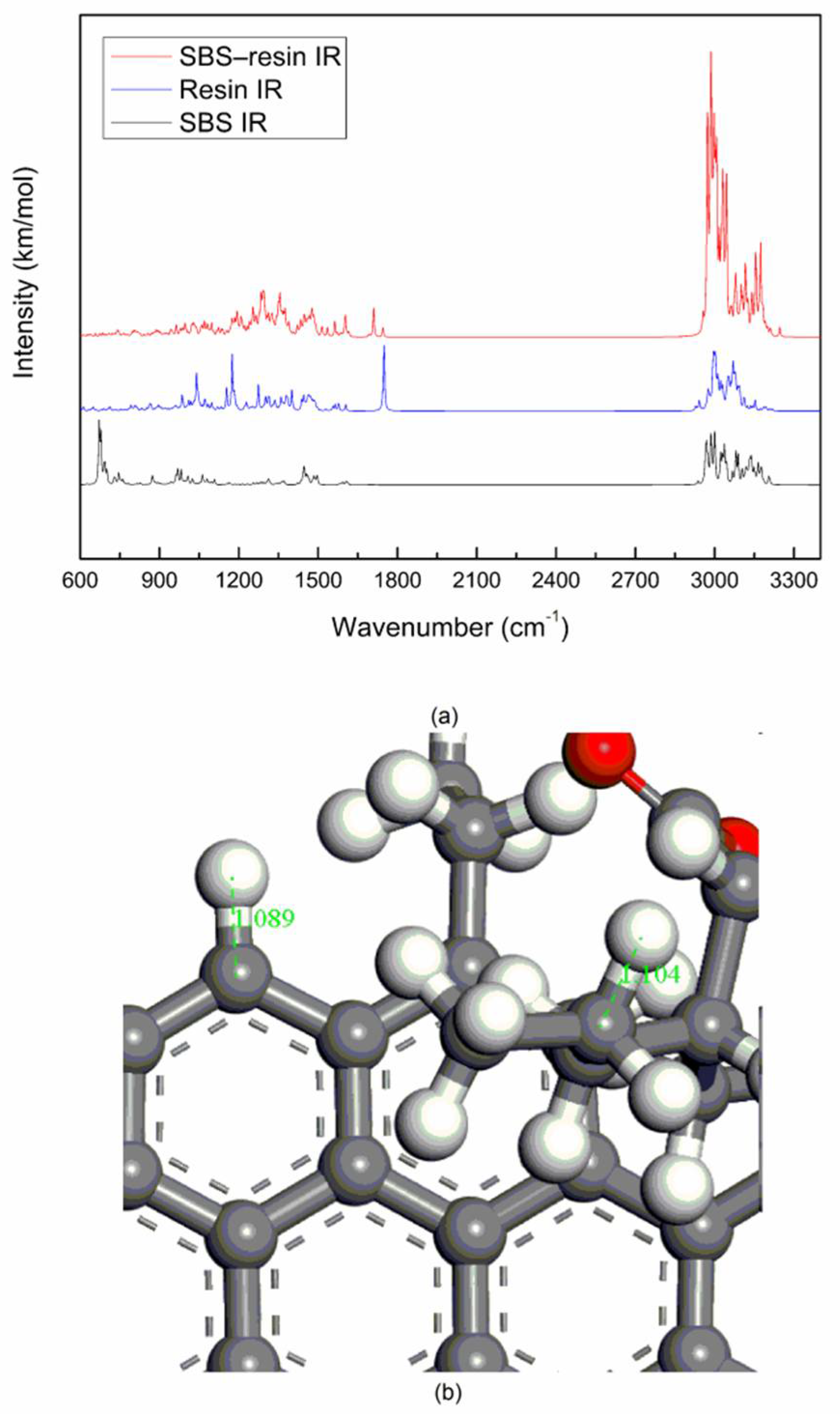
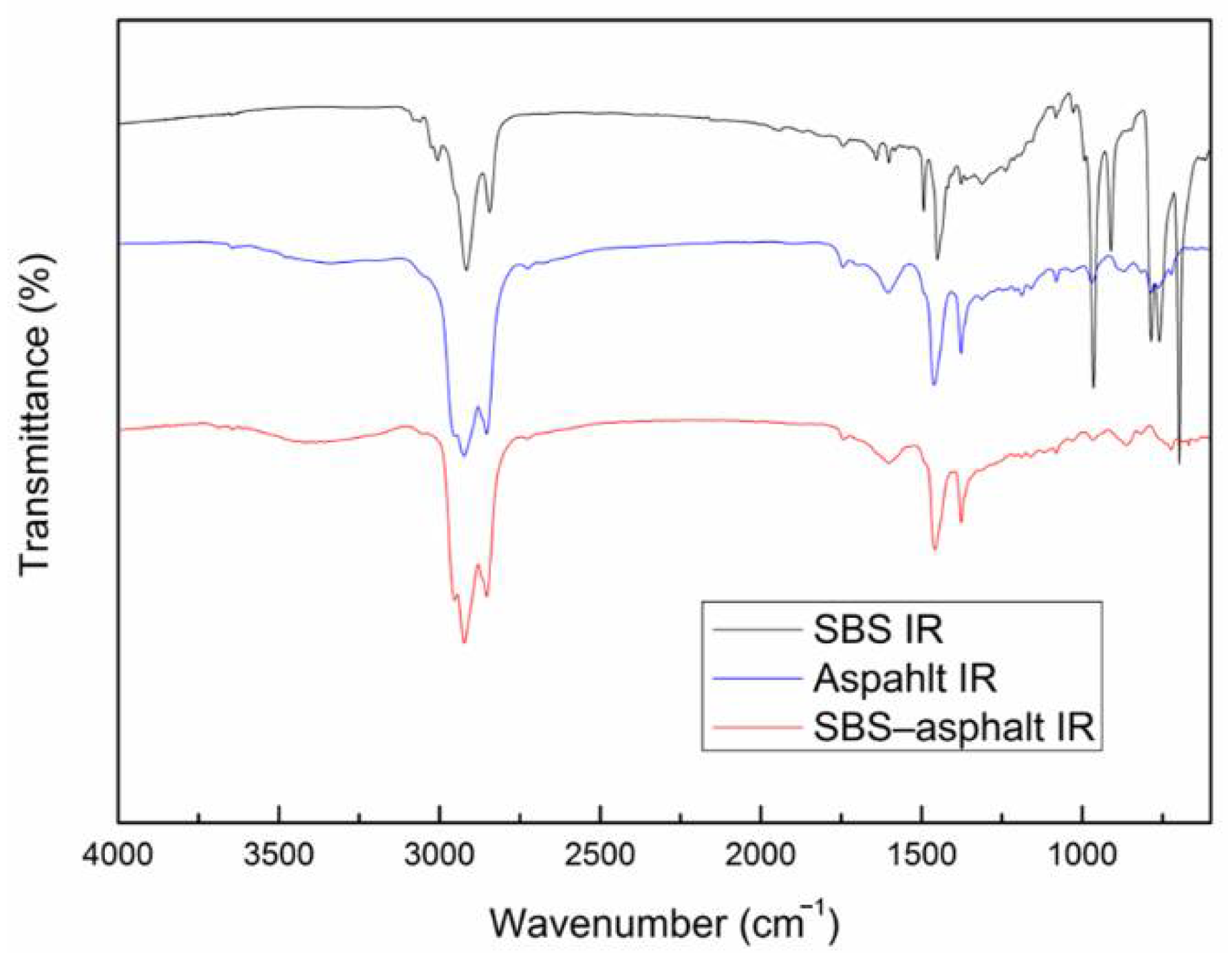
13] used infrared spectroscopy to find that an increase in hydroxyl groups and the formation of carbonyl compounds and sulfoxides after the aging of SBS modified asphalt. The content of the aromatic hydrocarbon decreased, while the content of the resin and asphaltene increased, resulting in hardening of the sample [
14]. However, the infrared spectroscopy is easily affected by the environment and equipment, while the simulation calculations are not subject to these constraints. They cover a wide range and can be studied at the microscopic molecular level. Ding et al. [
15] used molecular dynamics (MD) to study the molecular agglomeration behavior of SBS and asphalt, and found that the molecular agglomeration of asphalt with longer asphaltene alkane side branches is more regular. The agglomeration structure of the asphalt molecule is more susceptible to SBS when the asphaltene alkane side branches are longer. Most of the above studies used experimental data combined with microstructure characterization to explain their modification mechanism, which is limited by the experiment and characterization equipment. At the same time, because asphalt is an extremely complex mixture, some research results cannot be effectively verified. It is even more difficult to make research at the molecular level. Currently, there are relatively few researches involving asphalt simulation calculations, and they all use MD method of the first-principles. The DFT method of the first-principles is a powerful alternative to conventional theoretical research and molecular simulation and has become a regular tool used by many researchers in chemistry, physics, chemical engineering, materials science, and other disciplines. DMol3 of Materials Studio software using DFT method to study the molecular interfacial properties, electronic structure and energetics, a board range of systems can be studied, including inorganic, organic, crystal, metal, solid, covalent solids, and infinite surfaces of a material, also can predict structure, reaction barriers, reaction energies, thermodynamic properties, and vibration spectrum [
16]. It provides another effective method to study SBS modified asphalt. Moreover, the analysis and simulation of the molecular structure at the microscopic level are helpful to better understand the macroscopic experimental phenomena.
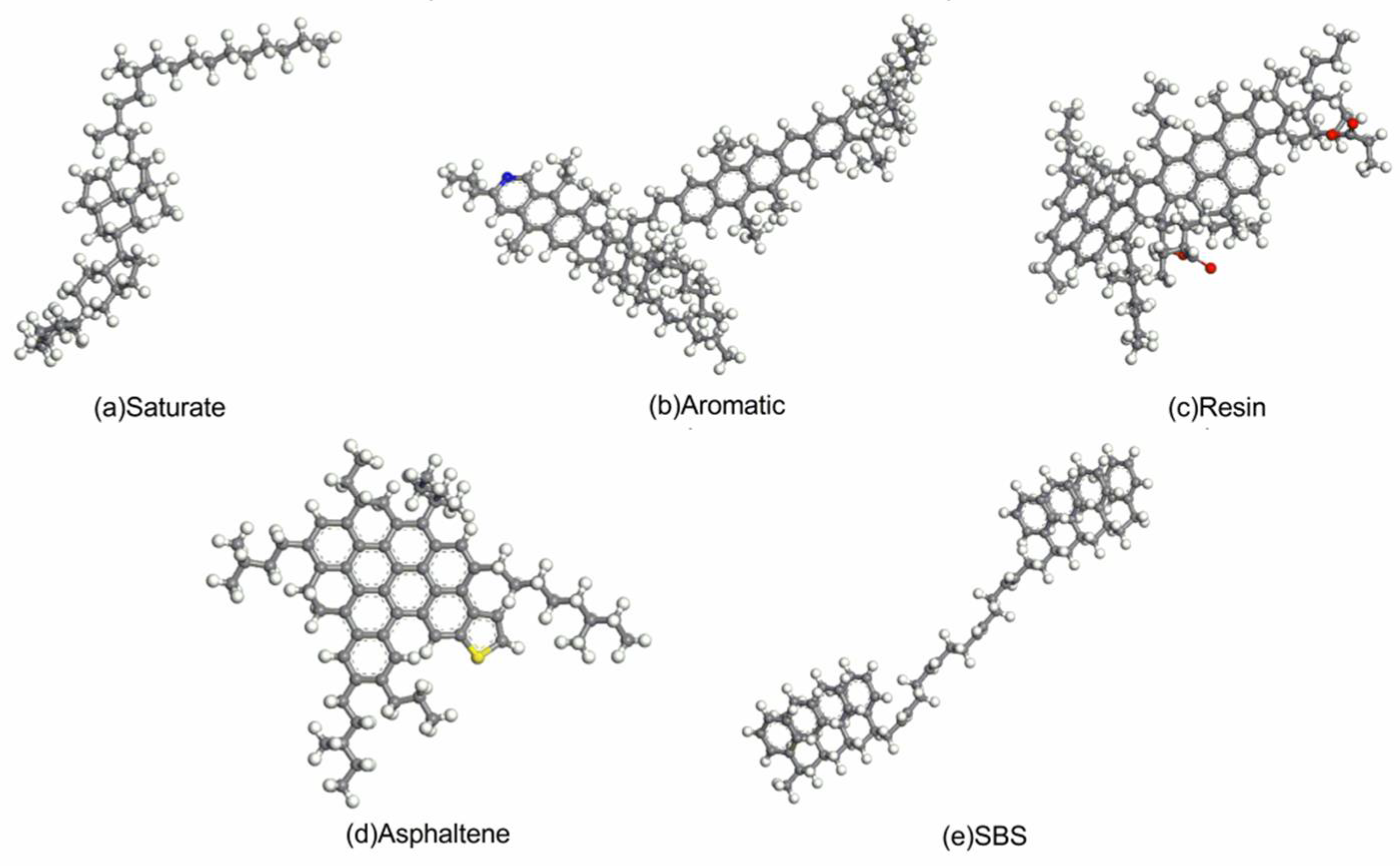
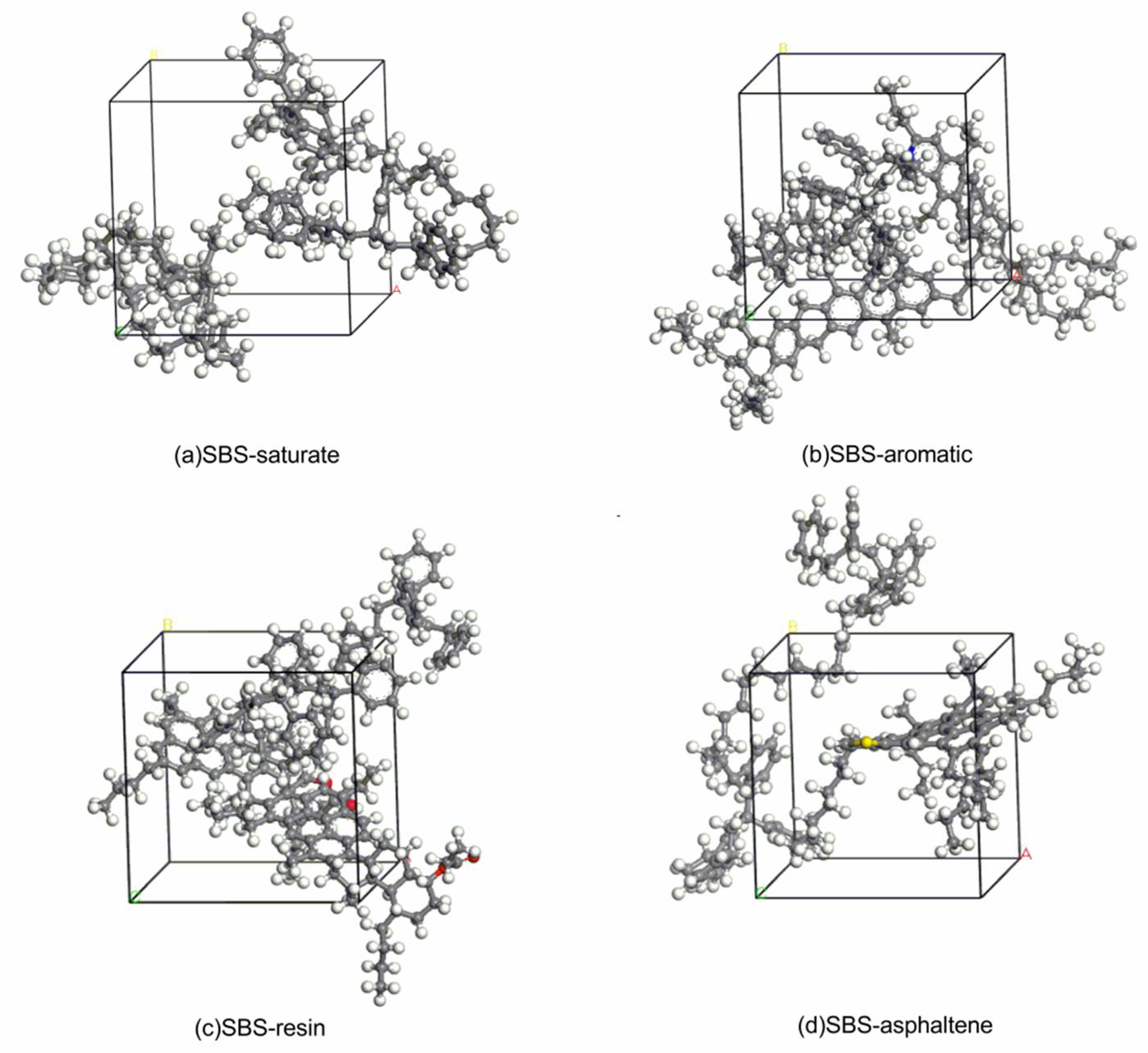
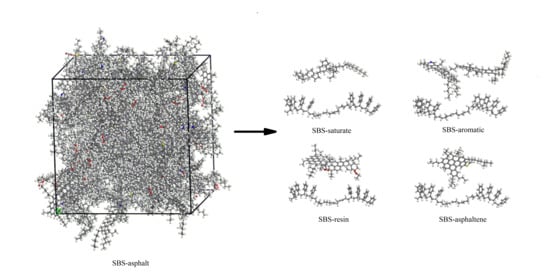
In this paper, the method of the DFT was used to observe the interaction between modifier and asphalt from a microscopic molecular scale. In order to solve the problem of difficult research on asphalt as a complex mixing system, asphalt was divided into the saturate, aromatic, resin and asphaltene. The models of the saturate, aromatic, resin, asphaltene and linear SBS with an S/B ratio of 4:4 were built. The mechanism of action between SBS and different components was studied separately. Then, SBS modified asphalt was prepared, the FTIR of SBS, asphalt, and SBS modified asphalt composite before and after the experiment were measured, and the experimental data were comprehensively analyzed. From the perspectives of molecular vibration spectrum and the binding energy between the SBS and each component of asphalt, the modification mechanism of asphalt modified by SBS at the molecular level was discussed, to some extent to make up for the deficiencies of existing researches.
4. Conclusions
Based on the first-principles, this paper analyzed the molecular vibration spectrums of the linear SBS molecule with an S/B ratio of 4:4, saturate molecule, aromatic molecule, resin molecule, asphaltene molecule, SBS–saturate system, SBS–aromatic system, SBS–resin system, and SBS–asphaltene system, and analyzed the interaction between SBS and each component of asphalt from the molecular level. At the same time, the binding strength between SBS and each component of asphalt was analyzed by binding energy. The main conclusions are as follows:
- (1)
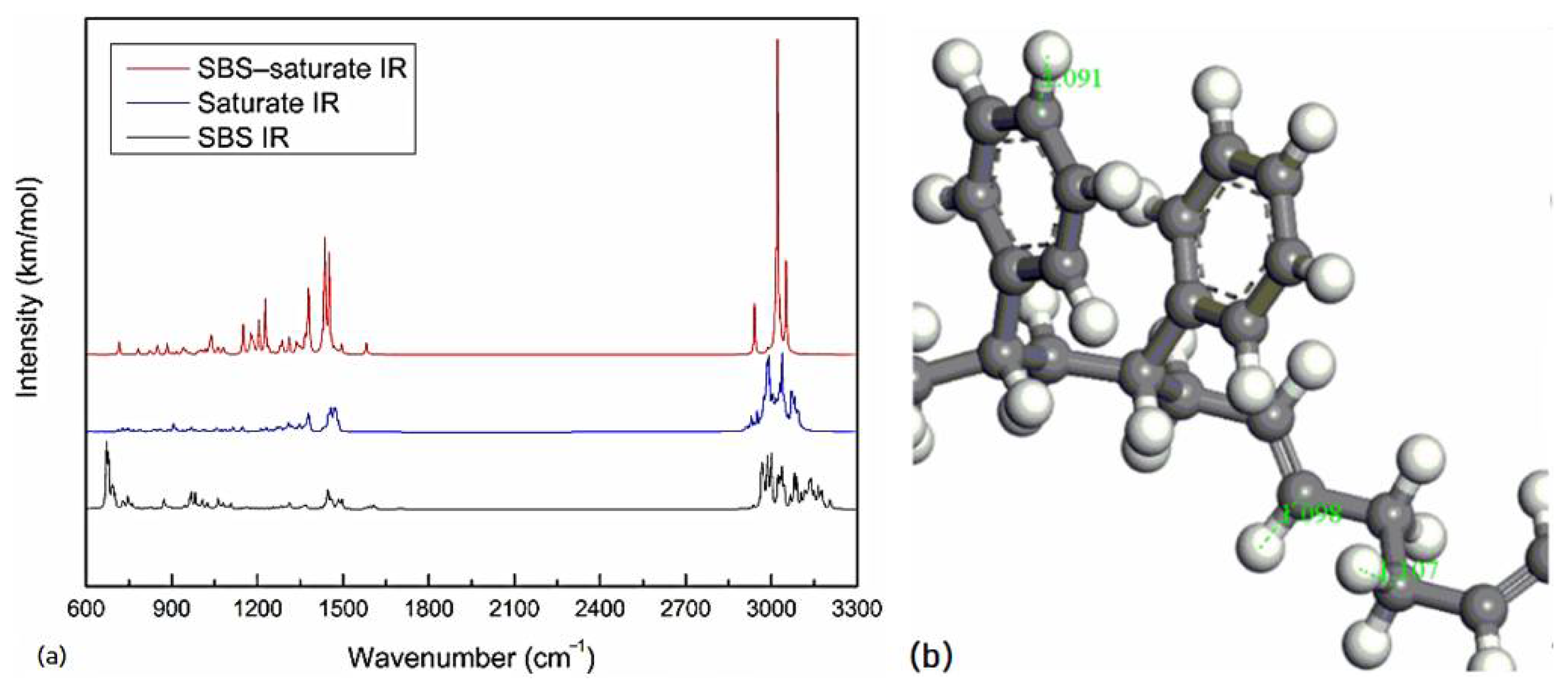
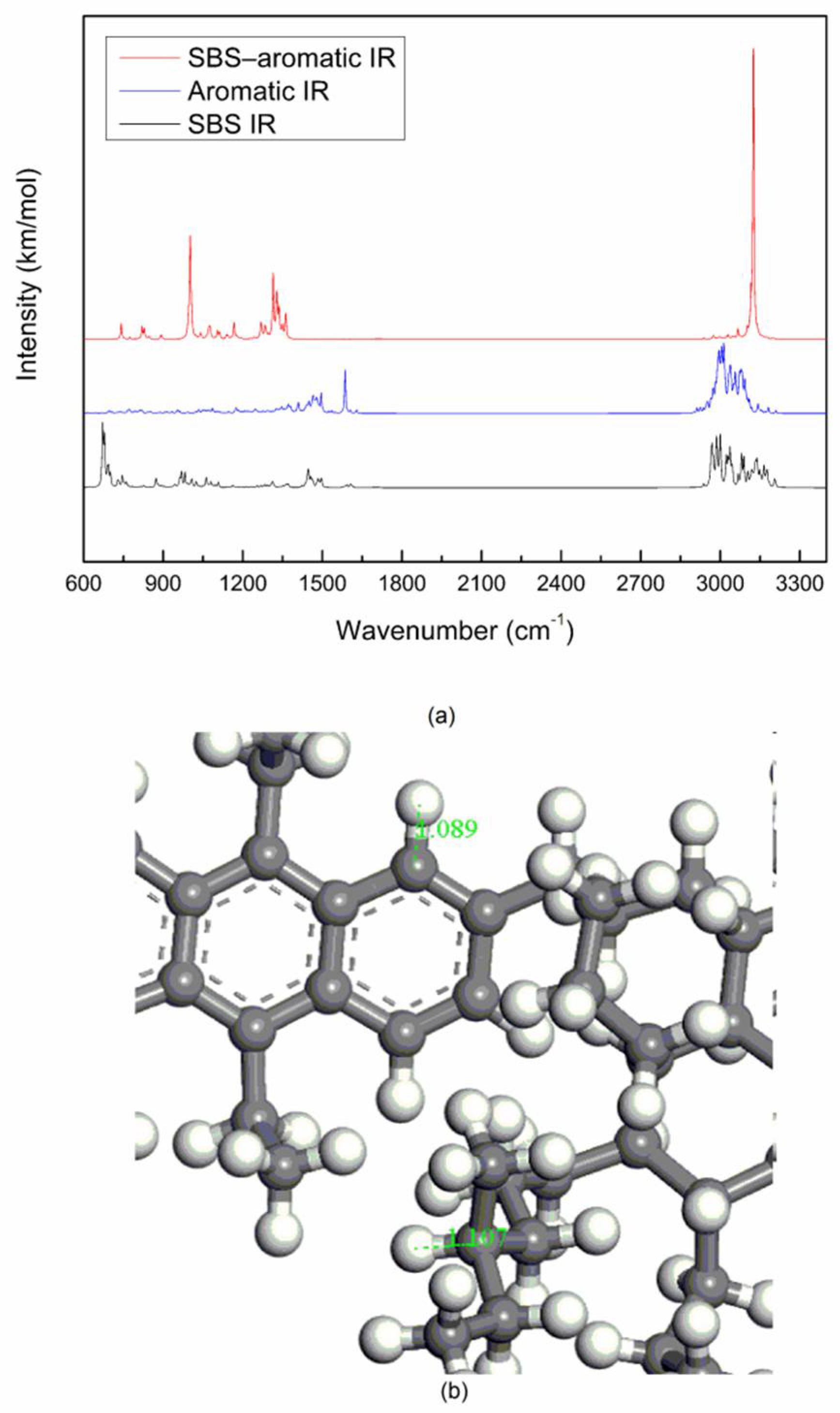
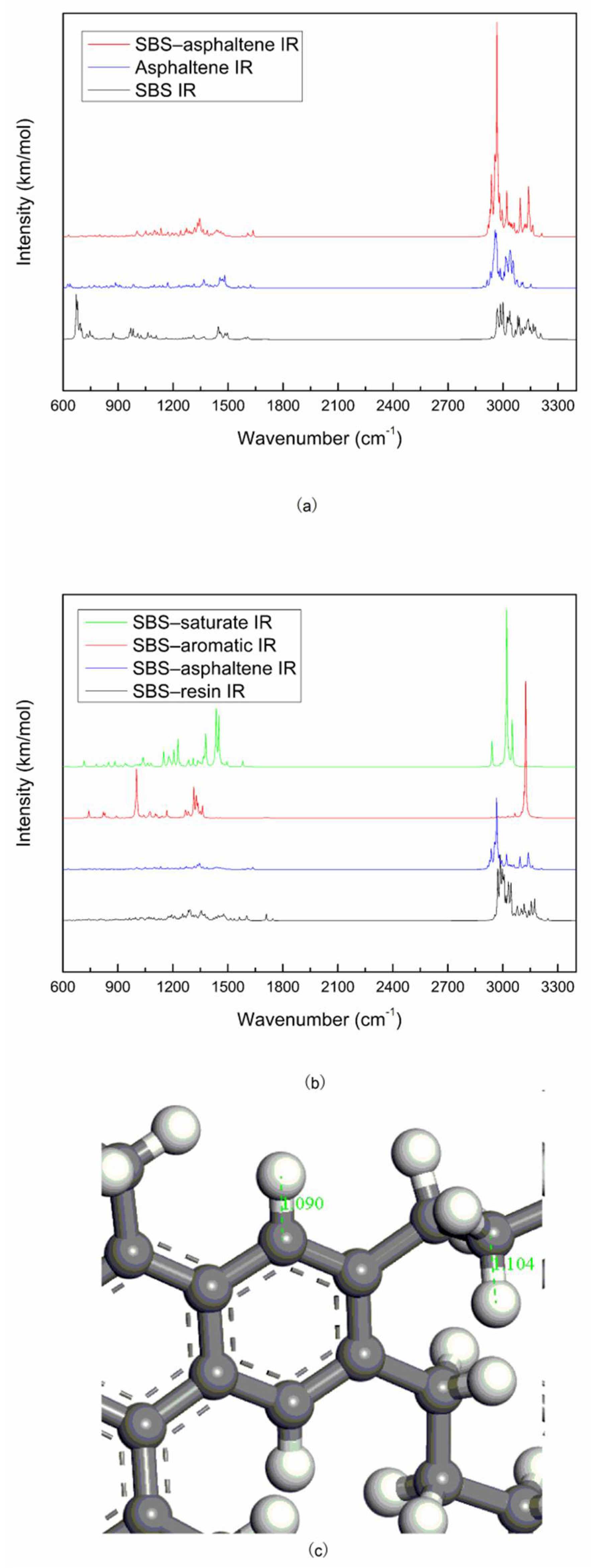
The spectrum of the mixed system of SBS and asphalt is mostly the superposition of the spectrum of SBS and asphalt. The peak position of the mixed system does not change significantly, and the physical effect is mainly between SBS and asphalt.
- (2)
The vibrational peak intensity of SBS and the light components of asphalt (saturate and aromatic) is stronger than that of SBS and the heavy components of asphalt (resin and asphaltene), which may be due to the interaction of PB blocks and the light components of asphalt, the interaction of PS blocks and the heavy components of asphalt, while the molecular interaction of the former is stronger than that of the latter.
- (3)
SBS and each component of asphalt can be combined spontaneously. From the absolute value of the binding energy between SBS and each component of asphalt, the stability of the binding between SBS and the saturate is the worst. SBS mainly produces physical adsorption with molecules containing aromatic ring structure in asphalt to achieve a uniform mixing state. This adsorption may be the interaction of aromatic ring packing.