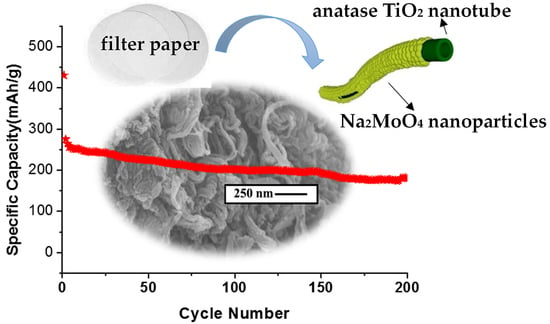
A Bio-Inspired Nanotubular Na2MoO4/TiO2 Composite as a High-Performance Anodic Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Na2MoO4/TiO2 Nanocomposites
2.3. Characterizations
2.4. Electrochemical Measurements
3. Results and Discussion
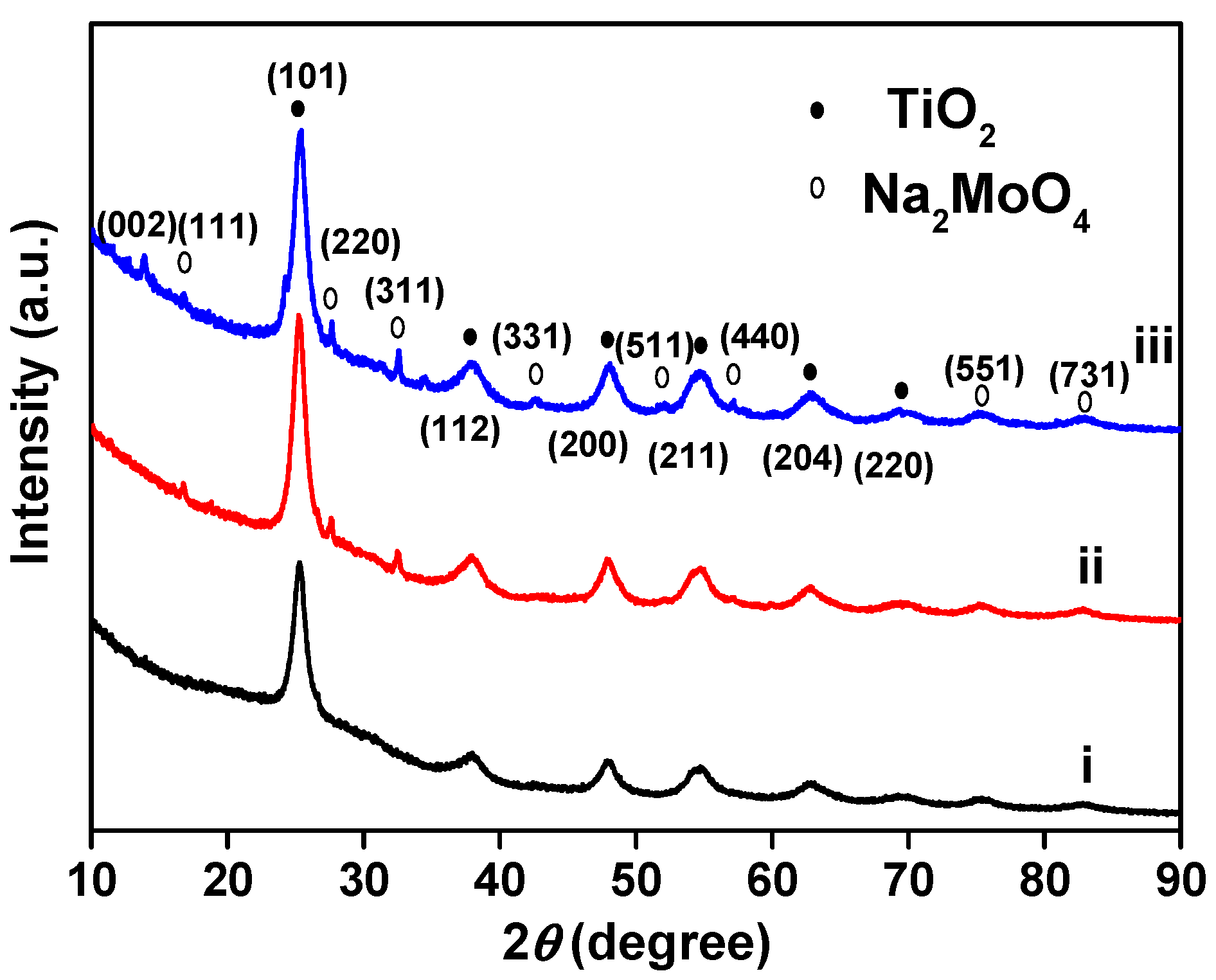
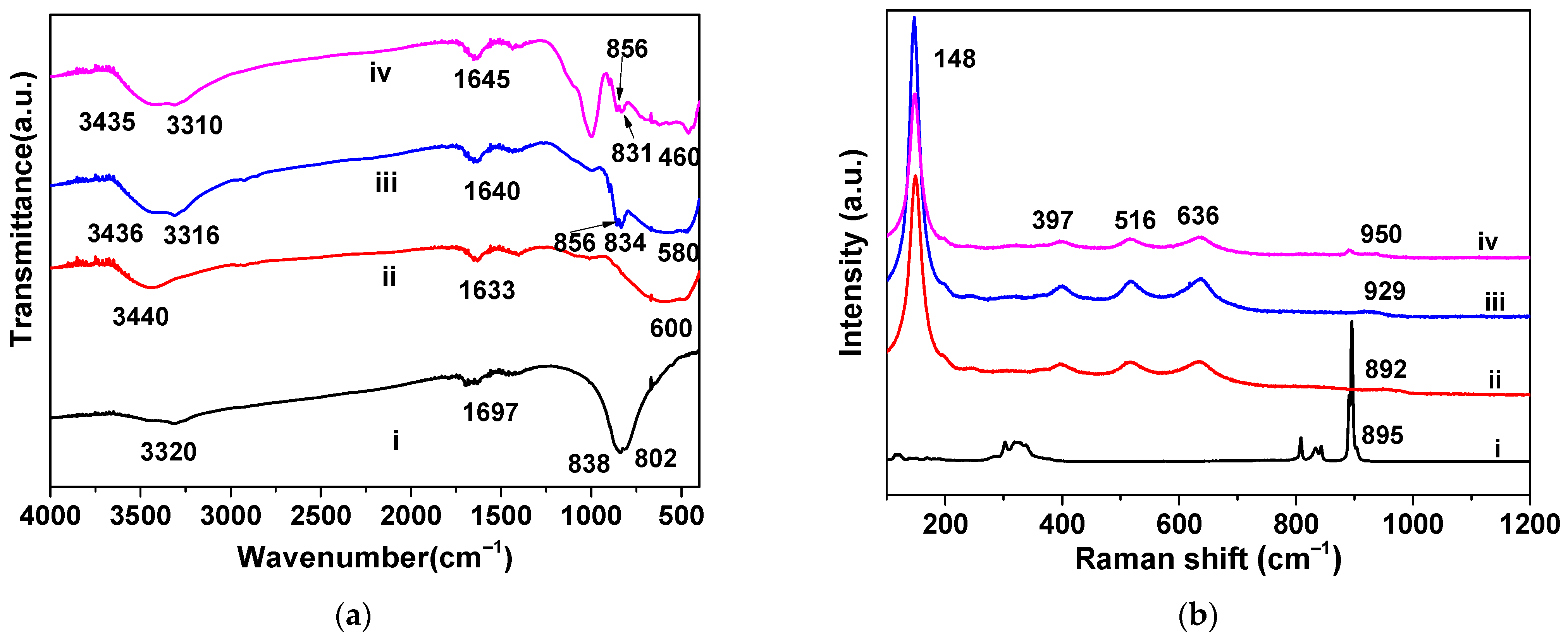
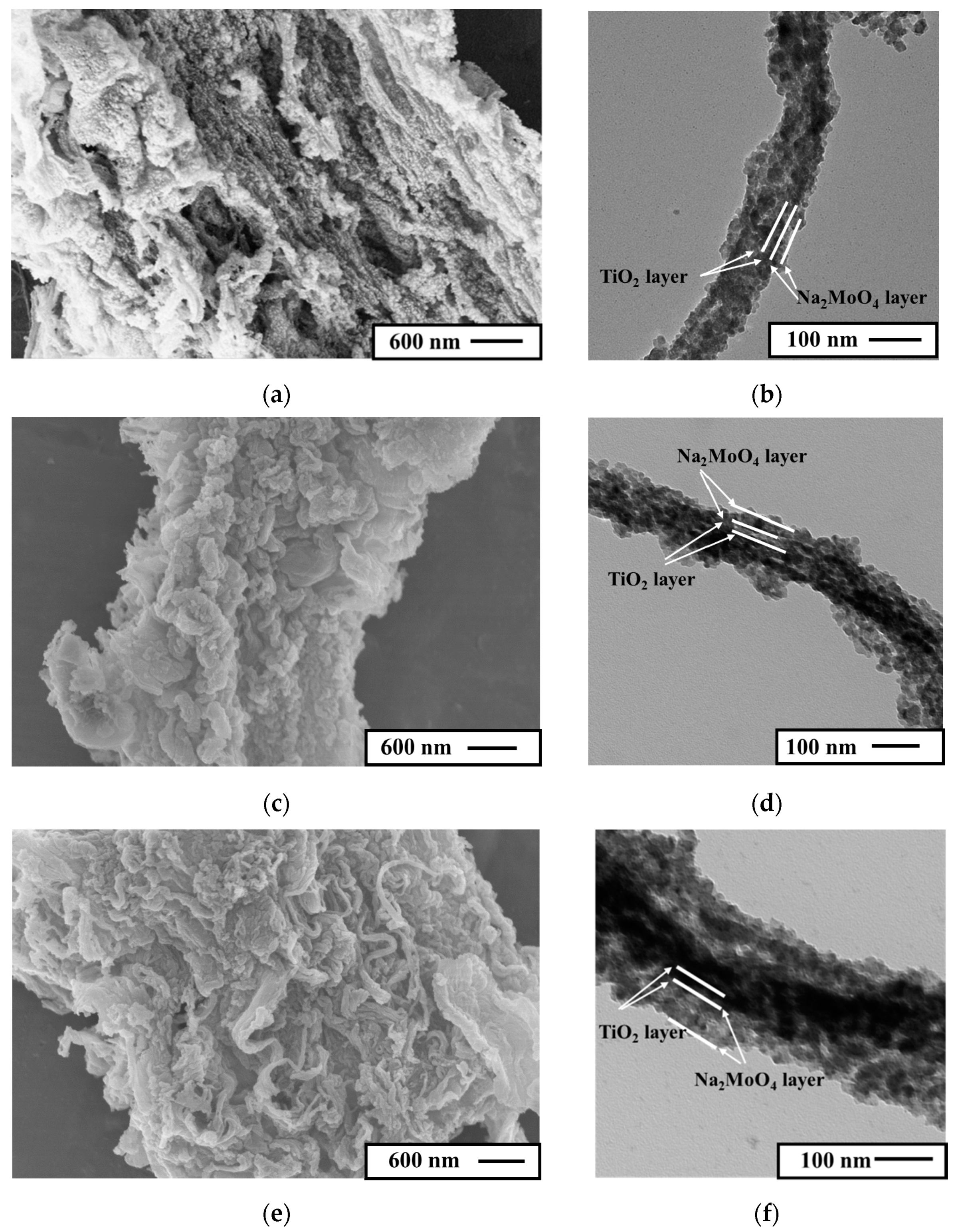
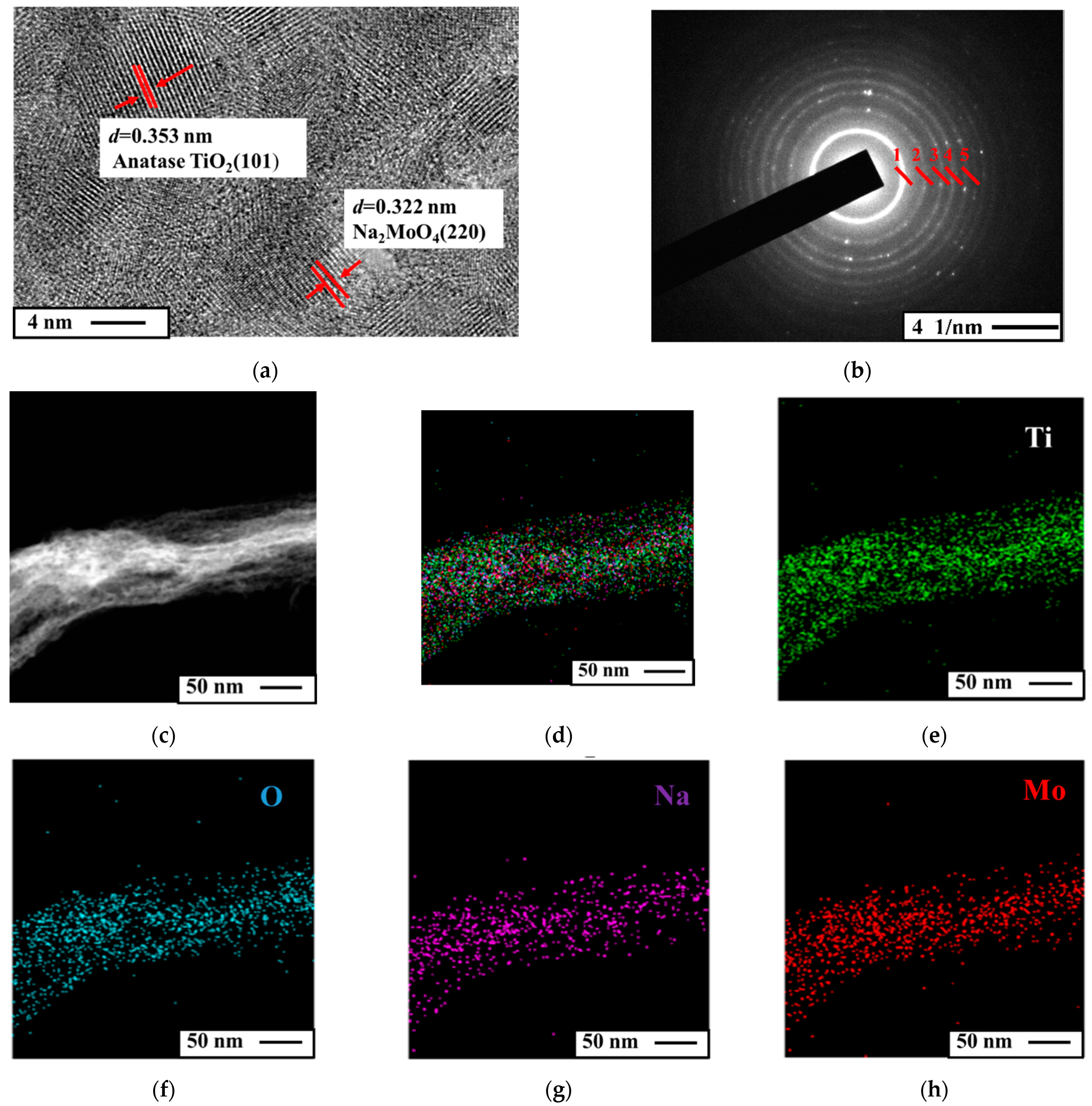
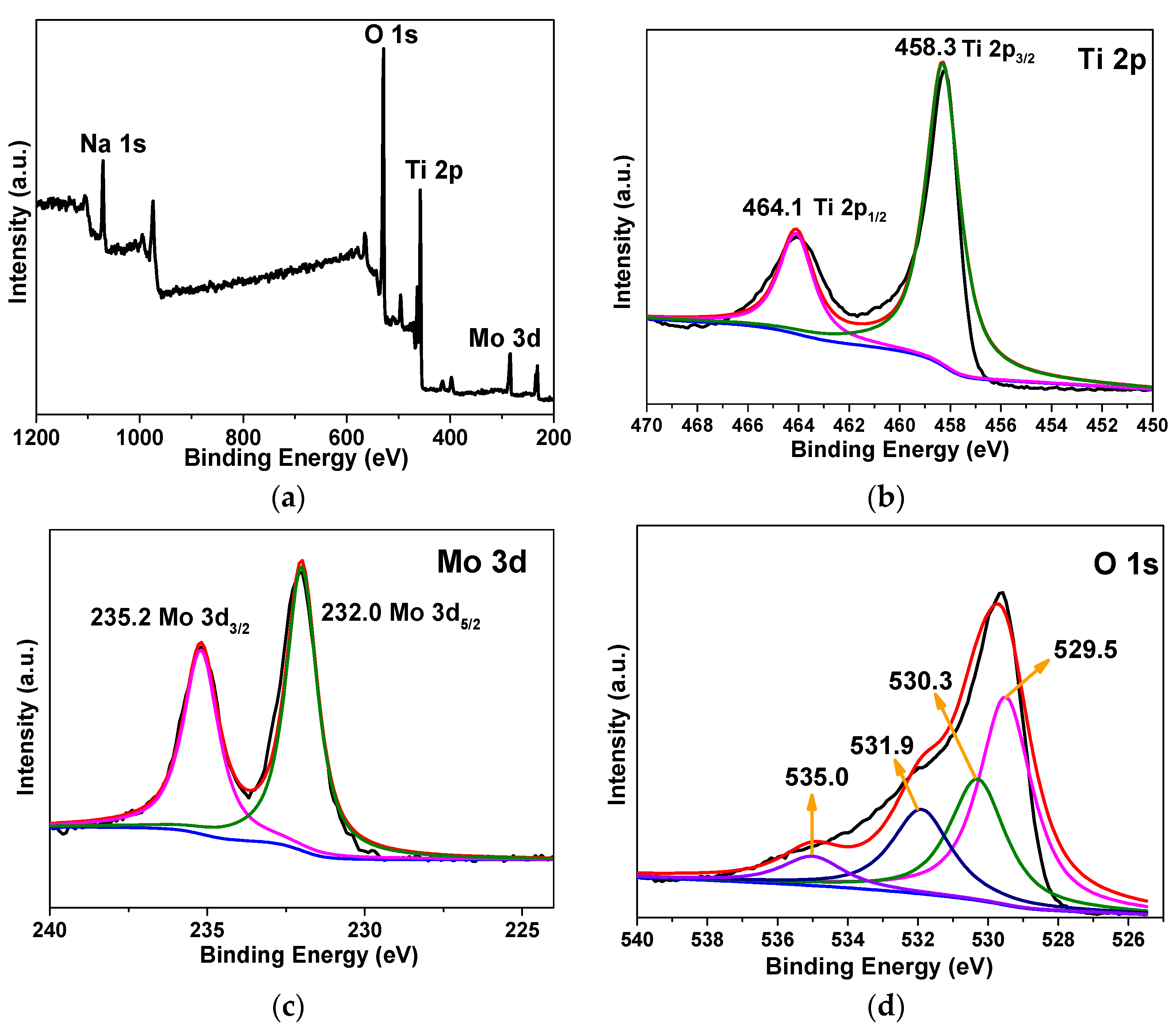
3.1. Structural Characterizations of the Nanotubular Na2MoO4/TiO2 Composites
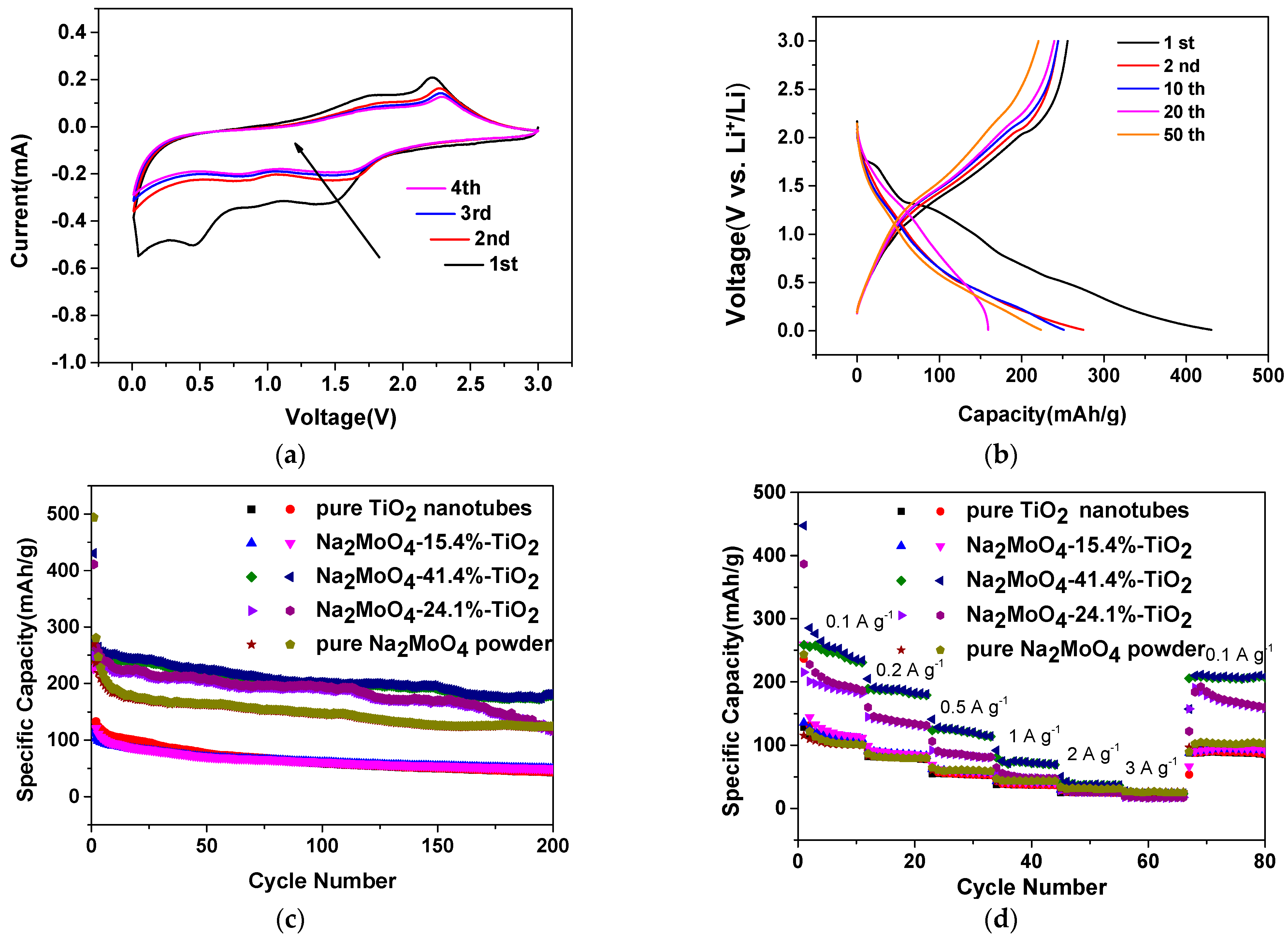
3.2. Electrochemical Study of the Nanotubular Na2MoO4/TiO2 Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Lu, J.; Chen, Z.; Ma, Z.; Pan, F.; Curtiss, L.A.; Amine, K. The role of nanotechnology in the development of battery materials for electric vehicles. Nat. Nanotechnol. 2016, 11, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.-S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Bilich, A.; Langham, K.; Geyer, R.; Goyal, L.; Hansen, J.; Krishnan, A.; Bergesen, J.; Sinha, P. Life cycle assessment of solar photovoltaic microgrid systems in off-grid communities. Environ. Sci. Technol. 2017, 51, 1043–1052. [Google Scholar] [CrossRef]
- Gao, B.; Li, X.; Ding, K.; Huang, C.; Li, Q.; Chu, P.K.; Huo, K. Recent progress in nanostructured transition metal nitrides for advanced electrochemical energy storage. J. Mater. Chem. A 2019, 7, 14–37. [Google Scholar] [CrossRef]
- Shao, J.; Zhou, H.; Feng, J.; Zhu, M.; Yuan, A. Facile synthesis of MOF-derived hollow NiO microspheres integrated with graphene foam for improved lithium-storage properties. J. Alloys Compd. 2019, 784, 869–876. [Google Scholar] [CrossRef]
- Qiu, Y.; Huang, H.; Song, W.; Gan, Y.; Wang, K.; Zhang, J.; Xia, Y.; Liang, C.; He, X.; Zhang, W. In-situ electrolytic synthesis and superior lithium storage capability of Ni–NiO/C nanocomposite by sacrificial nickel anode in molten carbonates. J. Alloys Compd. 2020, 834, 155111. [Google Scholar] [CrossRef]
- Sheng, L.; Liang, S.; Wei, T.; Chang, J.; Jiang, Z.; Zhang, L.; Zhou, Q.; Zhou, J.; Jiang, L.; Fan, Z. Space-confinement of MnO nanosheets in densely stacked graphene: Ultra-high volumetric capacity and rate performance for lithium-ion batteries. Energy Storage Mater. 2018, 12, 94–102. [Google Scholar] [CrossRef]
- Ko, I.-H.; Jin, A.; Kim, M.K.; Park, J.-H.; Kim, H.S.; Yu, S.-H.; Sung, Y.-E. The keys for effective distribution of intergranular voids of peapod-like MnO@C core-shell for lithium ion batteries. J. Alloys Compd. 2020, 817, 152760. [Google Scholar] [CrossRef]
- Kong, Y.; Jiao, R.; Zeng, S.; Cui, C.; Li, H.; Xu, S.; Wang, L. Study on the synthesis of Mn3O4 nanooctahedrons and their performance for lithium ion batteries. Nanomaterials 2020, 10, 367. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Yang, J.; Yuan, Z.; Guo, L.; Sui, Z.; Wang, M. Binder-free 3D porous Fe3O4–Fe2P–Fe@C films as high-performance anode materials for lithium-ion batteries. Ceram. Int. 2020, 46, 17469–17477. [Google Scholar] [CrossRef]
- Hu, Z.; Cui, H.; Li, J.; Lei, G.; Li, Z. Constructing three-dimensional Li-transport channels within the Fe3O4@SiO2@RGO composite to improve its electrochemical performance in Li-ion batteries. Ceram. Int. 2020, 46, 18868–18877. [Google Scholar] [CrossRef]
- Qu, D.; Sun, Z.; Xu, J.; Song, Z.; Kong, H.; Zhao, B.; Dong, X.; Niu, L. Rational construction of 2D Fe3O4@carbon core-shell nanosheets as advanced anode materials for high-performance lithium-ion half/full cells. Chem. Eur. J. 2020, 26, 8121–8128. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Fu, B.; Yuan, G.; Ma, M.; Jin, H.; Xie, S.; Li, J. Three-dimensional mesoporous γ-Fe2O3@carbon nanofiber network as high performance anode material for lithium- and sodium-ion batteries. Nanotechnology 2020, 31, 155401. [Google Scholar]
- Wang, Y.; Guo, W.; Yang, Y.; Yu, Y.; Li, Q.; Wang, D.; Zhang, F. Rational design of SnO2@C@MnO2 hierarchical hollow hybrid nanospheres for a Li-ion battery anode with enhanced performances. Electrochim. Acta 2018, 262, 1–8. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, L.; Xu, G.; Zhang, X.; Zhao, A. Synthesis of cobalt-doped V2O3 with a hierarchical yolk–shell structure for high-performance lithium-ion batteries. CrystEngComm 2020, 22, 1705–1711. [Google Scholar]
- Ren, Y.; Ma, Z.; Bruce, P.G. Ordered mesoporous metal oxides: Synthesis and applications. Chem. Soc. Rev. 2012, 41, 4909–4927. [Google Scholar] [CrossRef]
- Chen, N.; Gao, Y.; Zhang, M.; Meng, X.; Wang, C.; Wei, Y.; Du, F.; Chen, G. Electrochemical properties and sodium-storage mechanism of Ag2Mo2O7 as the anode material for sodium-ion batteries. Chem. Eur. J. 2016, 22, 7248–7254. [Google Scholar] [CrossRef]
- Zhang, J.; Li, R.; Chen, Q.; Zhao, G.; Jia, J. Porous carbon-coated Li2MoO4 as high-performance anode materials for lithium-ion batteries. Mater. Lett. 2018, 233, 302–305. [Google Scholar] [CrossRef]
- Verma, R.; Ramanujam, K.; Varadaraju, U.V. Nanocrystalline Na2Mo2O7: A new high performance anode material. Electrochim. Acta 2016, 215, 192–199. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Y.; Dong, Y.; Kuang, Q.; Liang, Z.; Lin, X.; Yan, D.; Liu, H. Synthesis of carbon-coated nanoplate α-Na2MoO4 and its electrochemical lithiation process as anode material for lithium-ion batteries. Electrochim. Acta 2015, 154, 94–101. [Google Scholar] [CrossRef]
- Li, J.; Chen, Q.; Zhou, Q.; Shen, N.; Li, M.; Guo, C.; Zhang, L. Engineering Na-Mo-O/graphene oxide composites with enhanced electrochemical performance for lithium ion batteries. ChemistryOpen 2019, 8, 1225–1229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, C.; Liu, T.; Peng, N.; Zheng, R.; Zhang, J.; Cheng, X.; Yu, H.; Long, N.; Shu, J. Facile synthesis of Y2(MoO4)3 nanowires as anode materials towards enhanced lithium storage performance. J. Electroanal. Chem. 2019, 841, 111–118. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.; He, J.; Wang, T.; Yang, J.; Shen, X.; Cao, L.; Huang, J. MoO3/carbon dots composites for Li-ion battery anodes. ChemNanoMat 2019, 5, 921–925. [Google Scholar] [CrossRef]
- Qi, D.; Zhang, Y.; Jia, D.; Huang, J. Self-assembly approach for synthesis of nanotubular molybdenum trioxide/titania composite anode for lithium-ion batteries. Energy Technol. 2017, 5, 2015–2025. [Google Scholar] [CrossRef]
- Zhang, L.; He, W.; Ling, M.; Shen, K.; Liu, Y.; Guo, S. Cu@MoO2@C nanocomposite with stable yolk-shell structure for high performance lithium-ion batteries. J. Alloys Compd. 2018, 768, 714–721. [Google Scholar] [CrossRef]
- Wang, W.; Shi, G.; Cai, H.; Zhao, C.; Wu, J.; Yu, Y.; Hu, J.; Fang, Z.; Yan, J.; Liu, B. Yolk-shell structured Mo/MoO2 composite microspheres function as high-performance anode materials for lithium-ion batteries. J. Alloys Compd. 2019, 792, 191–202. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, Y.; Wang, S.; Shi, J.; Liu, X.; Li, L. Rational construction of MoS2/Mo2N/C hierarchical porous tubular nanostructures for enhanced lithium storage. J. Mater. Chem. A 2019, 7, 23886–23894. [Google Scholar] [CrossRef]
- Xia, S.; Wang, Y.; Liu, Y.; Wu, C.; Wu, M.; Zhang, H. Ultrathin MoS2 nanosheets tightly anchoring onto nitrogen-doped graphene for enhanced lithium storage properties. Chem. Eng. J. 2018, 332, 431–439. [Google Scholar]
- Song, Y.; Wang, H.; Li, Z.; Ye, N.; Wang, L.; Liu, Y. Fe2(MoO4)3 nanoparticle-anchored MoO3 nanowires: Strong coupling via the reverse diffusion of heteroatoms and largely enhanced lithium storage properties. RSC Adv. 2015, 5, 16386–16393. [Google Scholar] [CrossRef]
- Han, C.; Ren, X.; Li, Q.; Luo, W.; Huang, L.; Zhou, L.; Mai, L. Graphene oxide-decorated Fe2(MoO4)3 microflowers as a promising anode for lithium and sodium storage. Nano. Res. 2018, 11, 1285–1293. [Google Scholar] [CrossRef]
- Xiao, K.; Xia, L.; Liu, G.; Wang, S.; Ding, L.-X.; Wang, H. Honeycomb-like NiMoO4 ultrathin nanosheet arrays for high-performance electrochemical energy storage. J. Mater. Chem. A 2015, 3, 6128–6135. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, S.; Zeng, H.; Zhao, H.; Sun, W.; Jiang, M.; Feng, C.; Liu, J.; Zhou, T.; Zheng, Y.; et al. Hierarchical porous NiO/β-NiMoO4 heterostructure as superior anode material for lithium storage. ChemPlusChem 2018, 83, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Liu, T.; Zheng, R.; Peng, N.; Zhang, J.; Cheng, X.; Yu, H.; Shui, M.; Shu, J. La2(MoO4)3@C as novel anode for lithium ion battery: Structural and chemical evolutions upon electrochemical cycling. Ceram. Int. 2019, 45, 7754–7760. [Google Scholar] [CrossRef]
- Tang, M.; Niu, Y.; Huang, J.; Li, C.M. Self-assembled flower-like ZnMoO4/graphene composite materials as anode in lithium-ion batteries. ChemistrySelect 2017, 2, 2144–2149. [Google Scholar] [CrossRef]
- Sharma, N.; Shaju, K.M.; Subba Rao, G.V.; Chowdari, B.V.R.; Dong, Z.L.; White, T.J. Carbon-coated nanophase CaMoO4 as anode material for Li ion batteries. Chem. Mater. 2004, 16, 504–512. [Google Scholar] [CrossRef]
- Menéndez, M.; Alvarez, P.; Botas, C.; Nacimiento, F.; Alcántara, R.; Tirado, J.L.; Ortiz, G.F. Self-organized amorphous titania nanotubes with deposited graphene film like a new heterostructured electrode for lithium ion batteries. J. Power Sources 2014, 248, 886–893. [Google Scholar] [CrossRef]
- Cabello, M.; Ortiz, G.F.; López, M.C.; Lavela, P.; Alcántara, R.; Tirado, J.L. Self-assembled Li4Ti5O12/TiO2/Li3PO4 for integrated Li–ion microbatteries. Electrochem. Commun. 2015, 56, 61–64. [Google Scholar] [CrossRef]
- Tang, K.; Farooqi, S.A.; Wang, X.; Yan, C. Recent progress on molybdenum oxides for rechargeable batteries. ChemSusChem 2019, 12, 755–771. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, W.; Liu, X.; Mei, Y.; Huang, Y. Nanostructured Mo-based electrode materials for electrochemical energy storage. Chem. Soc. Rev. 2015, 44, 2376–2404. [Google Scholar] [CrossRef]
- Knothe, D.W.; Van Riper, G.G. Acute toxicity of sodium molybdate dihydrate (Molyhibit 100) to selected saltwater organisms. Bull. Environ. Contam. Toxicol. 1988, 40, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Diamantino, T.C.; Guilhermino, L.; Almeida, E.; Soares, A.M. Toxicity of sodium molybdate and sodium dichromate to Daphnia magna straus evaluated in acute, chronic, and acetylcholinesterase inhibition tests. Ecotoxicol. Environ. Saf. 2000, 45, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Deng, S.; Fu, H. Sodium molybdate as a corrosion inhibitor for aluminium in H3PO4 solution. Corros. Sci. 2011, 53, 2748–2753. [Google Scholar] [CrossRef]
- Wubah, T.O.; Rubio, S.; Tirado, J.L.; Ortiz, G.F.; Akoi, B.J.; Huang, J.; Li, Q. Waste Pd/Fish-Collagen as anode for energy storage. Renew. Sustain. Energy Rev. 2020, 131, 109968. [Google Scholar] [CrossRef]
- Cabello, M.; Chyrka, T.; Klee, R.; Aragon, M.J.; Bai, X.; Lavela, P.; Vasylchenko, G.M.; Alcantara, R.; Tirado, J.L.; Ortiz, G.F. Treasure Na-ion anode from trash coke by adept electrolyte selection. J. Power Sources 2017, 347, 127–135. [Google Scholar] [CrossRef]
- Li, S.; Huang, J. Cellulose-rich nanofiber-based functional nanoarchitectures. Adv. Mater. 2016, 28, 1143–1158. [Google Scholar] [CrossRef]
- Wang, K.; Wang, M.; Huang, J. Natural-cellulose-derived tin-nanoparticle/carbon-nanofiber composite as anodic material in lithium-ion batteries. ChemNanoMat 2016, 2, 1040–1046. [Google Scholar] [CrossRef]
- Qi, D.; Li, S.; Chen, Y.; Huang, J. A hierarchical carbon@TiO2@MoS2 nanofibrous composite derived from cellulose substance as an anodic material for lithium-ion batteries. J. Alloys Compd. 2017, 728, 506–517. [Google Scholar] [CrossRef]
- Zhou, H.; Xia, X.; Lv, P.; Zhang, J.; Hou, X.; Zhao, M.; Ao, K.; Wang, D.; Lu, K.; Qiao, H.; et al. C@TiO2/MoO3 composite nanofibers with 1T-phase MoS2 nanograin dopant and stabilized interfaces as anodes for Li- and Na-ion batteries. ChemSusChem 2018, 11, 4060–4070. [Google Scholar] [CrossRef]
- Nakagaki, S.; Bail, A.; dos Santos, V.C.; de Souza, V.H.R.; Vrubel, H.; Nunes, F.S.; Ramos, L.P. Use of anhydrous sodium molybdate as an efficient heterogeneous catalyst for soybean oil methanolysis. Appl. Catal. A 2008, 351, 267–274. [Google Scholar] [CrossRef]
- Pillai, M.V.P.; Pradeep, T.; Bushiri, M.J.; Jayasree, R.S.; Nayar, V.U. Vibrational spectroscopic studies of FeClMoO4, Na2MoO4 and Na2MoO4 ·2H2O:D2O. Spectrochim. Acta A 1997, 53, 867–876. [Google Scholar] [CrossRef]
- Chatterjee, S.; Barik, S.K.; Choudhary, R.N.P. Studies of structural, spectroscopic and electrical properties of sodium molybdate ceramics. J. Mater. Sci. Mater. Electron. 2013, 24, 3359–3364. [Google Scholar] [CrossRef]
- Song, X.; Hu, Y.; Zheng, M.; Wei, C. Solvent-free in situ synthesis of g-C3N4/{001}TiO2 composite with enhanced UV- and visible-light photocatalytic activity for NO oxidation. Appl. Catal. B 2016, 182, 587–597. [Google Scholar] [CrossRef]
- Lin, Z.; Yu, B.; Huang, J. Cellulose-derived hierarchical g-C3N4/TiO2-nanotube heterostructured composites with enhanced visible-light photocatalytic performance. Langmuir 2020, 36, 5967–5978. [Google Scholar] [CrossRef]
- Shao, Y.; Cao, C.; Chen, S.; He, M.; Fang, J.; Chen, J.; Li, X.; Li, D. Investigation of nitrogen doped and carbon species decorated TiO2 with enhanced visible light photocatalytic activity by using chitosan. Appl. Catal. B 2015, 179, 344–351. [Google Scholar] [CrossRef]
- Ruso, J.M.; Verdinelli, V.; Hassan, N.; Pieroni, O.; Messina, P.V. Enhancing CaP biomimetic growth on TiO2 cuboids nanoparticles via highly reactive facets. Langmuir 2013, 29, 2350–2358. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, F.; Liu, Y.; Hong, Y.; Liu, P.; Ni, L. Construction of TiO2 hollow nanosphere/g-C3N4 composites with superior visible-light photocatalytic activity and mechanism insight. J. Ind. Eng. Chem. 2016, 41, 130–140. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, J.; Huang, J. Hierarchical nanofibrous anatase-titania–cellulose composite and its photocatalytic property. CrystEngComm 2014, 16, 464–471. [Google Scholar] [CrossRef]
- Colombo, K.; Maruyama, S.; Yamamoto, C.; Wypych, F. Intercalation of molybdate ions into Ni/Zn layered double hydroxide salts: Synthesis, characterization, and preliminary catalytic activity in methyl transesterification of soybean oil. J. Brazil. Chem. Soc. 2016, 28, 1315–1322. [Google Scholar]
- McCafferty, E.; Wightman, J.P. Determination of the concentration of surface hydroxyl groups on metal oxide films by a quantitative XPS method. Surf. Interface Anal. 1998, 26, 549–564. [Google Scholar] [CrossRef]
- Huang, J.; Ichinose, I.; Kunitake, T.; Nakao, A. Preparation of nanoporous titania films by surface sol−gel process accompanied by low-temperature oxygen plasma treatment. Langmuir 2002, 18, 9048–9053. [Google Scholar] [CrossRef]
- Liu, X.; Li, J.; Zhang, Y.; Huang, J. Bioinspired hierarchical nanotubular titania immobilized with platinum nanoparticles for photocatalytic hydrogen production. Chem. Eur. J. 2015, 21, 7345–7349. [Google Scholar] [CrossRef] [PubMed]
- Cotte, S.; Pelé, V.; Pecquenard, B.; Le Cras, F.; Grissa, R.; Bourgeois, L.; Sougrati, M.T.; Martinez, H. Iron molybdate thin films prepared by sputtering and their electrochemical behavior in Li batteries. J. Alloys Compd. 2018, 735, 1454–1462. [Google Scholar] [CrossRef] [Green Version]
- Ma, F.; Yuan, A.; Xu, J.; Hu, P. Porous α-MoO3/MWCNT nanocomposite synthesized via a surfactant-assisted solvothermal route as a lithium-ion-battery high-capacity anode material with excellent rate capability and cyclability. ACS Appl. Mater. Interfaces 2015, 7, 15531–15541. [Google Scholar] [CrossRef]
- Li, Y.; Hu, Y.; Shen, J.; Jiang, H.; Min, G.; Qiu, S.; Song, Z.; Sun, Z.; Li, C. Rapid flame synthesis of internal Mo(6+) doped TiO2 nanocrystals in situ decorated with highly dispersed MoO3 clusters for lithium ion storage. Nanoscale 2015, 7, 18603–18611. [Google Scholar] [CrossRef]
- Liu, C.; Jiang, Z.; Tong, Z.; Li, Y.; Yang, D. Biomimetic synthesis of inorganic nanocomposites by a de novo designed peptide. RSC Adv. 2014, 4, 434–441. [Google Scholar] [CrossRef]
- Schindler, M.; Hawthorne, F.C.; Freund, M.S.; Burns, P.C. XPS spectra of uranyl minerals and synthetic uranyl compounds. II: The O 1s spectrum. Geochim. Cosmochim. Acta 2009, 73, 2488–2509. [Google Scholar] [CrossRef]
- Liu, X.; Lyu, Y.; Zhang, Z.; Li, H.; Hu, Y.; Wang, Z.; Zhao, Y.; Kuang, Q.; Dong, Y.; Liang, Z.; et al. Nanotube Li2MoO4: A novel and high-capacity material as a lithium-ion battery anode. Nanoscale 2014, 6, 13660–13667. [Google Scholar] [CrossRef]
- Nazar, L.F.; Goward, G.; Leroux, F.; Duncan, M.; Huang, H.; Kerr, T.; Gaubicher, J. Nanostructured materials for energy storage. Int. J. Inorg. Mater. 2001, 3, 191–200. [Google Scholar] [CrossRef]
- Leroux, F.; Nazar, L.F. Uptake of lithium by layered molybdenum oxide and its tin exchanged derivatives: High volumetric capacity materials. Solid State Ionics 2000, 133, 37–50. [Google Scholar] [CrossRef]
- Leroux, F.; Goward, G.R.; Power, W.P.; Nazar, L.F. Understanding the nature of low-potential Li uptake into high volumetric capacity molybdenum oxides. Electrochem. Solid-State Lett. 1998, 1, 255–258. [Google Scholar] [CrossRef]
- Qiu, S.; Lu, G.; Liu, J.; Lyu, H.; Hu, C.; Li, B.; Yan, X.; Guo, J.; Guo, Z. Enhanced electrochemical performances of MoO2 nanoparticles composited with carbon nanotubes for lithium-ion battery anodes. RSC Adv. 2015, 5, 87286–87294. [Google Scholar] [CrossRef]
- Cherian, C.T.; Reddy, M.V.; Haur, S.C.; Chowdari, B.V. Interconnected network of CoMoO(4) submicrometer particles as high capacity anode material for lithium ion batteries. ACS Appl. Mater. Interfaces 2013, 5, 918–923. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.; Lin, Z.; Huang, J. A Bio-Inspired Nanotubular Na2MoO4/TiO2 Composite as a High-Performance Anodic Material for Lithium-Ion Batteries. Materials 2021, 14, 357. https://doi.org/10.3390/ma14020357
Yu B, Lin Z, Huang J. A Bio-Inspired Nanotubular Na2MoO4/TiO2 Composite as a High-Performance Anodic Material for Lithium-Ion Batteries. Materials. 2021; 14(2):357. https://doi.org/10.3390/ma14020357
Chicago/Turabian StyleYu, Bo, Zehao Lin, and Jianguo Huang. 2021. "A Bio-Inspired Nanotubular Na2MoO4/TiO2 Composite as a High-Performance Anodic Material for Lithium-Ion Batteries" Materials 14, no. 2: 357. https://doi.org/10.3390/ma14020357
APA StyleYu, B., Lin, Z., & Huang, J. (2021). A Bio-Inspired Nanotubular Na2MoO4/TiO2 Composite as a High-Performance Anodic Material for Lithium-Ion Batteries. Materials, 14(2), 357. https://doi.org/10.3390/ma14020357