Aluminothermic Reduction of Manganese Oxide from Selected MnO-Containing Slags
Abstract
1. Introduction
2. Experimental Procedure
2.1. Material Preparation
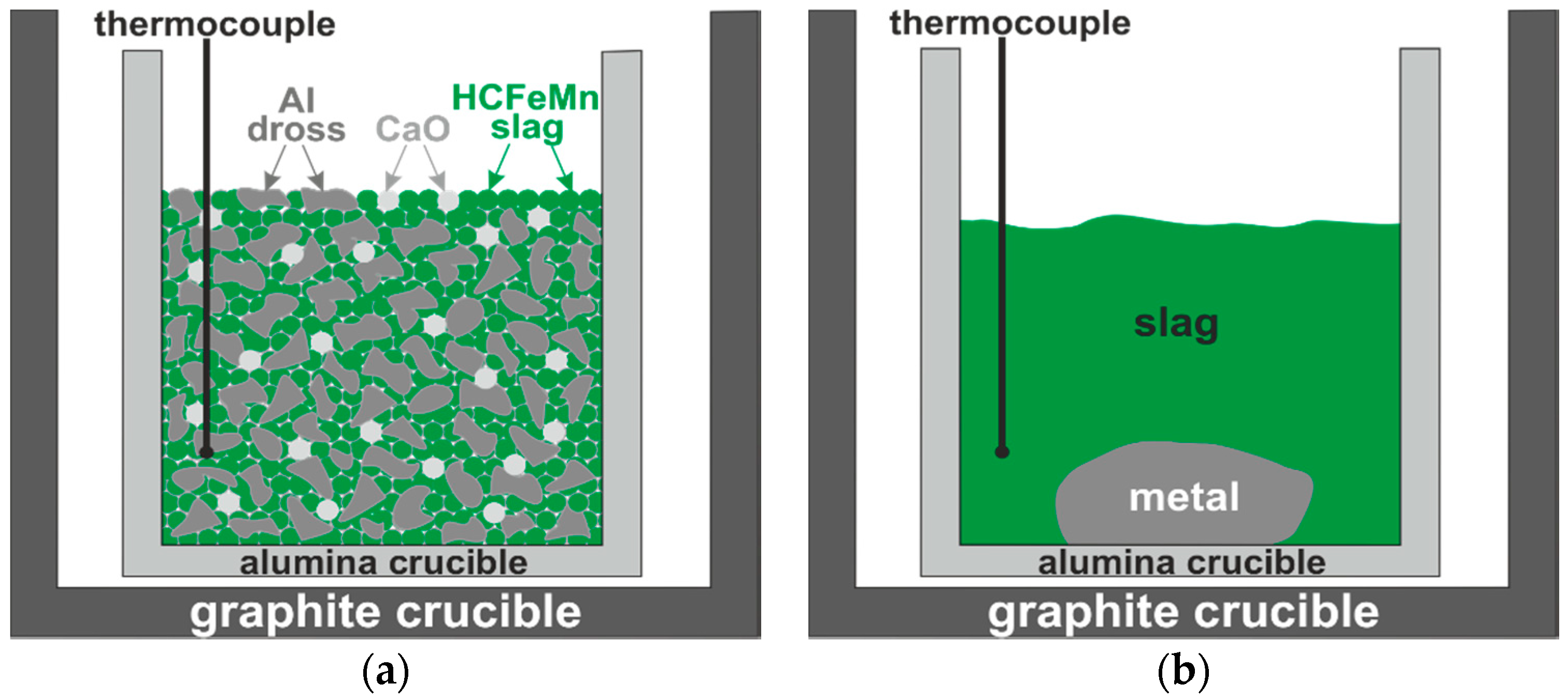
2.2. Aluminothermic Reduction
3. Results and Discussion
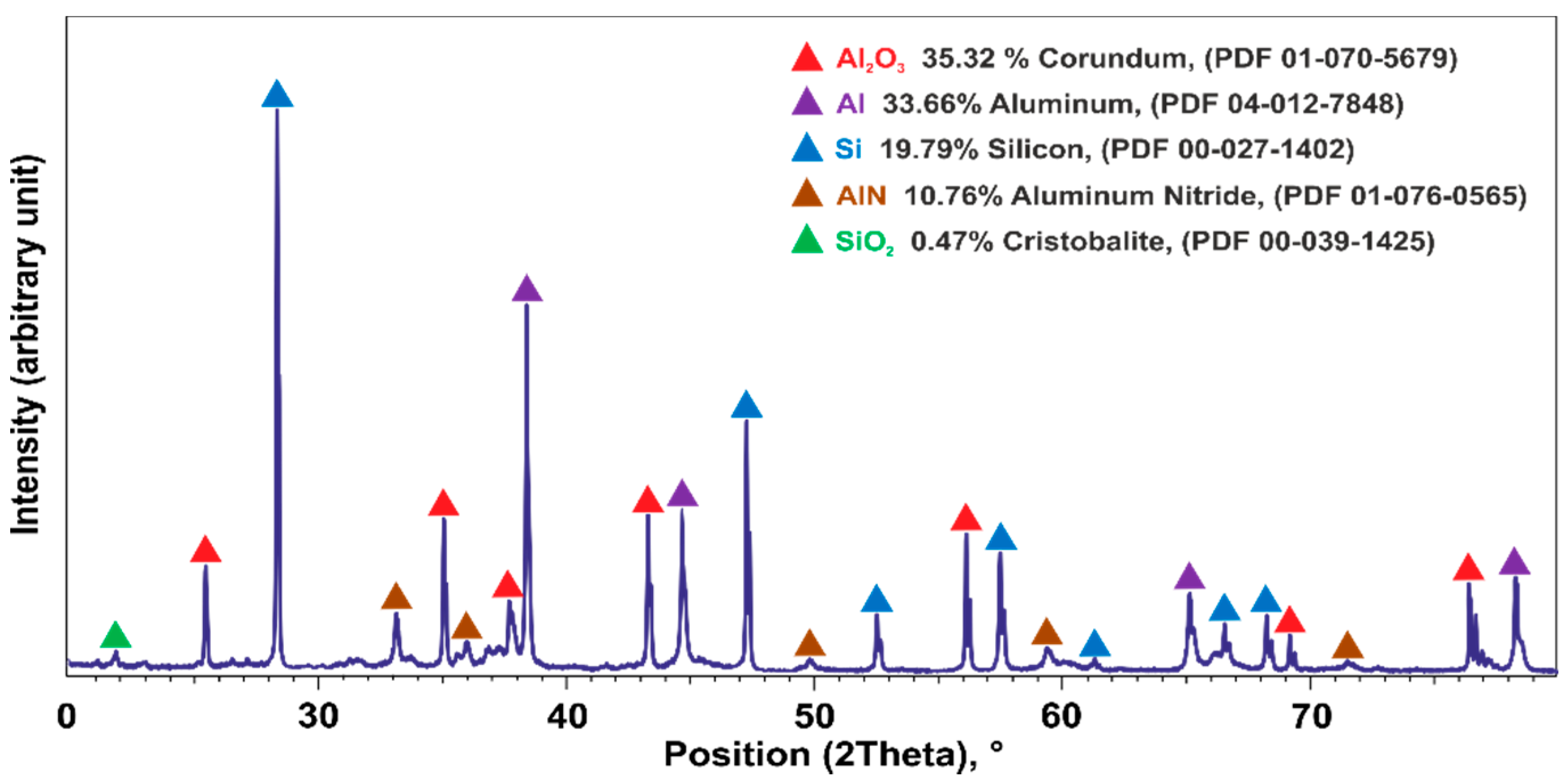
3.1. Reactant Materials Characteristics
3.2. Characteristics of the Products
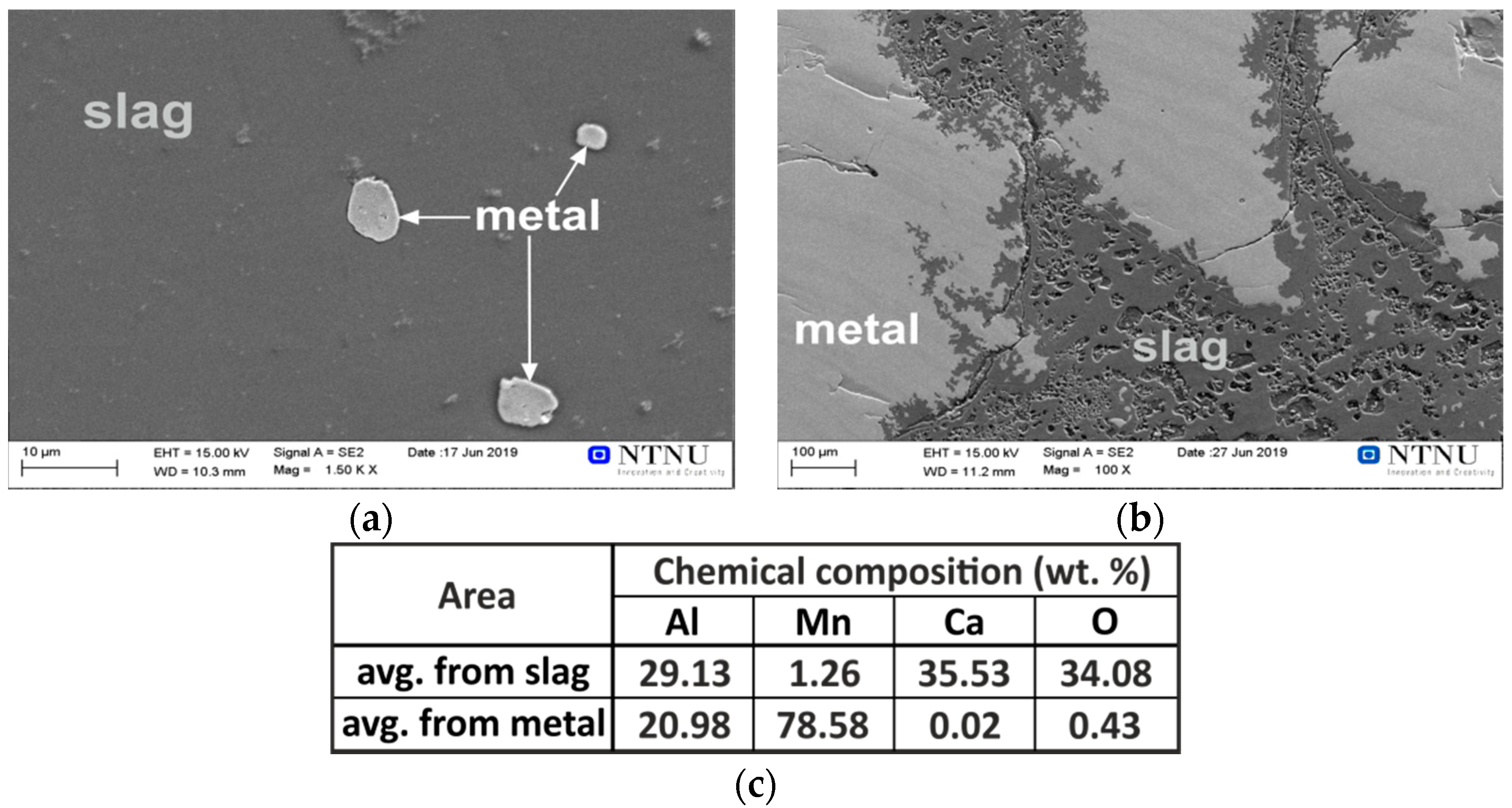
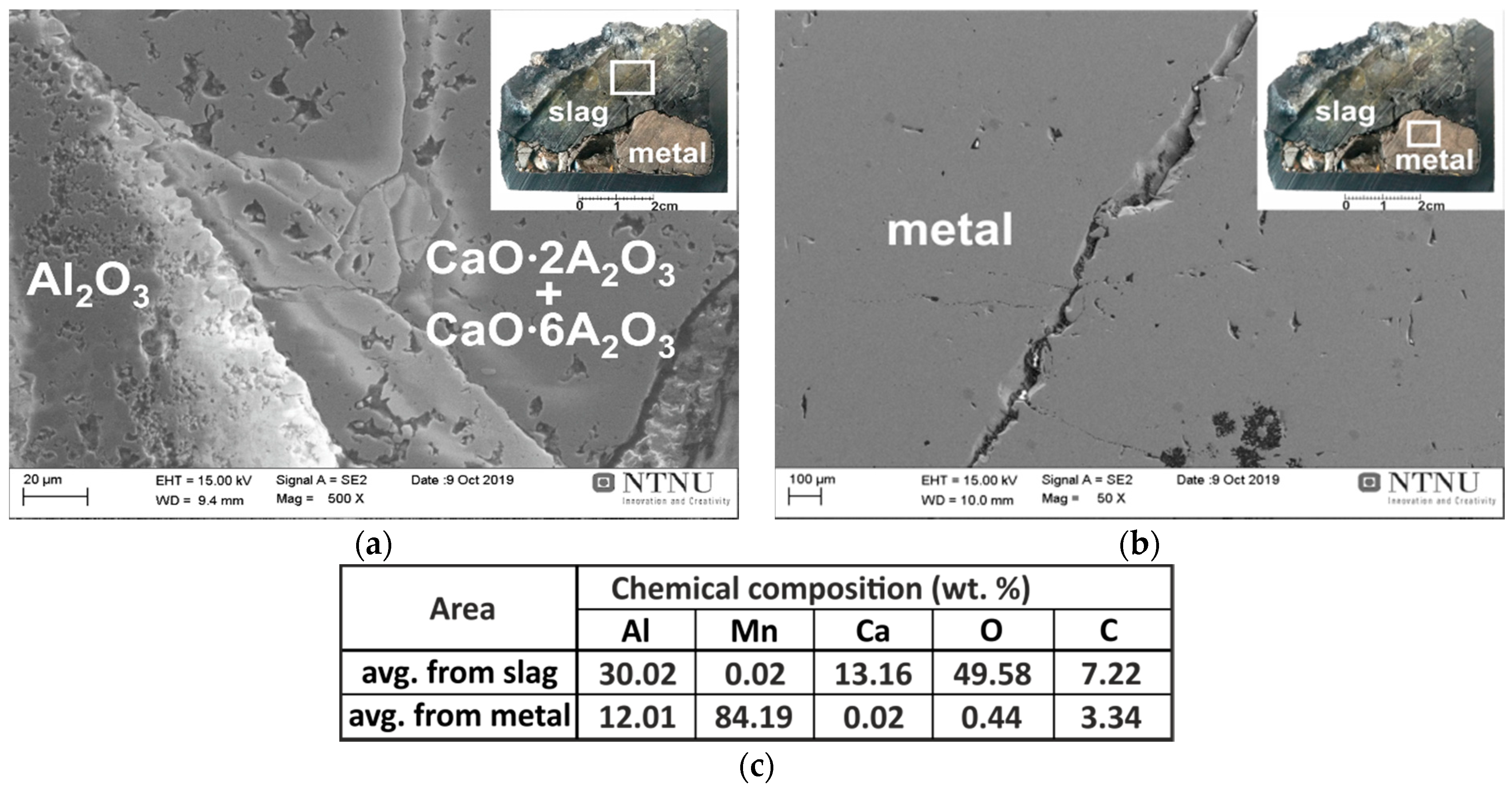
3.2.1. Interaction of Pure Al with Synthetic Slag
3.2.2. Interaction of Al Dross with Synthetic Slag
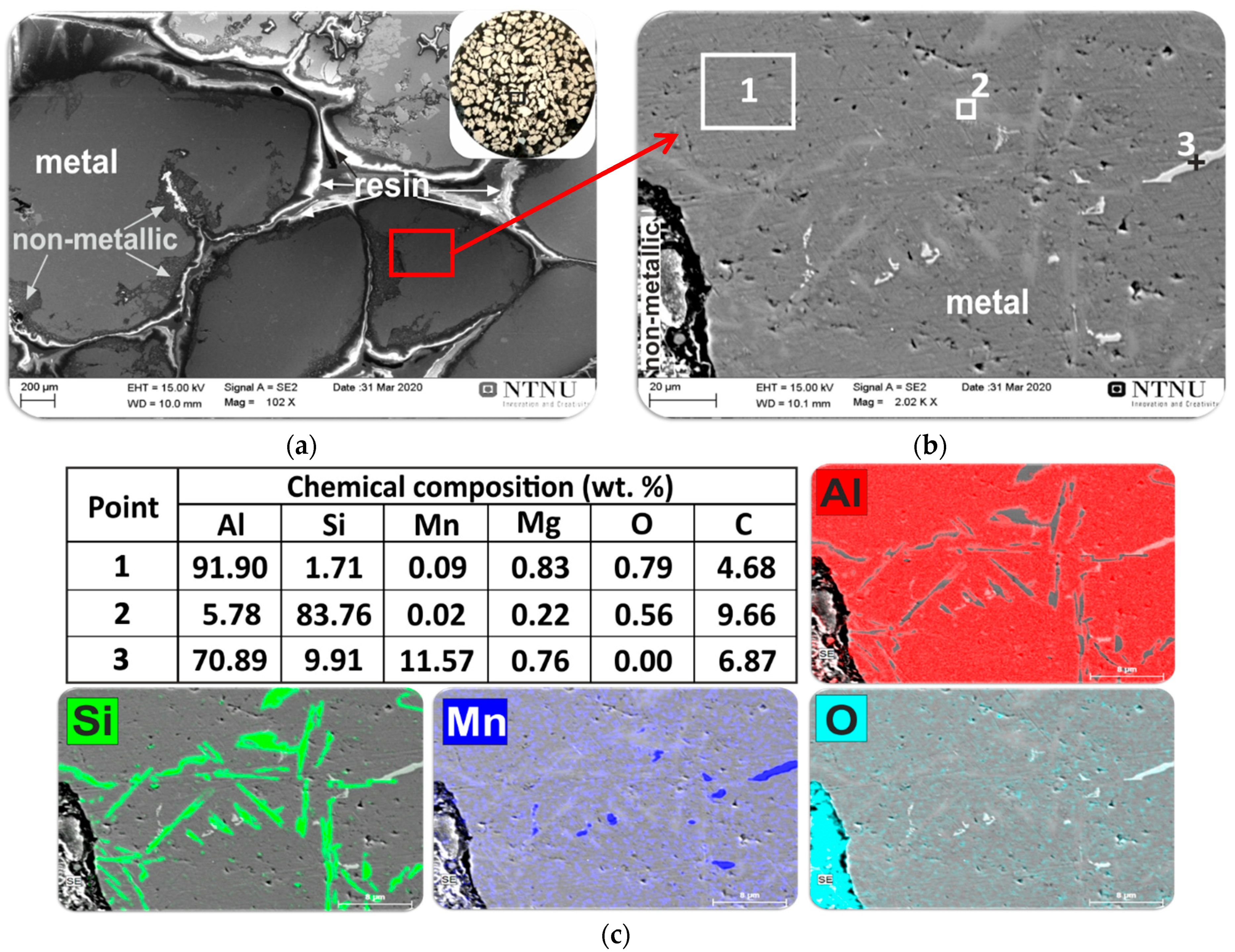
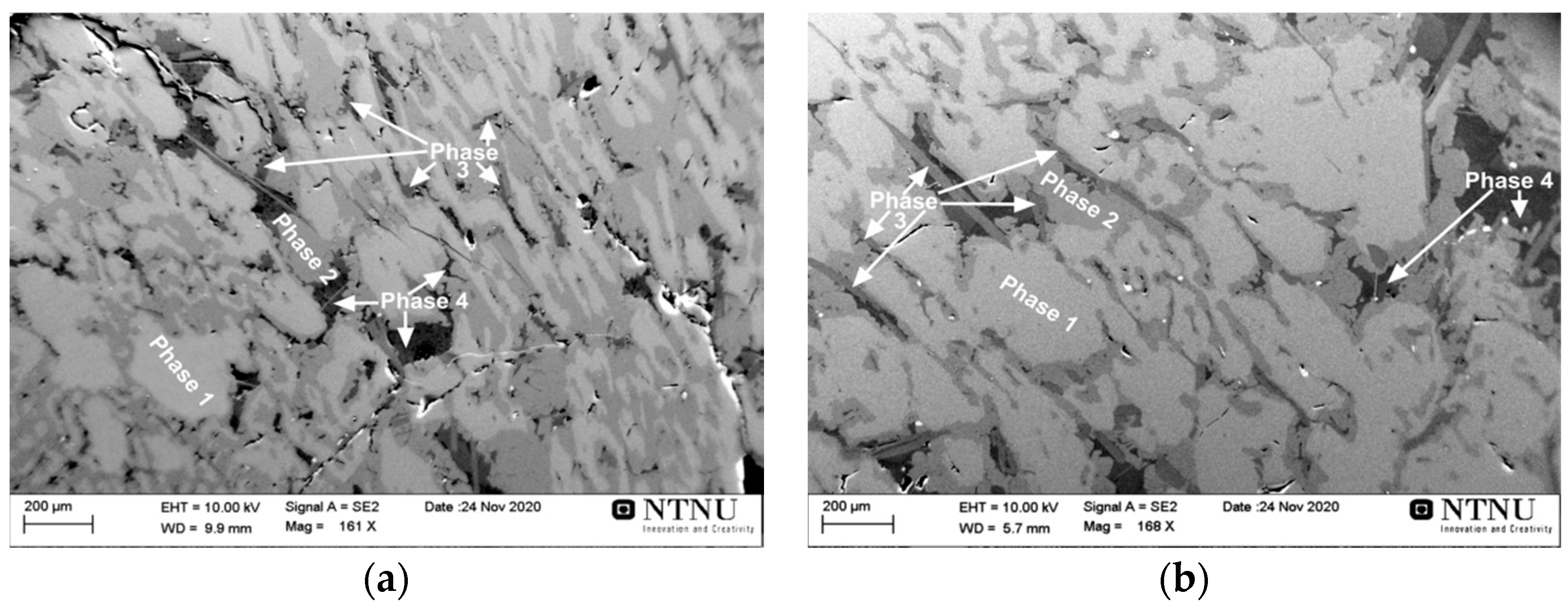
3.2.3. Interaction of Al-Dross with FeMn Slag
4. Process Evaluation
4.1. Process Flexibility
4.2. Energy Consumption
5. Conclusions
- Pure Mn-Al alloys were obtained via the aluminothermic reduction of highly pure synthetic CaO-MnO slag by pure Al, and with Al dross from the primary Al production process.
- The composition of the Mn-Al metal and the corresponding slag depends on the charge compositions and amounts.
- Mn-Al-Si alloys were produced using upgraded industrial Al dross and industrial ferromanganese slag, and the metal composition is slightly dependent on additional flux (lime) addition. More Ca is transferred to the metal when the CaO content of slag is increased.
- The composition and microstructure of the produced slag are very dependent on the charge mixture and can be easily engineered.
- The outlined process is very flexible, and a variety of charge mixtures can be used.
- The energy consumption of the process is low and is slightly affected by the target metal composition and the applied technology in practice regarding the energy savings.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torres, J.; Flores Valdés, A.; Almanza Robles, J.M. Elaboration of Al-Mn Alloys by Aluminothermic Reduction of Mn2O3. Mater. Today 2015, 2, 4963–4970. [Google Scholar] [CrossRef]
- Tabereaux, A.T.; Peterson, R.D. Chapter 2.5—Aluminum Production. In Treatise on Process Metallurgy; Seetharaman, S., Ed.; Royal Institute of Technology: Stockholm, Sweden, 2014; Volume 3, pp. 839–917. [Google Scholar]
- Mandin, P.; Wüthrich, R.; Roustan, H. Industrial Aluminium Production: The Hall-Heroult Process Modelling. ECS Trans. 2009, 19, 1–10. [Google Scholar] [CrossRef]
- Tsakiridis, P.E. Aluminum salt slag characterization and utilization—A review. J. Hazard. Mater. 2012, 2017–2018, 1–10. [Google Scholar] [CrossRef] [PubMed]
- International Aluminum Institute. Available online: https://www.world-aluminium.org/statistics/ (accessed on 11 December 2020).
- Jafari, N.H.; Stark, T.D.; Roper, R. Classification and reactivity of secondary aluminum production waste. J. Hazard. Toxic Radioact. Waste 2014, 18, 04014018. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Sekunowo, O.I.; Taiwo, O.O.; Ayoola, W.A.; Machado, A. Physical and Mechanical Properties of aluminum dross. Adv. Mater. 2014, 3, 6–10. [Google Scholar] [CrossRef]
- Manfredi, O.; Wuth, W.; Bohlinger, I. Characterizing the physical and chemical properties of aluminum dross. JOM 1997, 49, 48–51. [Google Scholar] [CrossRef]
- Masson, D.B.; Taghiei, M.M. Interfacial reactions between aluminum alloys and salt flux during melting. Mater. Trans. 1989, 30, 411–422. [Google Scholar] [CrossRef][Green Version]
- Maung, K.N.; Yoshida, T.; Liu, G.; Lwin, C.M.; Muller, D.B.; Hashimoto, S. Assessment of secondary aluminum reserves of nations. Resour. Conserv. Recycl. 2017, 126, 34–41. [Google Scholar] [CrossRef]
- Shinzato, M.C.; Hypolito, R. Effect of disposal of aluminum recycling waste in soil and water bodies. Environ. Earth Sci. 2016, 75, 628–638. [Google Scholar] [CrossRef]
- Mankhand, T.R. Recovery of valuable materials from aluminum dross. J. Sustain. Planet. 2012, 3, 86–94. [Google Scholar]
- Tsakiridis, P.E.; Oustadakis, P.; Agatzini-Leonardou, S. Aluminium recovery during black dross hydrothermal treatment. J. Environ. Chem. Eng. 2013, 1, 23–32. [Google Scholar] [CrossRef]
- Olsen, S.E.; Merete, T.; Lindstad, T. Production of Manganese Ferroalloys; Tapir Akademisk Forlag: Trondheim, Norway, 2007. [Google Scholar]
- International Manganese Institute, IMNI Statistics Report 2020. Available online: https://www.manganese.org/ (accessed on 11 December 2020).
- Safarian, J. Kinetics and Mechanisms of Reduction of MnO-Containing Silicate Slags by Selected Forms of Carbonaceous Materials. PhD Thesis, Norwegian University of Science and Technology (NTNU), Trondheim, Norway, 2007; p. 250. [Google Scholar]
- Dávila, O.F.; Torres, J.T.; Valdes, A.F. Effect of Mg Concentration on the Aluminothermic Reduction of Mn2O3 Particles Obtained from Cathodes of Discharged Alkaline Batteries: Mathematical Modeling and Experimental Results. Metals 2019, 9, 49. [Google Scholar] [CrossRef]
- Kavitha, R.; McDermid, J.R. On the in-situ aluminothermic reduction of manganese oxides in continuous galvanizing baths. Surf. Coat. Technol. 2012, 212, 152–158. [Google Scholar] [CrossRef]
- Cruz-Crespo, A.; Puchol, R.Q.; González, L.P.; Gómez Pérez, C.R.; Cedré, E.D.; Jacomino, J.G. Effect of CaO from the slag system MnO–SiO2–CaO on the chemical composition of weld metal. Weld. Int. 2010, 24, 518–523. [Google Scholar] [CrossRef]
- Monteiro, M.R.; Swinbourne, D.R.; Rankin, W.J. Metallothermic reduction of manganese-bearing slags. AusIMM Proc. 1998, 303, 1–5. [Google Scholar]
- Taşyürek, K.C.; Buğdaycı, M.; Yücel, O. Reduction Conditions of Metallic Calcium from Magnesium Production Residues. Metals 2018, 8, 383. [Google Scholar] [CrossRef]
- Jiaxing, S.; Tao, G.; Wen, D.; Yiming, M.; Xiang, F.; Hao, W. Study on Thermal Chemical Reaction of Al/MnO2 Thermite. IOP Conf. Ser. Earth Environ. Sci. 2018, 186, 1–6. [Google Scholar] [CrossRef]
- Sarangi, B.; Sarangi, A.; Ray, H.S. Kinetics of aluminothermic reduction of MnO2 and Fe2O3: A thermoanalytical investigation. ISIJ Int. 1996, 36, 1135–1141. [Google Scholar] [CrossRef]
- Bhoi, B.; Murthy, B.V.R.; Datta, P.; Rajeev; Jouhari, A.K. Studies on Aluminothermic Reduction of Manganese ore for Ferro-Manganese Making. In Proceeding: Ferro Alloy Industries in the Liberalised Economy; Vatsh, A.K., Singh, S.D., Gas, N.G., Ramachandrarao, P., Eds.; NML: Jainshedpur, India, 1997; pp. 66–70. [Google Scholar]
- Eveno, M.; Duran, A.; Castaing, J. A portable X-ray diffraction apparatus for in situ analyses of masters’ paintings. Appl. Phys. A 2010, 100, 577–584. [Google Scholar] [CrossRef]
- Azof, F.I.; Jinglin You, K.T.; Safarian, J. Synthesis and Characterization of 12CaO·7Al2O3 Slags: The Effects of Impurities and Atmospheres on the Phase Relations. Metall. Mater. Trans. B 2020, 51, 2689–2710. [Google Scholar] [CrossRef]
- Liu, X.J.; Ohnuma, I.; Kainuma, R.; Ishida, K. Thermodynamic assessment of the Aluminum-Manganese (Al-Mn) binary phase diagram. JPE 1999, 20, 45–56. [Google Scholar] [CrossRef]
- Bale, C.W.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Mahfoud, R.B.; Melançon, J.; Pelton, A.D.; Petersen, S. FactSage thermochemical software and databases. Calphad 2002, 26, 189–228. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; Pelton, A.D.; et al. FactSage thermochemical software and databases—Recent developments. Calphad 2009, 33, 295–311. [Google Scholar] [CrossRef]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. FactSage thermochemical software and databases, 2010–2016. Calphad 2016, 54, 35–53. [Google Scholar] [CrossRef]
- Jung, I.H.; Van Ende, M.A. Computational Thermodynamic Calculations: FactSage from CALPHAD Thermodynamic Database to Virtual Process Simulation. Metall. Mater. Trans. B 2020, 51, 1851–1874. [Google Scholar] [CrossRef]
- Safarian, J.; Kolbeinsen, L. Purity requirements for Mn-alloys for producing high manganese TRIP and TWIP steels. In Proceedings of the INFACON XIII: The Thirteenth International Ferroalloys Congress, Almaty, Kazakhstan, 9–12 June 2013; Volume II, pp. 175–183. [Google Scholar]
- Azof, F.I.; Safarian, J. Leaching kinetics and mechanism of slag produced from smelting-reduction of bauxite for alumina recovery. Hydrometallurgy 2020, 195, 1–13. [Google Scholar] [CrossRef]
- Keene, B.J.; Mills, K.C. Chapter 8—Densities of molten slags. In Slag Atlas, 2nd ed.; Stahleisen, V., Eisenhüttenleute, V.D., Eds.; Verlag Stahleisen GmbH: Dusseldorf, Germany, 1995; pp. 313–348. [Google Scholar]
- Ahmed, A.; Halfa, H.; El-Fawakhry, M.K.; El-Faramawy, H.; Eissa, M. Parameters Affecting Energy Consumption for Producing High Carbon Ferromanganese in a Closed Submerged Arc Furnace. J. Iron Steel Res. Int. 2014, 21, 666–672. [Google Scholar] [CrossRef]
- IEA. Aluminium. 2020. Available online: https://www.iea.org/reports/aluminium (accessed on 11 December 2020).




| Exp. Number | Synthetic Slag (g) | HCFeMn Slag (g) | CaO Addition (g) | Al Metal (g) | Al Dross (g) |
|---|---|---|---|---|---|
| 1 | 166 | - | - | 46 | - |
| 2 | 166 | - | - | - | Dross 1 *: 69 |
| 3 | - | 49.5 | 11 | - | Dross 2 **: 49.5 |
| 4 | - | 50.2 | 25.15 | - | Dross 2 **: 50.2 |
| Sample | MnO | SiO2 | CaO | Al2O3 | MgO | Fe2O3 | K2O | BaO | SO3 | Na2O | TiO2 | SrO | Rest |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FeMn slag | 46.18 | 19.24 | 13.52 | 10.94 | 4.11 | 2.29 | 0.96 | 0.94 | 0.75 | 0.39 | 0.33 | 0.29 | 0.07 |
| Al | Mn | Si | Mg | Fe | Ca | C | O | |
|---|---|---|---|---|---|---|---|---|
| Metal 3 overall composition | 41.33 | 31.12 | 21.80 | 0.59 | 2.54 | 0.62 | 1.60 | 0.41 |
| Metal 4 overall composition | 39.63 | 31.74 | 21.62 | 0.41 | 3.09 | 1.61 | 1.80 | 0.11 |
| Phase 1 | 39.35 | 42.25 | 13.81 | 0.45 | 3.44 | 0.02 | 0.69 | 0.00 |
| Phase 2 | 30.05 | 26.72 | 38.55 | 0.38 | 3.39 | 0.02 | 0.90 | 0.00 |
| Phase 3 | 32.01 | 0.44 | 39.70 | 0.36 | 0.20 | 25.95 | 1.06 | 0.30 |
| Phase 4 | 0.84 | 0.17 | 97.18 | 0.12 | 0.03 | 0.00 | 1.53 | 0.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudyba, A.; Akhtar, S.; Johansen, I.; Safarian, J. Aluminothermic Reduction of Manganese Oxide from Selected MnO-Containing Slags. Materials 2021, 14, 356. https://doi.org/10.3390/ma14020356
Kudyba A, Akhtar S, Johansen I, Safarian J. Aluminothermic Reduction of Manganese Oxide from Selected MnO-Containing Slags. Materials. 2021; 14(2):356. https://doi.org/10.3390/ma14020356
Chicago/Turabian StyleKudyba, Artur, Shahid Akhtar, Inge Johansen, and Jafar Safarian. 2021. "Aluminothermic Reduction of Manganese Oxide from Selected MnO-Containing Slags" Materials 14, no. 2: 356. https://doi.org/10.3390/ma14020356
APA StyleKudyba, A., Akhtar, S., Johansen, I., & Safarian, J. (2021). Aluminothermic Reduction of Manganese Oxide from Selected MnO-Containing Slags. Materials, 14(2), 356. https://doi.org/10.3390/ma14020356





