Efficient Separation of Photoexcited Charge at Interface between Pure CeO2 and Y3+-Doped CeO2 with Heterogonous Doping Structure for Photocatalytic Overall Water Splitting
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Doping Structures of Y3+-Doped CeO2 Prepared by SSR and CPT Methods
3.2. Investigation of Charge Separation Sites by Photo-Deposition Methods
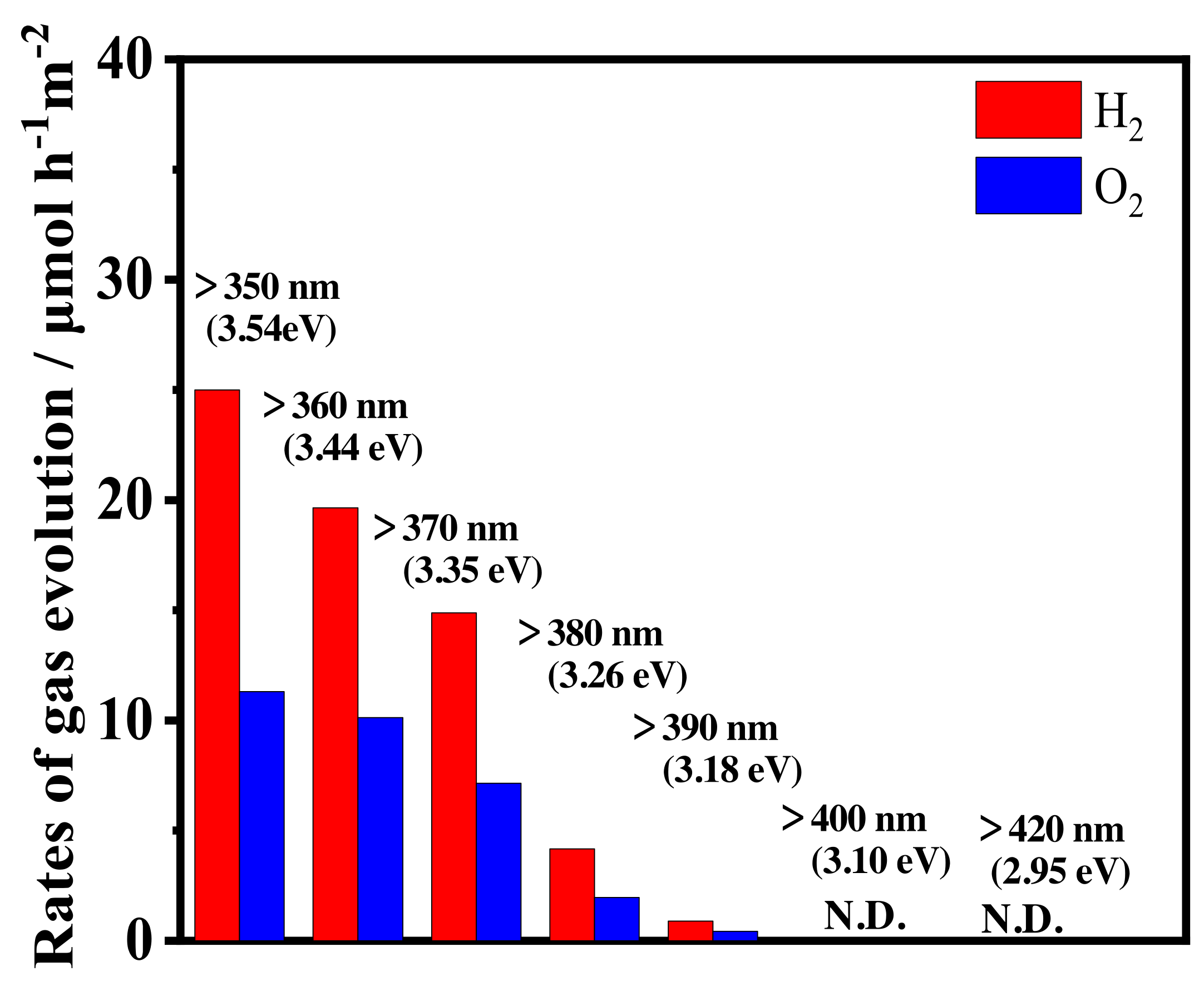
3.3. Photocatalytic Activity for Water Splitting on Y3+-Doped CeO2 with Different Doping Structure
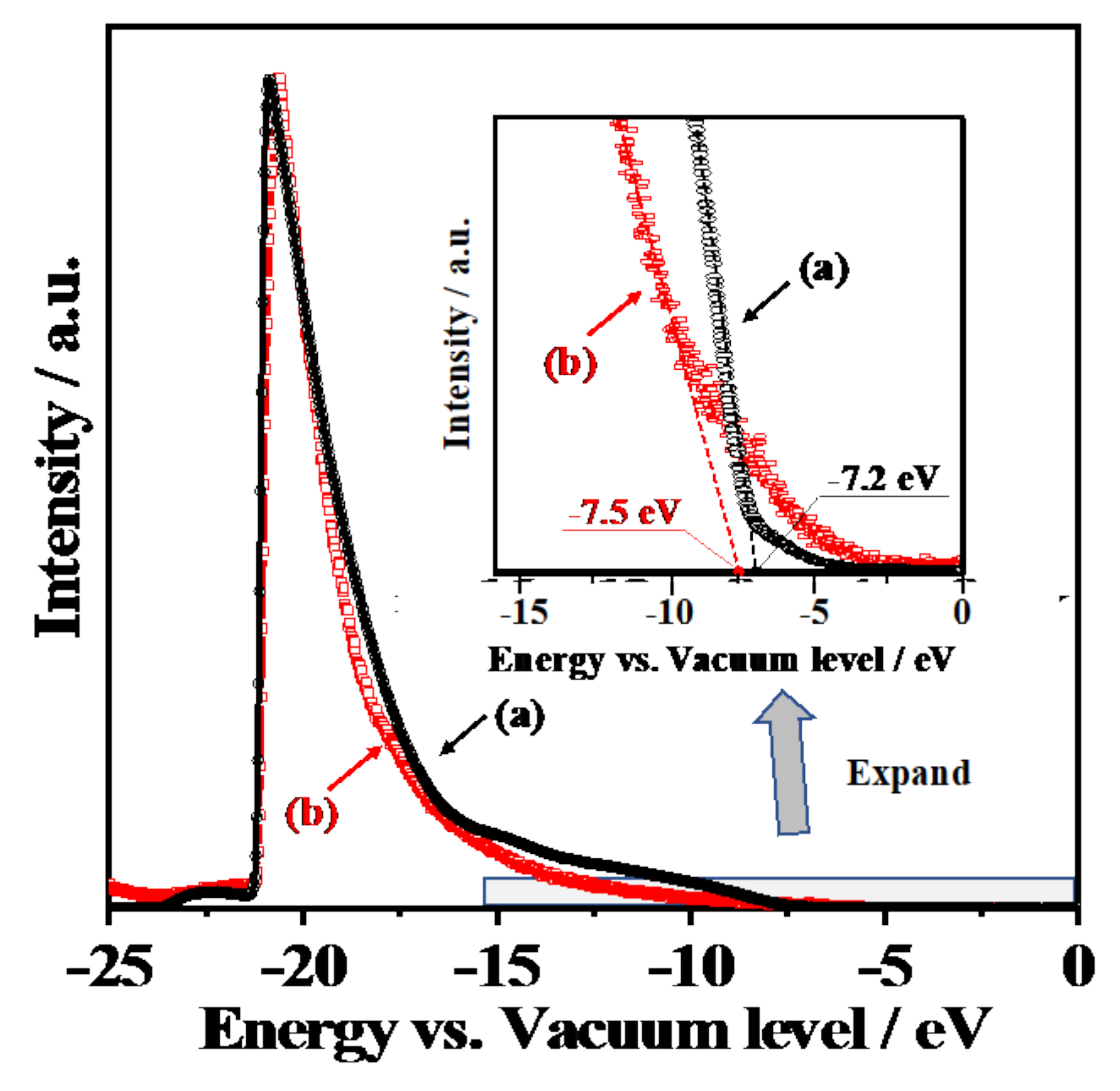
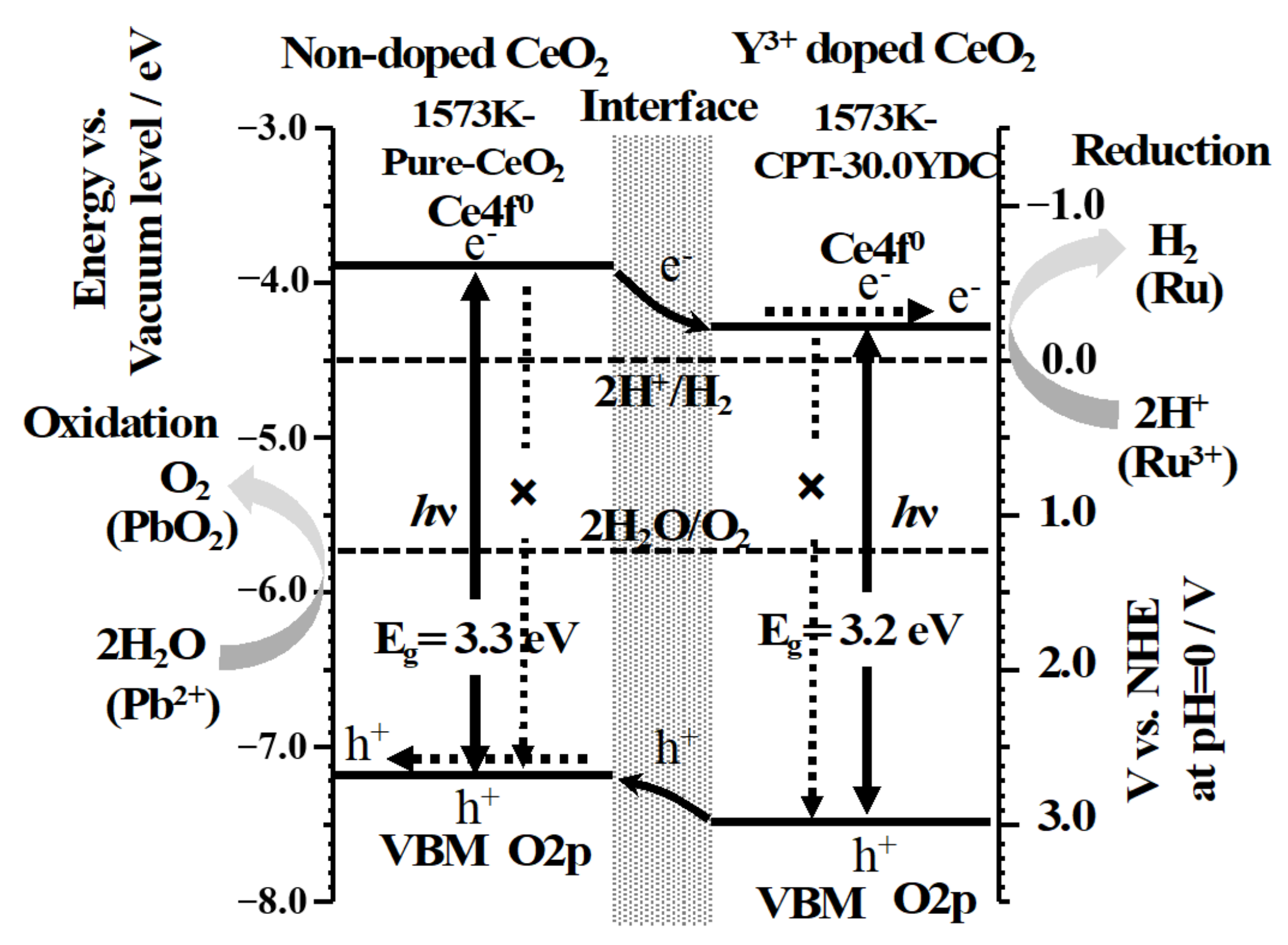
3.4. Electronic Band Structures and Schematic Model of Charge Separation at Interface between CeO2 and Y3+-Doped CeO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inoue, Y. Photocatalytic water splitting by RuO2-loaded metal oxides and nitrides with d0- and d10- related electronic configurations. Energy Environ. Sci. 2009, 2, 364–386. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Abe, R. Recent progress on photocatalytic and photoelectrochemical water splitting under visible light irradiation. J. Photochem. Photobiol. C 2010, 11, 179–209. [Google Scholar] [CrossRef]
- Maeda, K. Photocatalytic water splitting using semiconductor particles: History and recent developments. J. Photochem. Photobiol. C 2011, 12, 237–268. [Google Scholar] [CrossRef]
- Chen, S.S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050–17066. [Google Scholar] [CrossRef]
- Wang, Z.; Inoue, Y.; Hisatomi, T.; Ishikawa, R.; Wang, Q.; Takata, T.; Chen, S.; Shibata, N.; Ikuhara, Y.; Domen, K. Overall Water splitting by Ta3N5 nanorod single crystals grown on the edges of KTaO3 particles. Nat. Catal. 2018, 1, 756–763. [Google Scholar] [CrossRef]
- Wang, Z.; Li, C.; Domen, K. Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem. Soc. Rev. 2019, 48, 2109–2125. [Google Scholar] [CrossRef]
- Wang, Q.; Domen, K. Particulate photocatalysts for light driven water splitting: Mechanisms, challenges, and design strategies. Chem. Rev. 2020, 120, 919–985. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water at a semiconductor electrode. Nature 1972, 238, 37–38. [Google Scholar] [CrossRef]
- Takata, T.; Jiang, J.Z.; Sakata, Y.; Nakabayashi, M.; Shibata, N.; Nandal, V.; Seki, K.; Hisatomi, T.; Domen, K. Photocatalytic water splitting with a quantum efficiency of almost unity. Nature 2020, 581, 411–414. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, Q.; Feng, Z.; Li, M.; Li, C. Importance of the relationship between surface phases and photocatalytic activity of TiO2. Angew. Chem. Int. Ed. 2008, 47, 1766–1769. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, C. Roles of phase junction in photocatalysis and photoelectrocatalysis. J. Phys. Chem. C 2018, 122, 21083–21096. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Q.; Li, M.R.; Shen, S.; Wang, X.L.; Wang, Y.C.; Feng, Z.C.; Shi, J.Y.; Han, H.X.; Li, C. Photocatalytic overall water splitting promoted by an α-β phase junction on Ga2O3. Angew. Chem. Int. Ed. 2012, 51, 13089–13092. [Google Scholar] [CrossRef] [PubMed]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef] [PubMed]
- Bamwenda, G.R.; Sayama, K.; Arakawa, H. The Photoproduction of O2 from a Suspension Containing CeO2 and Ce4+ Cations as an Electron Acceptor. Chem. Lett. 1999, 28, 1047–1048. [Google Scholar] [CrossRef]
- Lu, X.H.; Zhai, T.; Cui, H.; Shi, J.Y.; Xie, S.L.; Huang, Y.Y.; Liang, C.L.; Tong, Y.X. Redox cycles promoting photocatalytic hydrogen evolution of CeO2 nanorods. J. Mater. Chem. 2011, 21, 5569–5572. [Google Scholar] [CrossRef]
- Kadowaki, H.; Saito, N.; Nishiyama, H.; Inoue, Y. RuO2-loaded Sr2+-doped CeO2 with d0 electronic configuration as a new photocatalyst for overall water splitting. Chem. Lett. 2007, 36, 440–441. [Google Scholar] [CrossRef]
- Hou, H.H.; Watanabe, K.; Furuno, H.; Nishikawa, M.; Saito, N. Photocatalytic overall water splitting on RuO2-loaded Sm3+-doped CeO2 with heterogenous doping structure. Chem. Lett. 2018, 48, 200–203. [Google Scholar] [CrossRef]
- Yabe, S.; Yamashita, M.; Momose, S.; Tahira, K.; Yoshida, S.; Li, R.; Yin, S.; Sato, T. Synthesis and UV-shielding properties of metal oxide doped ceria via soft solution chemical processes. Int. J. Inorg. Mater. 2001, 3, 1003–1008. [Google Scholar] [CrossRef]
- Hong, S.J.; Virkar, A.V. Lattice parameters and densities of rare-earth oxide doped ceria electrolytes. J. Am. Ceram. Soc. 1995, 78, 433–439. [Google Scholar] [CrossRef]
- Rey, J.; Muccillo, E. Lattice parameters of yttria-doped ceria solid electrolytes. J. Eur. Ceram. Soc. 2004, 24, 1287–1290. [Google Scholar] [CrossRef]
- Dirstine, R.T.; Blumenthal, R.N.; Kuech, T.F. Ionic conductivity of calcia, yttria, and rare earth-doped cerium dioxide. J. Electrochem. Soc. 1979, 126, 264–269. [Google Scholar] [CrossRef]
- Yang, D.; Wang, L.; Sun, Y.Z.; Zhou, K.B. Synthesis of one-dimensional Ce1-xYxO2-x/2 (0≤ x≤ 1) solid solutions and their catalytic properties: The role of oxygen vacancies. J. Phys. Chem. C 2010, 114, 8926–8932. [Google Scholar] [CrossRef]
- Lee, W.; Chen, S.Y.; Chen, Y.S.; Dong, C.L.; Lin, H.J.; Chen, C.T.; Gloter, A. Defect structure guided room temperature ferromagnetism of Y-Doped CeO2 Nanoparticles. J. Phys. Chem. C 2014, 118, 26359–26367. [Google Scholar] [CrossRef]
- Nolan, M. Charge compensation and Ce3+ formation in trivalent doping of the CeO2 (110) surface: The key role of dopant ionic radius. J. Phys. Chem. C 2014, 118, 6671–6681. [Google Scholar] [CrossRef] [Green Version]
- Murray, A.D.; Murch, G.E.; Catlow, C.R.A. A new hybrid scheme of computer simulation based on Hades and Monte Carlo: Application to ionic conductivity in Y3+ doped CeO2. Solid State Ion. 1986, 18, 196–202. [Google Scholar] [CrossRef]
- Wang, Q.; Hisatomi, T.; Jia, Q.X.; Tokudome, H.; Zhong, M.; Wang, C.Z.; Pan, Z.H.; Takata, T.; Nakabayashi, M.; Shibata, N.; et al. Scalable water splitting on particulate photocatalyst sheets with a solar-to-hydrogen energy conversion efficiency exceeding 1%. Nat. Mater. 2016, 15, 611–615. [Google Scholar] [CrossRef]
- Koelling, D.D.; Boring, A.M.; Wood, J.H. The electronic structure of CeO2 and PrO2. Solid State Commun. 1983, 47, 227–232. [Google Scholar] [CrossRef]
- Pfau, A.; Schierbaum, K.D. The electronic structure of stoichiometric and reduced CeO2 surfaces: An XPS, UPS and HREELS study. Surf. Sci. 1994, 321, 71–80. [Google Scholar] [CrossRef]
- Mullins, D.R.; Overbury, S.H.; Huntley, D.R. Electron spectroscopy of single crystal and polycrystalline cerium oxide surfaces. Surf. Sci. 1998, 409, 307–319. [Google Scholar] [CrossRef]
- Kullgren, J.; Castleton, C.W.M.; Muller, C.; Ramo, D.M.; Hermansson, K. B3LYP calculations of cerium oxides. J. Chem. Phys. 2010, 132, 054110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorenbos, P. The electronic structure of lanthanide doped compounds with 3d, 4d, 5d, or 6d conduction band states. J. Lumin. 2014, 151, 224–228. [Google Scholar] [CrossRef]
- Yamazaki, S.; Matsui, T.; Ohashi, T.; Arita, Y. Defect structures in doped CeO2 studied by using XAFS spectrometry. Solid State Ion. 2000, 136, 913–920. [Google Scholar] [CrossRef]
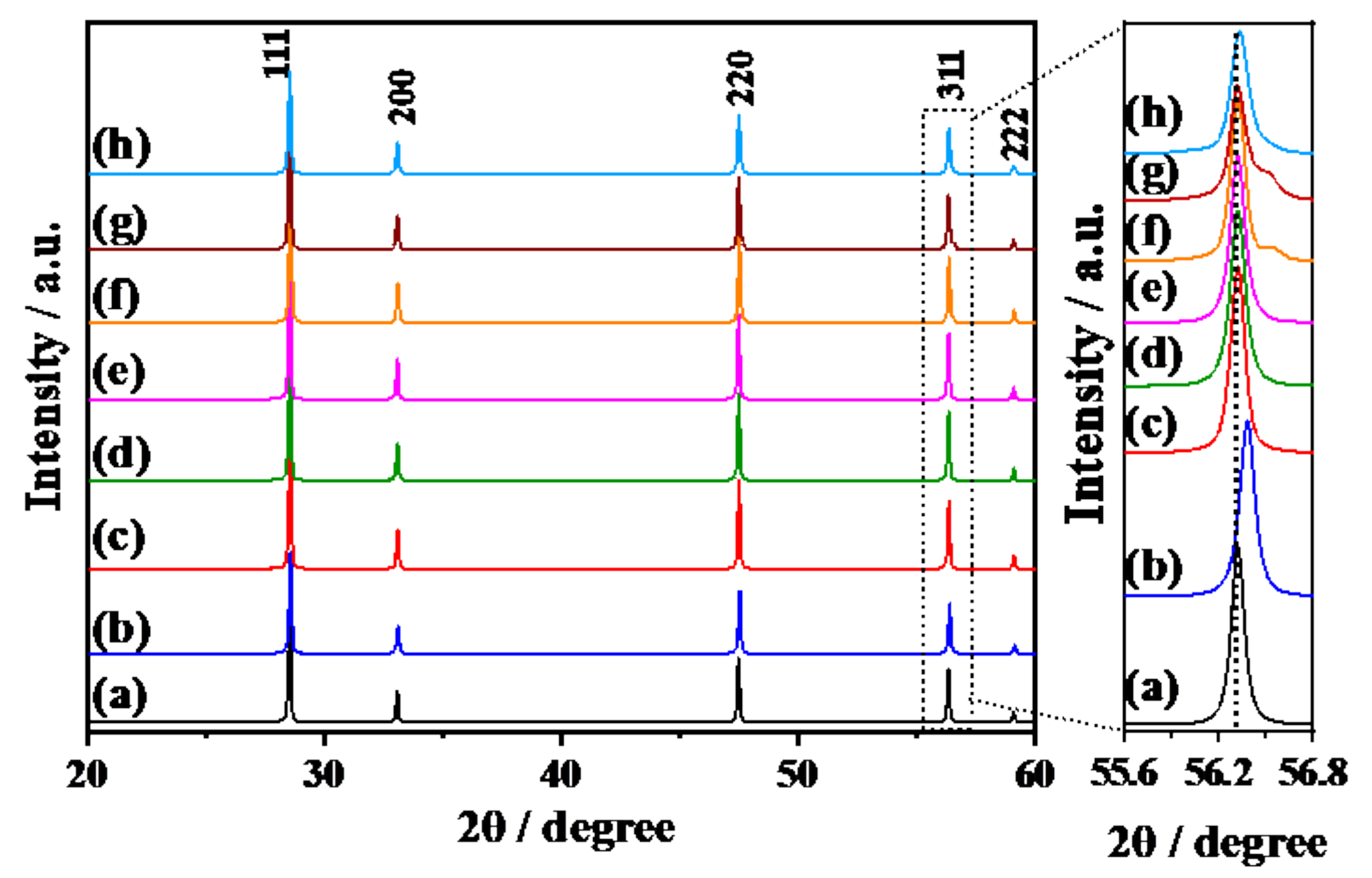
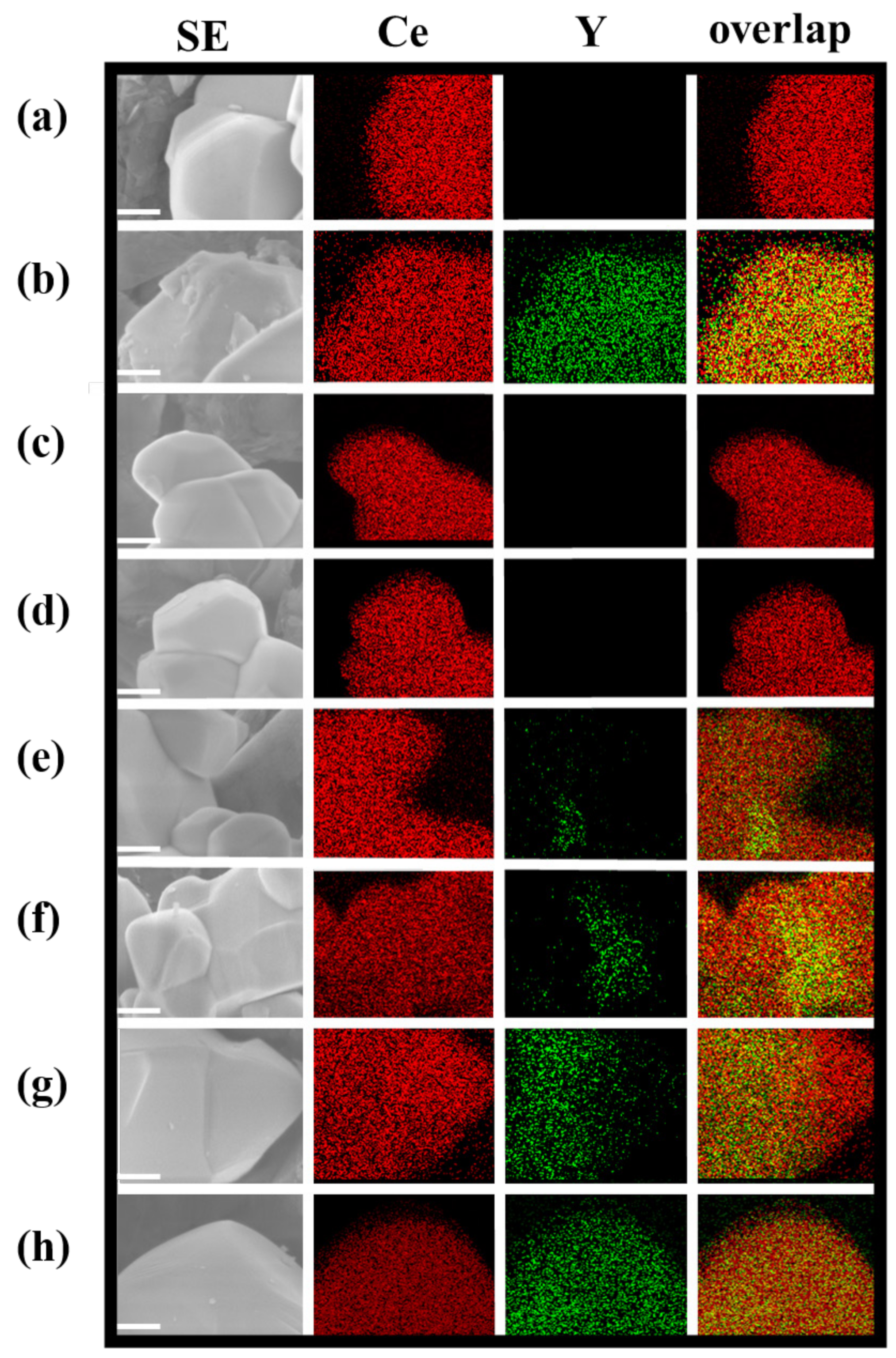

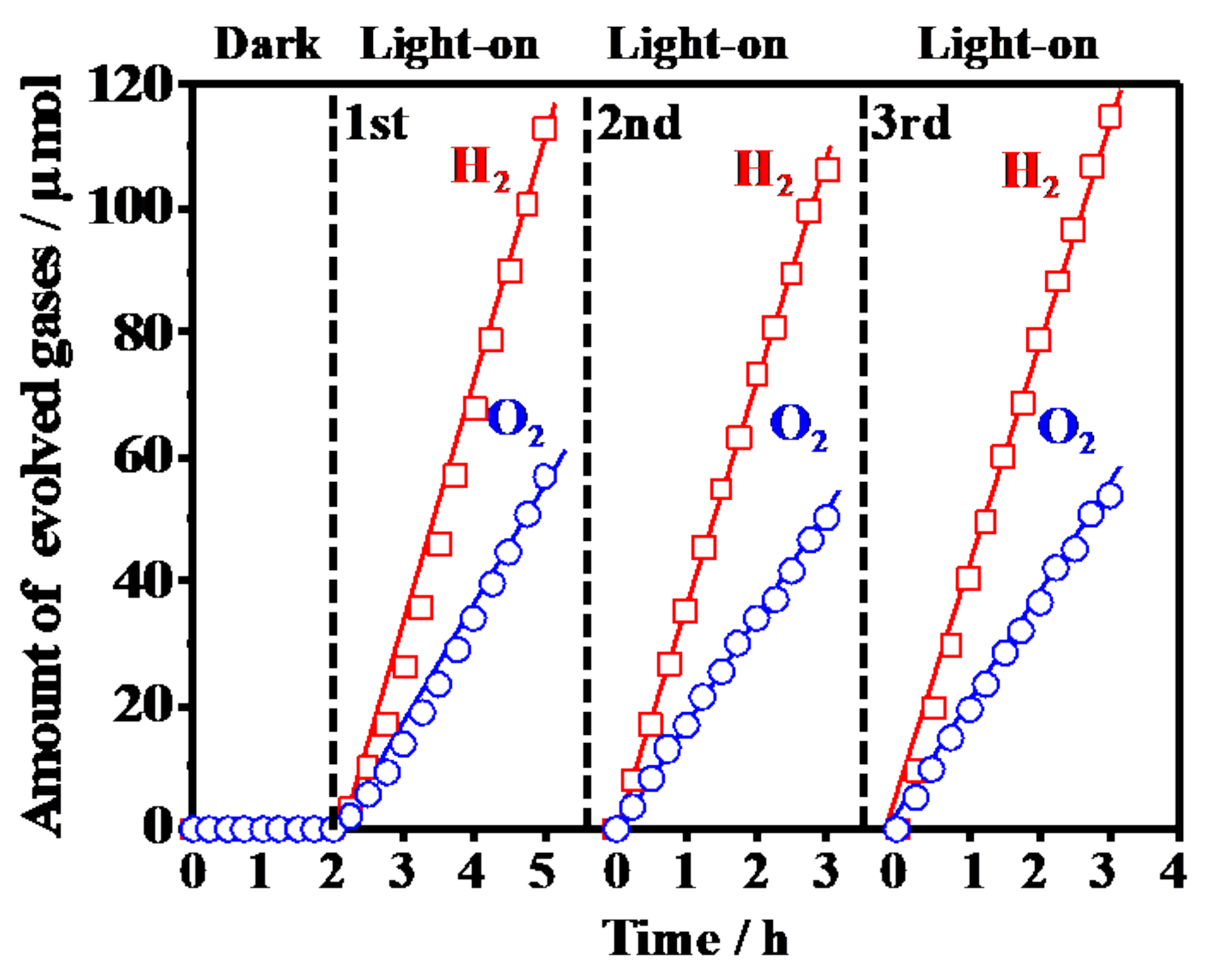
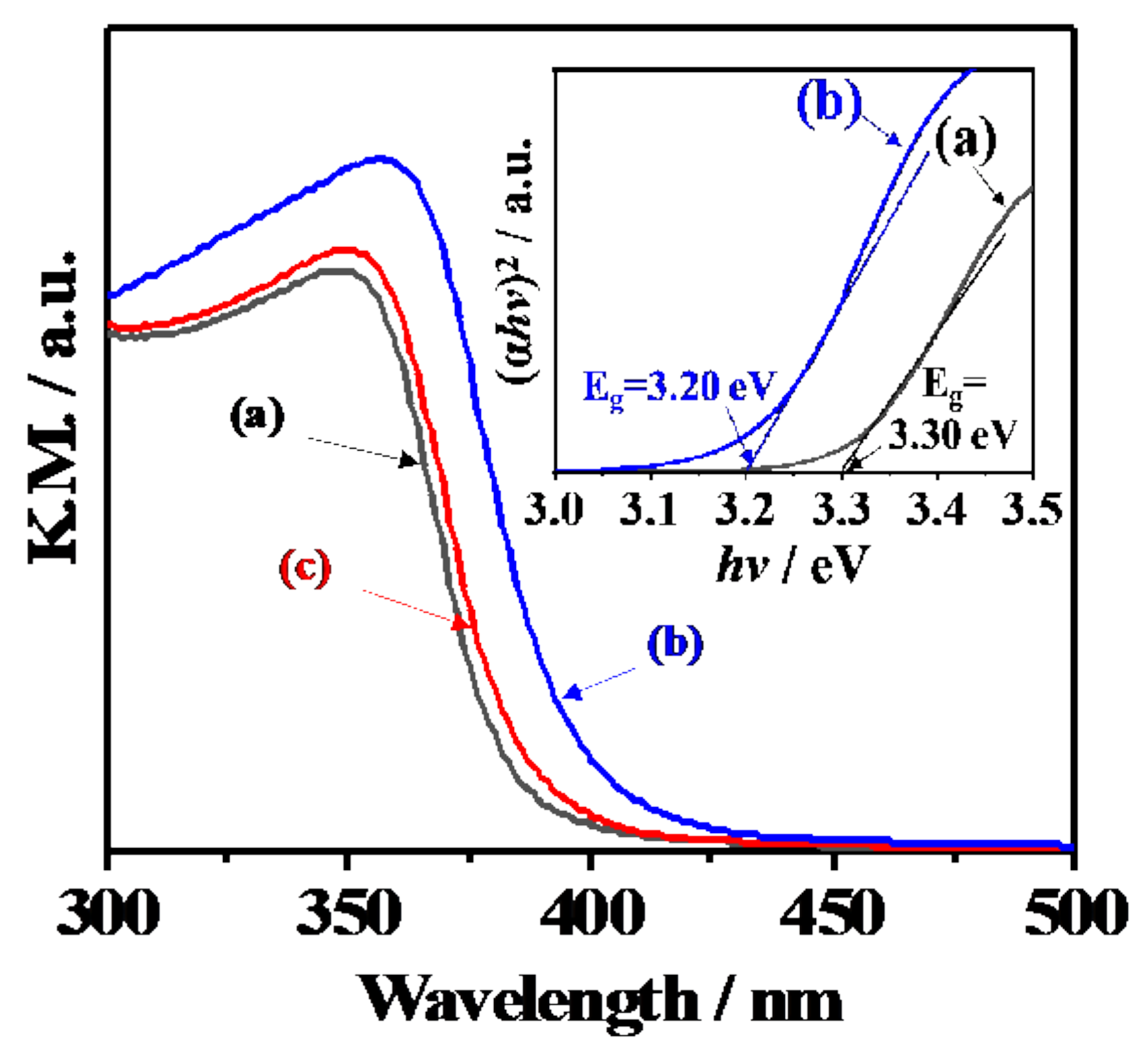
| Sample | Lattice Constant of Y3+-Doped Phase/Å | Percentage of Doped Y3+/mol % | Percentage of Doped Phase/% *c | |
|---|---|---|---|---|
| (Doped Phase) *a | (Average) *b | |||
| 1573K-Pure-CeO2 | - | - | - | - |
| 1573K-CPT | 5.408 | 10 | 10 | 100 |
| 1273K-SSR | - | - | 1.5 | - |
| 1373K-SSR | - | - | 2.2 | - |
| 1473K-SSR | - | - | 3.7 | - |
| 1573K-SSR | 5.393 | 44 | 7.9 | 11 |
| 1673K-SSR | 5.396 | 36 | 9.4 | 21 |
| 1773K-SSR | - | - | 9.8 | - |
| Sample | Percentage of Doped Y3+ on Surface (XPS)/mol% *a | BET Specific Surface Area/m2g−1 |
|---|---|---|
| 1573K-Pure-CeO2 | - | 4.3 |
| 1573K-CPT | 24 | 0.8 |
| 1273K-SSR | 6 | 3.5 |
| 1373K-SSR | 30 | 2.4 |
| 1473K-SSR | 34 | 2.1 |
| 1573K-SSR | 42 | 1.2 |
| 1673K-SSR | 44 | 0.8 |
| 1773K-SSR | 22 | 0.7 |
| Sample | Doping Structures Determined by XRD and EDS | Activities/μmol h−1m−2 | |
|---|---|---|---|
| H2 | O2 | ||
| 1573K-Pure-CeO2 | Non-doped | N.D. | N.D. |
| 1273 K-CPT | Homogeneous | N.D. | N.D. |
| 1373 K-CPT | Homogeneous | N.D. | N.D. |
| 1473 K-CPT | Homogeneous | N.D. | N.D. |
| 1573 K-CPT | Homogeneous | N.D. | N.D. |
| 1673 K-CPT | Homogeneous | N.D. | N.D. |
| 1773 K-CPT | Homogeneous | N.D. | N.D. |
| 1573 K-CPT-0.1YDC | Homogeneous | N.D. | N.D. |
| 1573 K-CPT-0.5YDC | Homogeneous | N.D. | N.D. |
| 1573 K-CPT-1.0YDC | Homogeneous | N.D. | N.D. |
| 1573 K-CPT-5.0YDC | Homogeneous | N.D. | N.D. |
| 1573 K-CPT-20.0YDC | Homogeneous | N.D. | N.D. |
| 1573 K-CPT-30.0YDC | Homogeneous | N.D. | N.D. |
| 1273 K-SSR | Heterogeneous | 1.5 | 0.7 |
| 1373 K-SSR | Heterogeneous | 5.3 | 2.6 |
| 1473 K-SSR | Heterogeneous | 8.5 | 4 |
| 1573 K-SSR | Heterogeneous | 30 | 14.4 |
| 1673 K-SSR | Heterogeneous | 16.4 | 7.7 |
| 1773 K-SSR | Heterogeneous | 3.1 | 1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, H.; Yamada, H.; Nitta, A.; Murakami, Y.; Saito, N. Efficient Separation of Photoexcited Charge at Interface between Pure CeO2 and Y3+-Doped CeO2 with Heterogonous Doping Structure for Photocatalytic Overall Water Splitting. Materials 2021, 14, 350. https://doi.org/10.3390/ma14020350
Hou H, Yamada H, Nitta A, Murakami Y, Saito N. Efficient Separation of Photoexcited Charge at Interface between Pure CeO2 and Y3+-Doped CeO2 with Heterogonous Doping Structure for Photocatalytic Overall Water Splitting. Materials. 2021; 14(2):350. https://doi.org/10.3390/ma14020350
Chicago/Turabian StyleHou, Honghao, Hirohisa Yamada, Atsumi Nitta, Yoshinori Murakami, and Nobuo Saito. 2021. "Efficient Separation of Photoexcited Charge at Interface between Pure CeO2 and Y3+-Doped CeO2 with Heterogonous Doping Structure for Photocatalytic Overall Water Splitting" Materials 14, no. 2: 350. https://doi.org/10.3390/ma14020350
APA StyleHou, H., Yamada, H., Nitta, A., Murakami, Y., & Saito, N. (2021). Efficient Separation of Photoexcited Charge at Interface between Pure CeO2 and Y3+-Doped CeO2 with Heterogonous Doping Structure for Photocatalytic Overall Water Splitting. Materials, 14(2), 350. https://doi.org/10.3390/ma14020350




