Utilization of Treated Agricultural Residue Ash as Sodium Silicate in Alkali Activated Slag Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
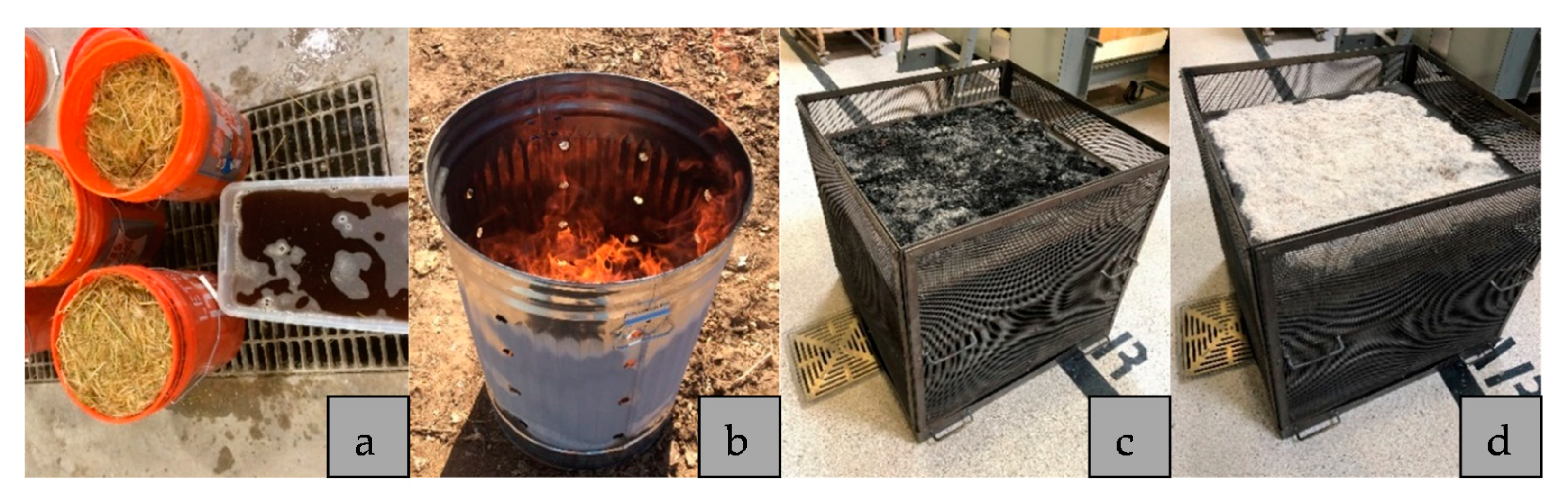
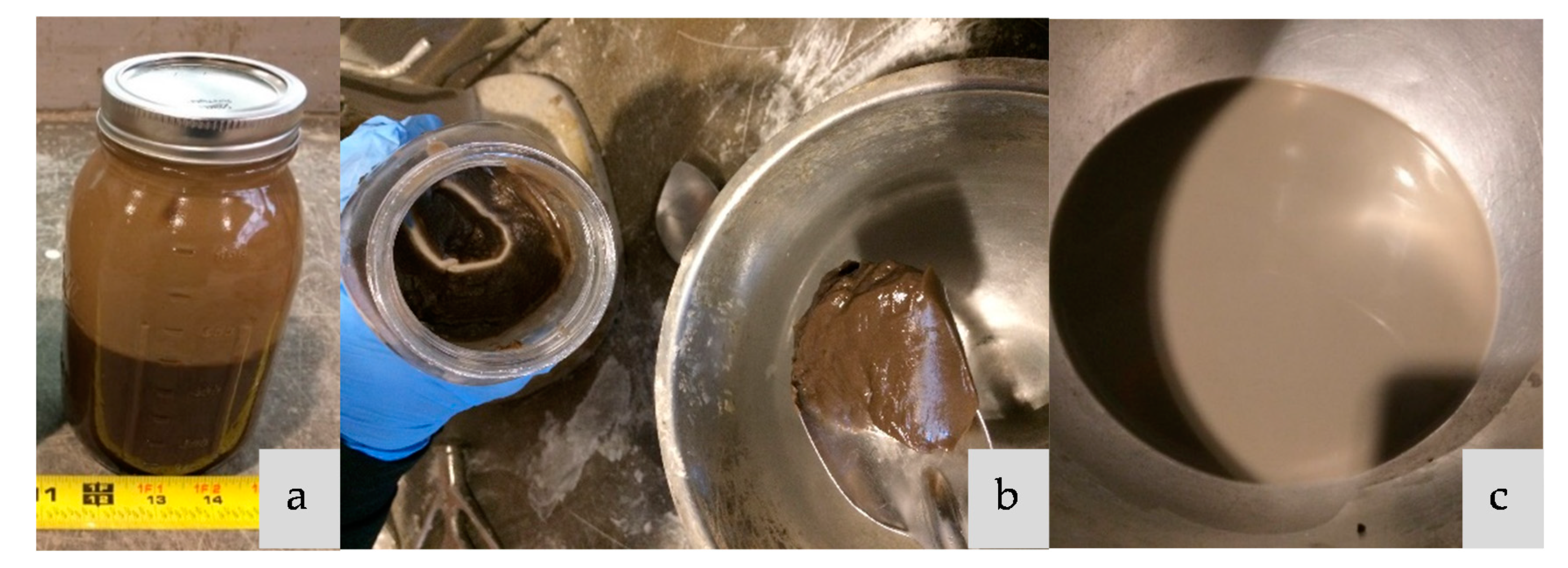
2.2. Methods
3. Results and Discussion
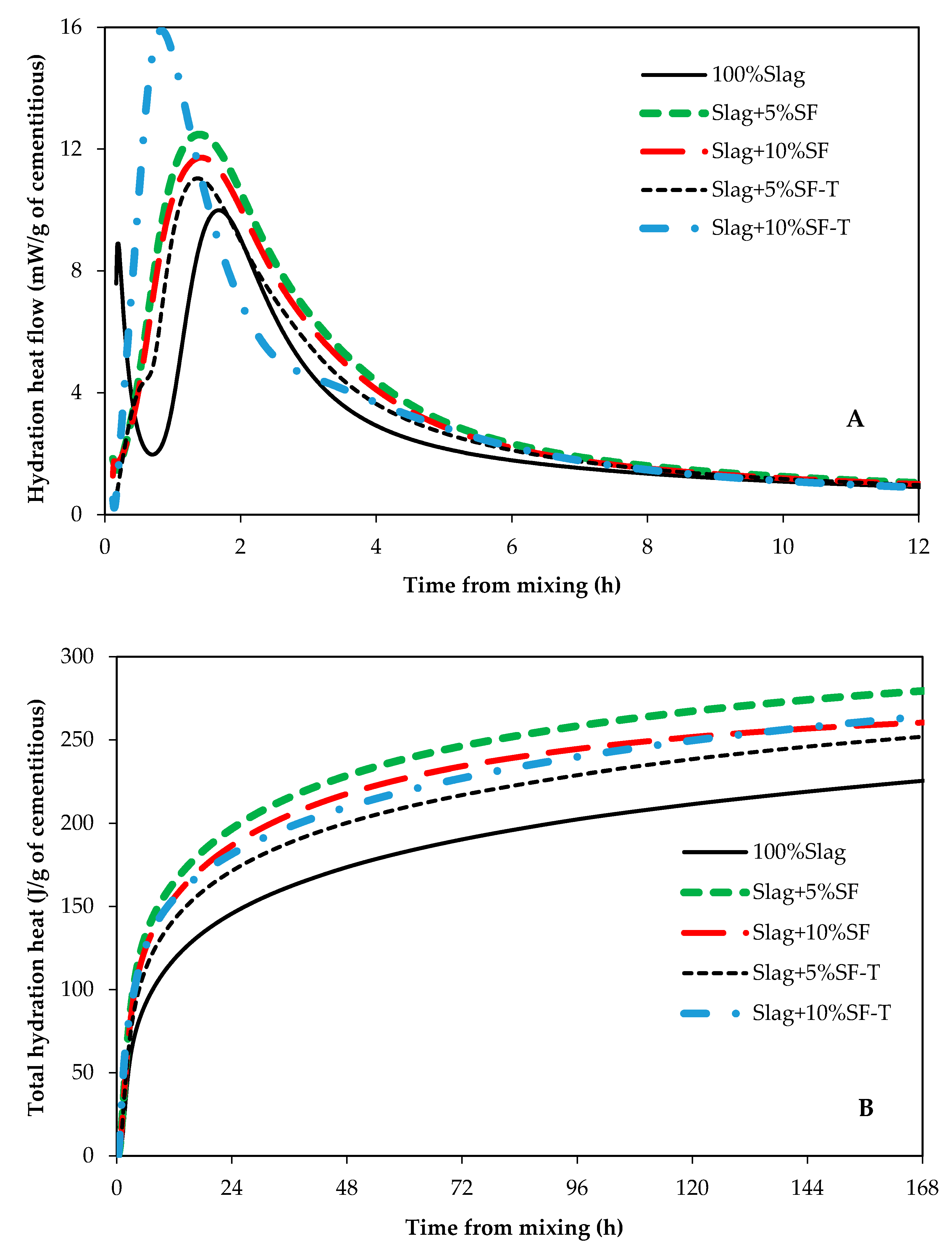
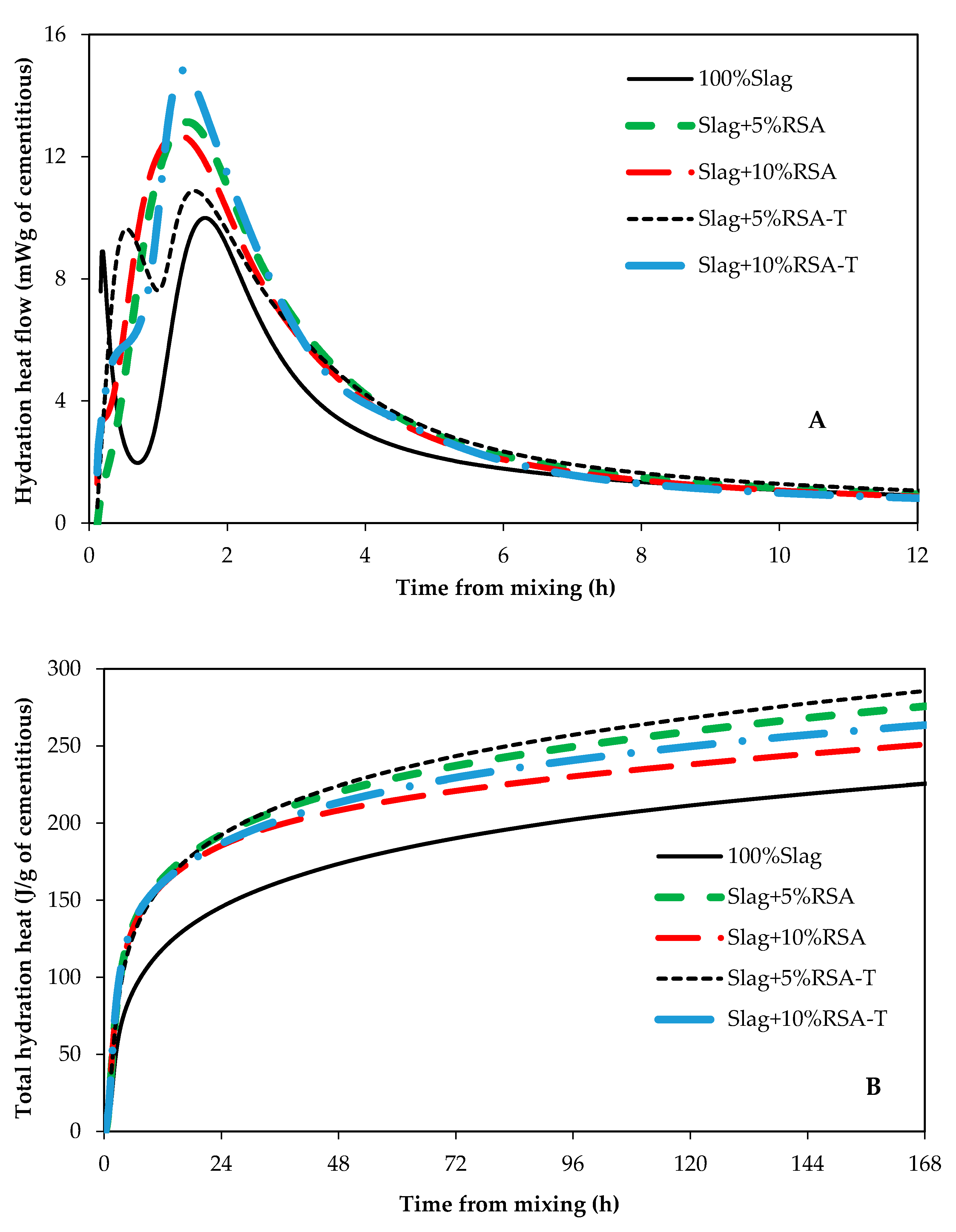
3.1. Impact of RSA, RHA, and SF on Hydration Kinetics
3.2. Impact of RSA, RHA, and SF on Mortar Flow
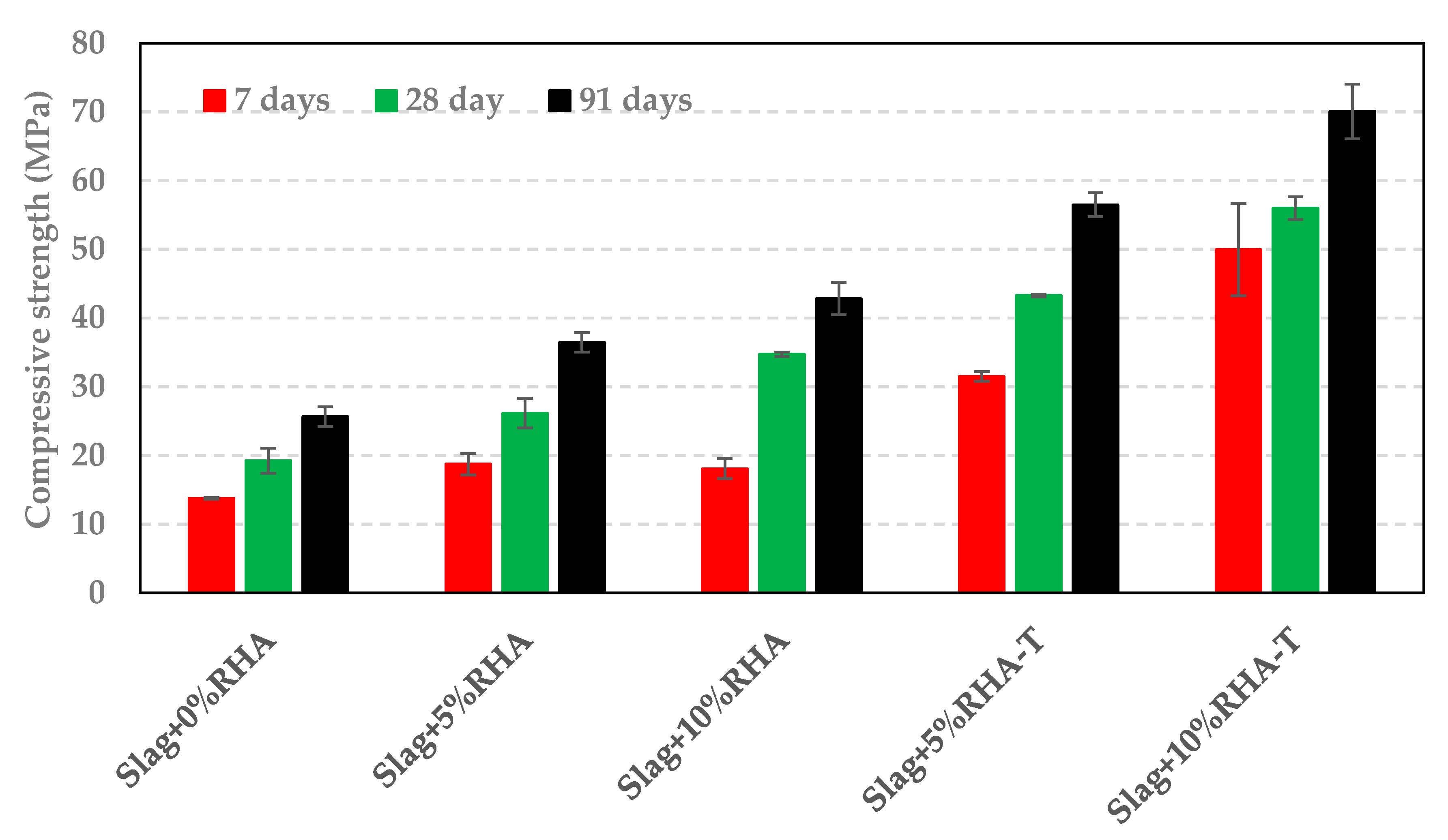
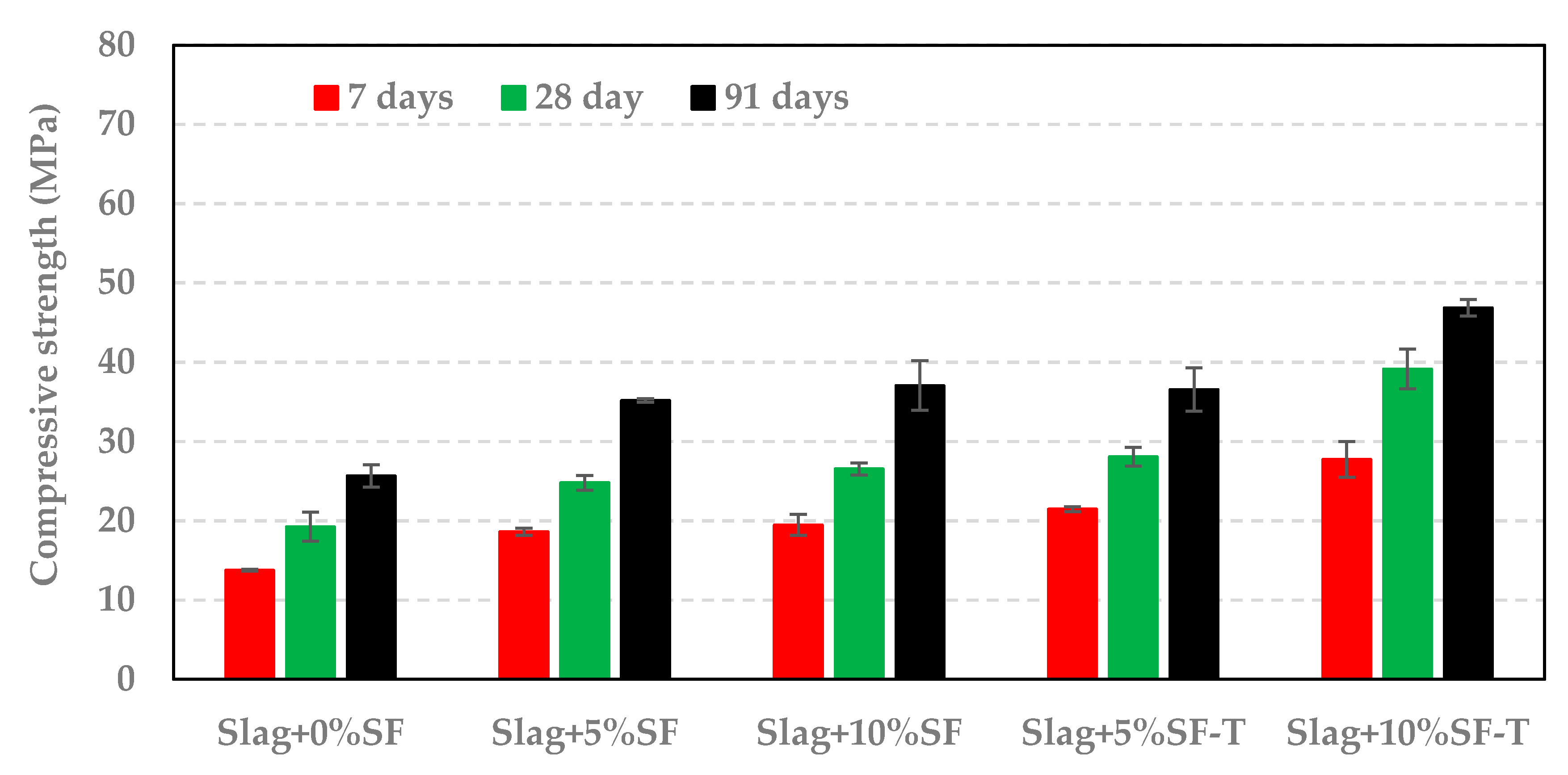
3.3. Impact of RSA, RHA, and SF on Compressive Strength
3.4. Impact of RSA, RHA, and SF on Drying Shrinkage
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Scrivener, K.; John, V.M.; Gartner, E. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Luukkonen, T.; Abdollahnejad, Z.; Yliniemi, J.; Kinnunen, P.; Illikainen, M. One-part alkali-activated materials: A review. Cem. Concr. Res. 2018, 103, 21–34. [Google Scholar] [CrossRef]
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217. [Google Scholar] [CrossRef]
- Flatt, R.J.; Roussel, N.; Cheeseman, C.R. Concrete: An eco material that needs to be improved. J. Eur. Ceram. Soc. 2012, 32, 2787–2798. [Google Scholar] [CrossRef]
- Provis, J.L. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part 1. Historical background, terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Awoyera, P.; Adesina, A. A critical review on application of alkali activated slag as a sustainable composite binder. Case Stud. Constr. Mater. 2019, 11, e00268. [Google Scholar] [CrossRef]
- Longo, F.; Cascardi, A.; Lassandro, P.; Aiello, M.A. A New Fabric Reinforced Geopolymer Mortar (FRGM) with Mechanical and Energy Benefits. Fibers 2020, 8, 49. [Google Scholar] [CrossRef]
- Gomaa, E.; Gheni, A.; ElGawady, M. Repair of ordinary Portland cement concrete using ambient-cured alkali-activated concrete: Interfacial behavior. Cem. Concr. Res. 2020, 129, 105968. [Google Scholar] [CrossRef]
- Habert, G.; De Lacaillerie, J.D.; Roussel, N. An environmental evaluation of geopolymer based concrete production: Reviewing current research trends. J. Clean. Prod. 2011, 19, 1229–1238. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Krizan, D.; Zivanovic, B. Effects of dosage and modulus of water glass on early hydration of alkali–slag cements. Cem. Concr. Res. 2002, 32, 1181–1188. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.J.; Peethamparan, S. Temperature and activator effect on early-age reaction kinetics of alkali-activated slag binders. Constr. Build. Mater. 2016, 113, 783–793. [Google Scholar] [CrossRef]
- Chen, W.; Li, B.; Wang, J.; Thom, N. Effects of alkali dosage and silicate modulus on autogenous shrinkage of alkali-activated slag cement paste. Cem. Concr. Res. 2021, 141, 106322. [Google Scholar] [CrossRef]
- Zhang, S.; Keulen, A.; Arbi, K.; Ye, G. Waste glass as partial mineral precursor in alkali-activated slag/fly ash system. Cem. Concr. Res. 2017, 102, 29–40. [Google Scholar] [CrossRef]
- Moraes, J.; Tashima, M.; Akasaki, J.; Melges, J.; Monzó, J.; Borrachero, M.; Soriano, L. Increasing the sustainability of alkali-activated binders: The use of sugar cane straw ash (SCSA). Constr. Build. Mater. 2016, 124, 148–154. [Google Scholar] [CrossRef]
- Puertas, F.; Torres-Carrasco, M. Use of glass waste as an activator in the preparation of alkali-activated slag. Mechanical strength and paste characterisation. Cem. Concr. Res. 2014, 57, 95–104. [Google Scholar] [CrossRef]
- Mejía, J.; Gutiérrez, R.M.d.; Puertas, F. Rice husk ash as a source of silica in alkali-activated fly ash and granulated blast furnace slag systems. Mater. Constr. 2013, 63, 361–375. [Google Scholar]
- ASTM-C778. Standard Specification for Standard Sand; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Ataie, F.; Riding, K.A. Influence of agricultural residue ash on early cement hydration and chemical admixtures adsorption. Constr. Build. Mater. 2016, 106, 274–281. [Google Scholar] [CrossRef]
- Ataie, F.F.; Riding, K.A. Thermochemical Pretreatments for Agricultural Residue Ash Production for Concrete. J. Mater. Civ. Eng. 2013, 25, 1703–1711. [Google Scholar] [CrossRef]
- ASTM-C109. Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- ASTM-C1437. Standard Test Method for Flow of Hydraulic Cement Mortar; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- ASTM-C596. Standard Test Method for Drying Shrinkage of Mortar Containing Hydraulic Cement; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Wang, S.-D.; Scrivener, K.L. Hydration Product of Alkali Activated Slage Cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Haha, M.B.; Lothenbach, B.; Le Saout, G.; Winnefeld, F. Influence of slag chemistry on the hydration of alkali-activated blast-furnace slag—Part I: Effect of MgO. Cem. Concr. Res. 2011, 41, 955–963. [Google Scholar] [CrossRef]
- Wu, Z.; Shi, C.; Khayat, K. Influence of silica fume content on microstructure development and bond to steel fiber in ultra-high strength cement-based materials (UHSC). Cem. Concr. Compos. 2016, 71, 97–109. [Google Scholar] [CrossRef]
- Wu, Z.; Khayat, K.; Shi, C. Changes in rheology and mechanical properties of ultra-high performance concrete with silica fume content. Cem. Concr. Res. 2019, 123, 100–105. [Google Scholar] [CrossRef]
- Gebregziabiher, B.S.; Thomas, R.J.; Peethamparan, S. Very early-age reaction kinetics and microstructural development in alkali-activated slag. Cem. Concr. Compos. 2015, 55, 91–102. [Google Scholar] [CrossRef]
- Ye, H.; Radlińska, A. Shrinkage mechanisms of alkali-activated slag. Cem. Concr. Res. 2016, 88, 126–135. [Google Scholar] [CrossRef]
- Atis, C.D.; Bilim, C.; Çelik, Ö.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Collins, F.; Sanjayan, J.G. Effect of pore size distribution on drying shrinking of alkali-activated slag concrete. Cem. Concr. Res. 2000, 30, 1401–1406. [Google Scholar] [CrossRef]
- Li, Z.; Lu, T.; Liang, X.; Dong, H.; Ye, G. Mechanisms of autogenous shrinkage of alkali-activated slag and fly ash pastes. Cem. Concr. Res. 2020, 135, 106107. [Google Scholar] [CrossRef]
- Ben Haha, M.; Le Saout, G.; Winnefeld, F.; Lothenbach, B. Influence of activator type on hydration kinetics, hydrate assemblage and microstructural development of alkali activated blast-furnace slags. Cem. Concr. Res. 2011, 41, 301–310. [Google Scholar] [CrossRef]
- Rostami, M.; Behfarniam, K. The effect of silica fume on durability of alkali activated slag concrete. Constr. Build. Mater. 2017, 134, 262–268. [Google Scholar] [CrossRef]




| SF | RHA | RSA | Slag | ||
|---|---|---|---|---|---|
| SiO2 | % | 98.5 | 93.8 | 87.0 | 31.9 |
| Al2O3 | % | 0.2 | 0.1 | 0.4 | 11.6 |
| Fe2O3 | % | 0.14 | 0.14 | 0.46 | 0.71 |
| CaO | % | 0.0 | 0.0 | 1.2 | 38.3 |
| MgO | % | 0.2 | 0.4 | 0.9 | 7.6 |
| SO3 | % | 0.05 | 0.04 | 0.18 | 3.98 |
| LOI | % | 2.3 | 4.0 | 4.6 | 3.8 |
| Na2O | % | 0.10 | 0.15 | 0.60 | 0.16 |
| K2O | % | 0.60 | 0.62 | 1.60 | 0.28 |
| P2O5 | % | 0.10 | 0.33 | 0.78 | 0.00 |
| Solution Type | (SiO2/Na2O) | Na2O % |
|---|---|---|
| NaOH (4 M) | 0.00 | 8.68 |
| NaOH + 5%RSA-T | 0.28 | 8.68 |
| NaOH + 10%RSA-T | 0.56 | 8.68 |
| NaOH + 5%RHA-T | 0.30 | 8.68 |
| NaOH + 10%RHA-T | 0.60 | 8.68 |
| NaOH + 5%SF-T | 0.32 | 8.68 |
| NaOH + 10%SF-T | 0.63 | 8.68 |
| 12.5% Na-Si | 0.29 | 8.38 |
| 20% Na-Si | 0.58 | 8.52 |
| Sample ID | Flow (%) | Sample ID | Flow (%) | Sample ID | Flow (%) |
|---|---|---|---|---|---|
| Slag | 144 | Slag | 144 | Slag | 144 |
| Slag + 5%RSA | 136 | Slag + 5%RHA | 134 | Slag + 5%SF | 152 |
| Slag + 10%RSA | 132 | Slag + 10%RHA | 132 | Slag + 10%SF | 144 |
| Slag + 5%RSA-T | 110 | Slag + 5%RHA-T | 150 | Slag + 5%SF-T | 128 |
| Slag + 10%RSA-T | 90 | Slag + 10%RHA-T | 57 | Slag + 10%SF-T | 131 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ataie, F.F. Utilization of Treated Agricultural Residue Ash as Sodium Silicate in Alkali Activated Slag Systems. Materials 2021, 14, 329. https://doi.org/10.3390/ma14020329
Ataie FF. Utilization of Treated Agricultural Residue Ash as Sodium Silicate in Alkali Activated Slag Systems. Materials. 2021; 14(2):329. https://doi.org/10.3390/ma14020329
Chicago/Turabian StyleAtaie, Feraidon F. 2021. "Utilization of Treated Agricultural Residue Ash as Sodium Silicate in Alkali Activated Slag Systems" Materials 14, no. 2: 329. https://doi.org/10.3390/ma14020329
APA StyleAtaie, F. F. (2021). Utilization of Treated Agricultural Residue Ash as Sodium Silicate in Alkali Activated Slag Systems. Materials, 14(2), 329. https://doi.org/10.3390/ma14020329




