Clinical and Histological Differences between Guided Tissue Regeneration with Acellular Dermal Matrix of Porcine Origin and Autologous Connective Tissue: An Animal Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
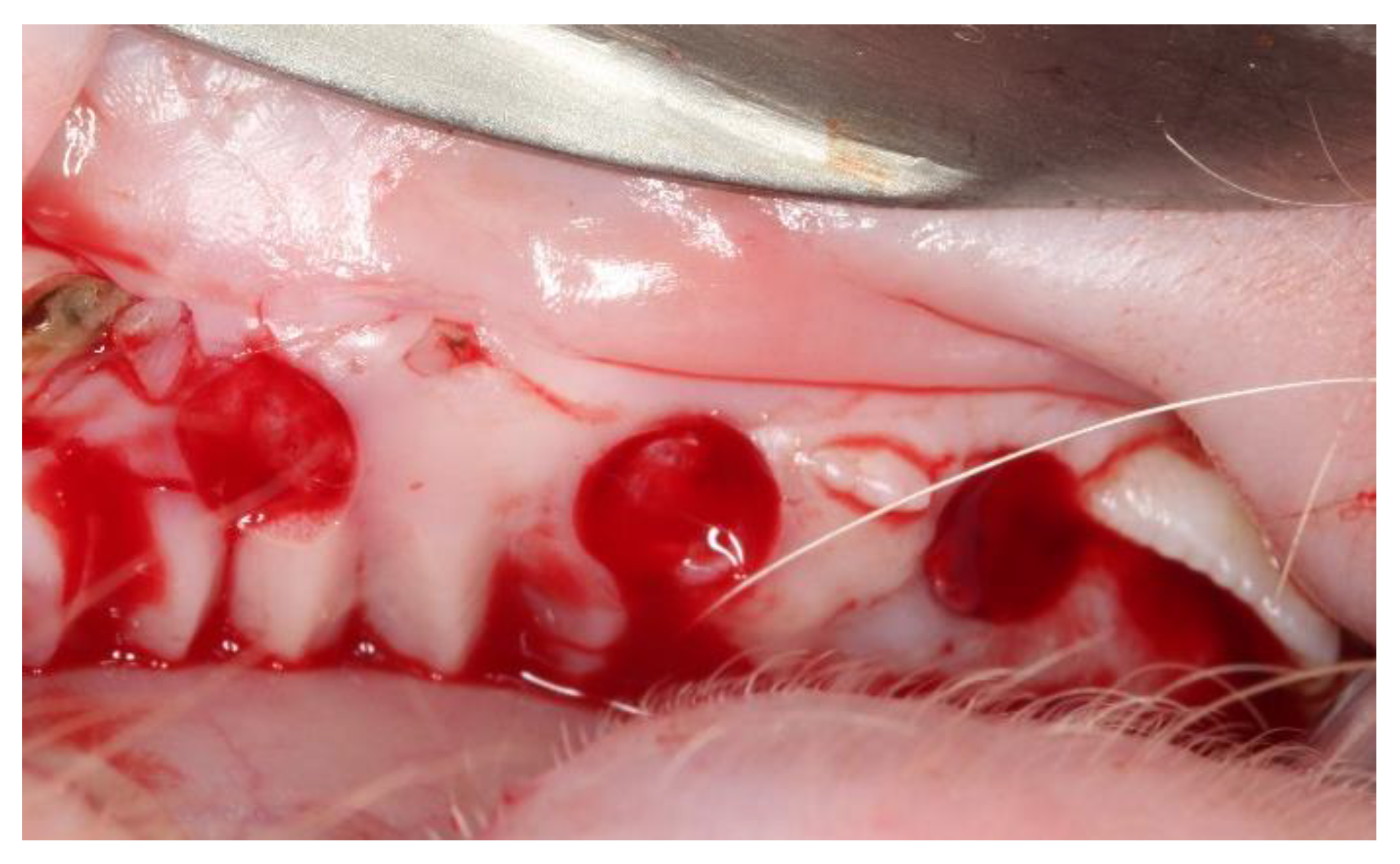
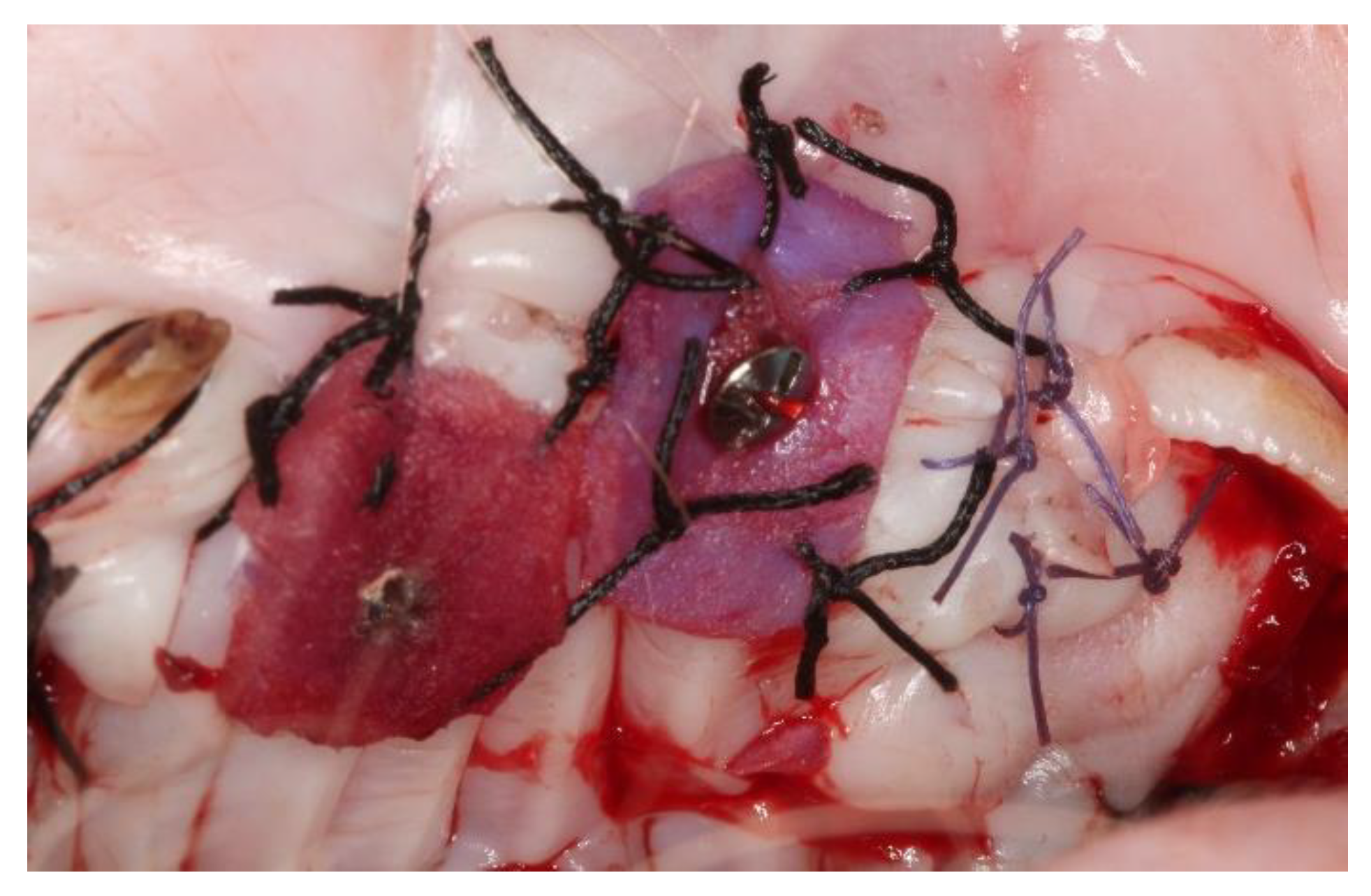
2.2. Surgical Procedures
2.3. Euthanasia
2.4. Histological Evaluation
- the thickness of the keratin layer, and
- the thickness of the epithelial tissue, measured from the most coronal part of the granular layer to the more apical part of the epithelial crest.
2.5. Clinical Evaluation
2.6. Statistical Analysis
3. Results
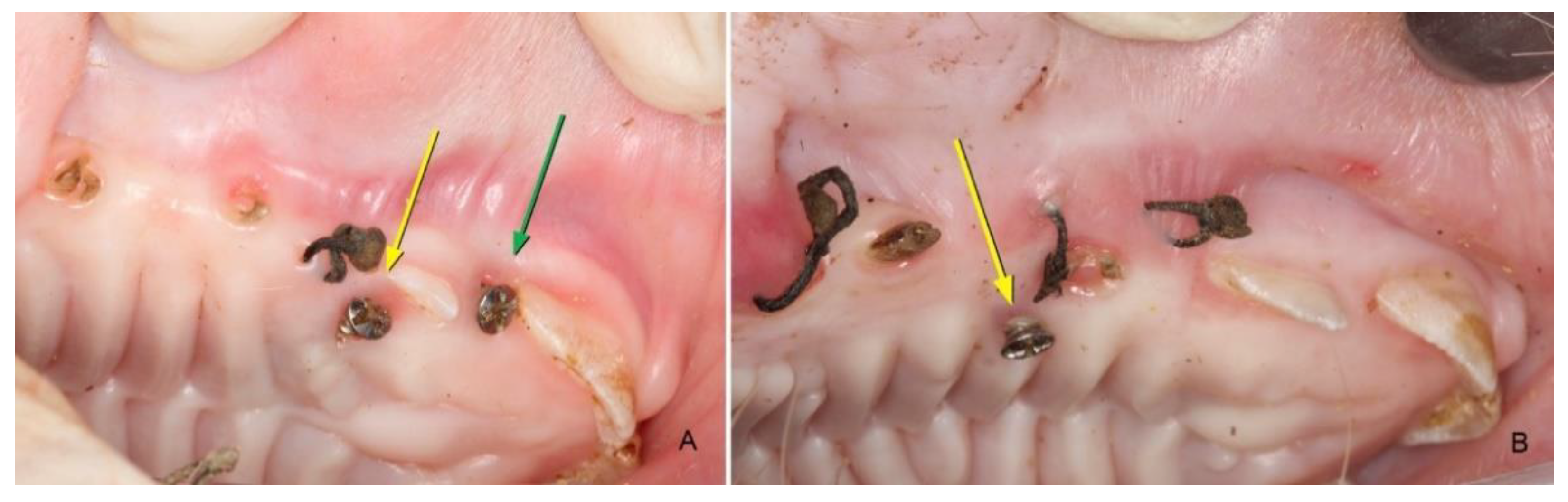
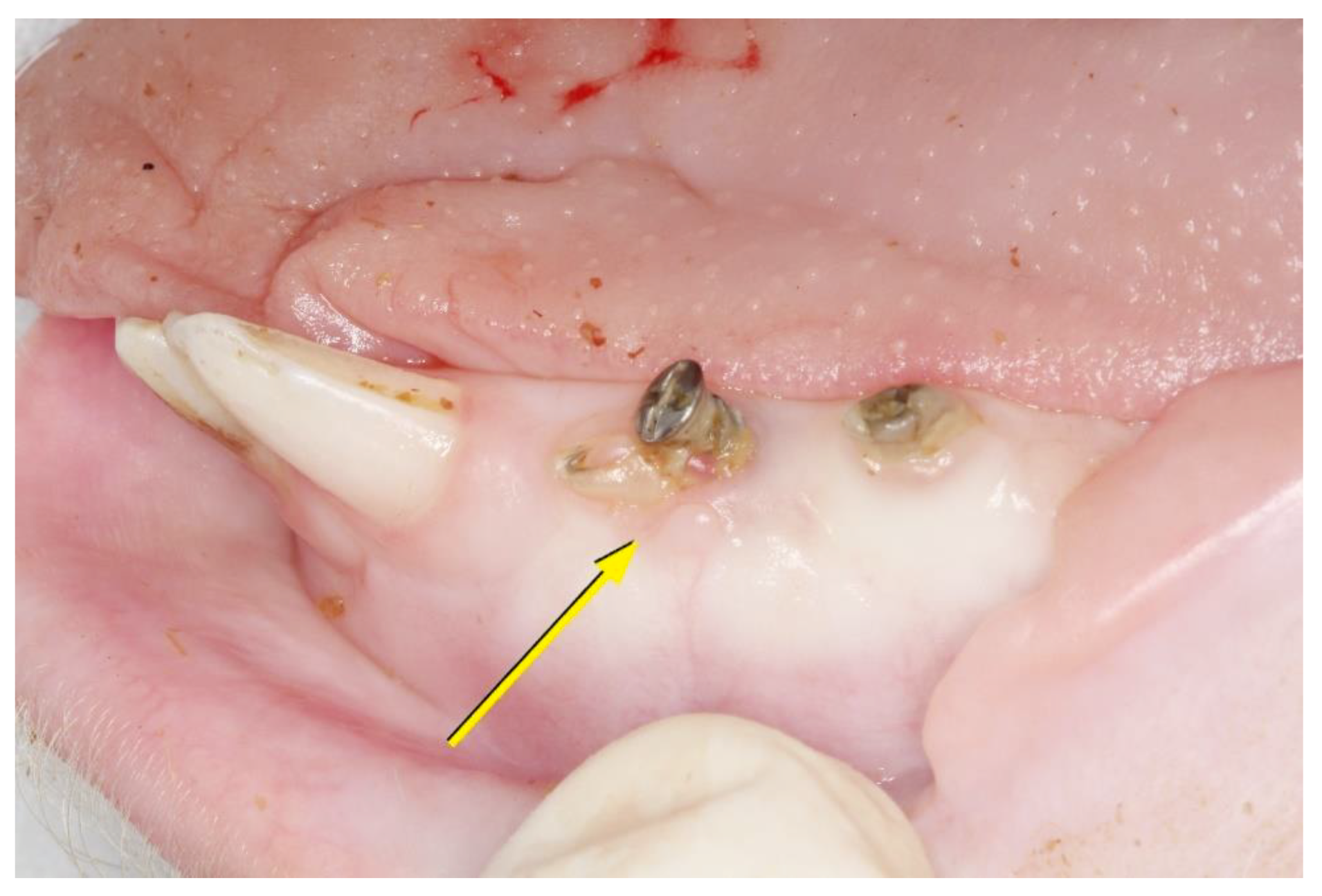
3.1. Clinical Evaluation
3.1.1. After 15 Days
3.1.2. After 45 Days
3.1.3. After 90 Days
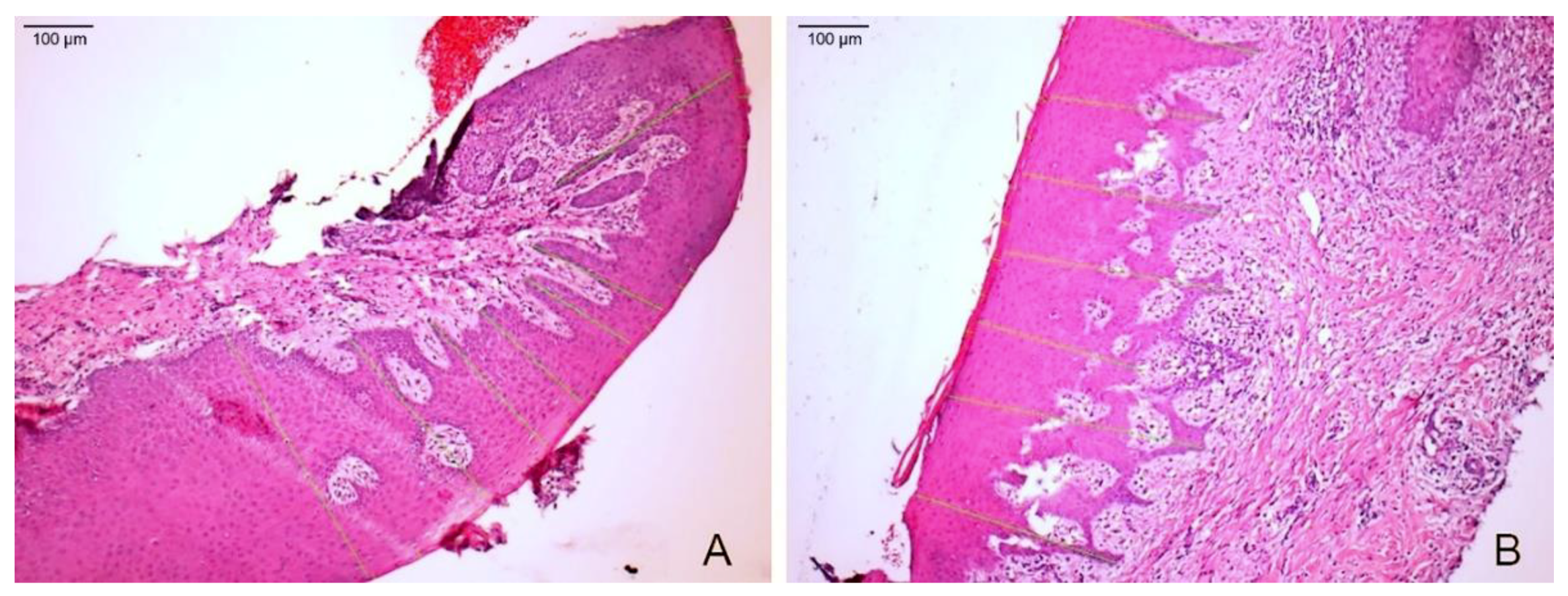
3.2. Histological Evaluation
3.2.1. After 15 Days
3.2.2. After 45 Days
3.2.3. After 90 Days
3.3. Keratin Layer and Epithelial Tissue Thickness
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sanz, M.; Lorenzo, R.; Aranda, J.J.; Martin, C.; Orsini, M. Clinical evaluation of a new collagen matrix (Mucograft prototype) to enhance the width of keratinized tissue in patients with fixed prothetic restorations: A randomized prospective clinical trial. J. Clin. Periodontol. 2009, 36, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F. Periodontal plastic surgery of gingival recessions at single and multiple teeth. Periodontology 2000 2017, 75, 296–316. [Google Scholar] [CrossRef] [PubMed]
- Sculean, A.; Mihatovic, I.; Shirakata, Y.; Bosshardt, D.D.; Schwarz, F.; Iglhaut, G. Healing of localized gingival recessions treated with coronally advanced flap alone or combined with either a resorbable collagen matrix or subepithelial connective tissue graft. A preclinical study. Clin. Oral Investig. 2015, 19, 903–909. [Google Scholar] [CrossRef] [PubMed]
- Cairo, F.; Pagliaro, U.; Nieri, M. Treatment of gingival recession with coronally advanced flap procedures: A systematic review. J. Clin. Periodontol. 2008, 35, 136–162. [Google Scholar] [CrossRef] [PubMed]
- Balderrama, D.; Ferreira, R.; Rezende, D.R.; Nogueira, A.L.; Zangrando, M.S. Root coverage stability with acellular dermal matrix in multiple gingival recessions in esthetic zone: A clinical report with 12-year follow-up. J. Indian Soc. Periodontol. 2019, 23, 584–588. [Google Scholar] [CrossRef]
- Aguirre-Zorzano, L.A.; García-De La Fuente, A.M.; Estefanía-Fresco, R.; Marichalar-Mendía, X. Complications of harvesting a connective tissue graft from the palate. A retrospective study and description of a new technique. J. Clin. Exp. Dent. 2017, 9, e1439–e1445. [Google Scholar] [CrossRef]
- Harris, R.J. Root coverage with a connective tissue with partial thickness double pedicle graft and an acellular dermal matrix graft: A clinical and histological evaluation of a case report. J. Periodontol. 1998, 69, 1305–1311. [Google Scholar] [CrossRef]
- Mao, E.J. The applications of periodontal gingival surgery. Ⅱ: Alternative materials. Hua Xi Kou Qiang Yi Xue Za Zhi 2018, 36, 117–122. [Google Scholar]
- Cevallos, C.A.R.; de Resende, D.R.B.; Damante, C.A.; Sant’Ana, A.C.P.; De Rezende, M.L.R.; Greghi, S.L.A.; Zangrando, M.S.R. Free gingival graft and acellular dermal matrix for gingival augmentation: A 15-year clinical study. Clin. Oral Investig. 2020, 24, 1197–1203. [Google Scholar] [CrossRef]
- Ahmedbeyli, C.; Ipci, S.D.; Cakar, G.; Yilmaz, S. Laterally positioned flap along with acellular dermal matrix graft in the management of maxillary localized recessions. Clin. Oral Investig. 2019, 23, 595–601. [Google Scholar] [CrossRef]
- Chambrone, L.; Ortega, M.A.S.; Sukekava, F.; Rotundo, R.; Kalemaj, Z.; Buti, J.; Prato, G.P.P.; Zamira, K. Root coverage procedures for treating localised and multiple recession-type defects. Cochrane Database Syst. Rev. 2018, 10, CD007161. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Martín, I.; Encalada, C.; Sanz-Sánchez, I.; Aracil, J.; Sanz, M. Soft tissue augmentation at immediate implants using a novel xenogeneic collagen matrix in conjunction with immediate provisional restorations: A prospective case series. Clin. Implant. Dent. Relat. Res. 2019, 21, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Heidari, M.; Paknejad, M.; Jamali, R.; Nokhbatolfoghahaei, H.; Fekrazad, R.; Moslemi, N. Effect of laser photobiomodulation on wound healing and postoperative pain following free gingival graft: A split-mouth triple-blind randomized controlled clinical trial. J. Photochem. Photobiol. B 2017, 172, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Sebaoun, A.; Nemcovsky, C.E.; Beitlitum, I.; Moses, O. Modified Tunnel Double Papilla Procedure for Root Coverage in the Anterior Mandible. Int. J. Periodontics Restor. Dent. 2019, 39, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Scheyer, E.T.; Nevins, M.L.; Neiva, R.; Cochran, D.L.; Giannobile, W.V.; Woo, S.-B.; King, W.N.; Spitznagel, J.K.; Bates, D.; McGuire, M.K. Generation of site-appropriate tissue by a living cellular sheet in the treatment of mucogingival defects. J. Periodontol. 2014, 85, e57–e64. [Google Scholar] [CrossRef]
- McGuire, M.K.; Scheyer, E.T.; Nunn, M.E.; Lavin, P.T. A pilot study to evaluate a tissue engineered bi-layered cell therapy as an alternative to tissue from the palate. J. Periodontol. 2008, 79, 1847–1856. [Google Scholar] [CrossRef]
- McGuire, M.K.; Scheyer, E.T.; Nevins, M.L.; Neiva, R.; Cochran, D.L.; Mellonig, J.T.; Giannobile, W.V.; Bates, D. Living cellular construct for increasing the width of keratinized gingiva: Results from a randomized, withinpatient, controlled trial. J. Periodontol. 2011, 82, 1414–1423. [Google Scholar] [CrossRef]
- Nevins, M.L. Tissue-engineered bilayered cell therapy for the treatment of oral mucosal defects: A case series. Int. J. Periodontics Restor. Dent. 2010, 30, 31–39. [Google Scholar]
- Dragan, I.F.; Hotlzman, L.P.; Karimbux, N.Y.; Morin, R.A.; Bassir, S.H. Clinical Outcomes of Comparing Soft Tissue Alternatives to Free Gingival Graft: A Systematic Review and Meta-Analysis. J. Evid. Based Dent. Pract. 2017, 17, 370–380.e3. [Google Scholar] [CrossRef]
- Pabst, A.M.; Happe, A.; Callaway, A.; Ziebart, T.; Stratul, S.I.; Ackerman, M.; Konerding, M.A.; Willershausen, B.; Kasaj, A. In vitro and in vivo characterization of porcine acellular dermal matrix for gingival augmentation procedures. J. Periodontal. Res. 2013, 49, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Laurell, L.; Geivelis, M.; Lingen, M.; Maddalozzo, D. Acellular dermal matrix allografts to achieve increased attached gingiva. Part 1. A clinical study. J. Periodontol. 2000, 71, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Yogini, M.; Prabhuji, M.L.V.; Karthikeyan, B.V.; Sai Jyothsna, N. Comparison of Extracellular Matrix Membrane and Connective Tissue Graft for Root Coverage in Class I/II Gingival Recession Defects: A Split Mouth Study. J. Int. Acad. Periodontol. 2016, 18, 36–44. [Google Scholar]
- Kim, D.M.; Neiva, R. Periodontal Soft Tissue Non–Root Coverage Procedures: A Systematic Review From the AAP Regeneration Workshop Title. J. Periodontol. 2015, 86, S56–S72. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.M.; Tudor, C.; Kiener, K.; Wehrhan, F.; Schmitt, J.; Eitner, S.; Agaimy, A.; Schlegel, K.A. Vestibuloplasty; porcine collagen matrix versus free gingival graft: A clinical and histologic study. J. Periodontol. 2013, 84, 914–923. [Google Scholar] [CrossRef] [PubMed]
- Suárez-López Del Amo, F.; Rodriguez, J.C.; Asa’ad, F.; Wang, H.L. Comparison of two soft tissue substitutes for the treatment of gingival recession defects: An animal histological study. J. Appl. Oral Sci. 2019, 27, e20180584. [Google Scholar] [CrossRef]
- De Resende, D.R.B.; Greghi, S.L.A.; Siqueira, A.F.; Benfatti, C.A.M.; Damante, C.A.; Ragghianti Zangrando, M.S. Acellular dermal matrix allograft versus free gingival graft: A histological evaluation and split-mouth randomized clinical trial. Clin. Oral Investig. 2019, 23, 539–550. [Google Scholar] [CrossRef]
- Kantarci, A.; Hasturk, H.; Van Dyke, T.E. Animal models for periodontal regeneration and peri-implant responses. Periodontology 2000 2015, 68, 66–82. [Google Scholar] [CrossRef]
- Scheyer, E.T.; Sanz, M.; Dibart, S.; Greenwell, H.; John, V.; Kim, D.M.; Langer, L.; Neiva, R.; Rasperini, G. Periodontal Soft Tissue Non–Root Coverage Procedures: A Consensus Report From the AAP Regeneration Workshop. J. Periodontol. 2015, 86, S73–S76. [Google Scholar] [CrossRef]
- Agudio, G.; Nieri, M.; Rotundo, R.; Cortellini, P.P.P.G. Free gingival grafts to increase keratinized tissue: A retrospective long-term evaluation (10 to 25 years) of outcomes. J. Periodontol. 2008, 79, 587–594. [Google Scholar] [CrossRef]
- Agudio, G.; Nieri, M.; Rotundo, R.; Franceschi, D.; Cortellini, P.; Pini Prato, G.P. Periodontal conditions of sites treated with gingival-augmentation surgery compared to untreated contralateral homologous sites: A 10- to 27-year long-term study. J. Periodontol. 2009, 80, 1399–1405. [Google Scholar] [CrossRef]
- Rusu, D.; Stratul, M.S.S.; Festila, M.S.D.; Surlin, P.; Kasaj, A. Histology and surface ultrastructure during early healing after gingival augmentation with a three- dimensional collagen matrix: A report of six cases. Quintessence Int. 2017, 48, 57–67. [Google Scholar] [PubMed]
- Nevins, M.; Nevins, M.L.; Camelo, M.; Camelo, J.M.; Schupbach, P.; Kim, D.M. The clinical efficacy of DynaMatrix extracellular membrane in augmenting keratinized tissue. Int. J. Periodontics Restor. Dent. 2010, 30, 151–161. [Google Scholar]
- Matoh, U.; Petelin, M.; Gašperšič, R. Split-Mouth Comparison of Coronally Advanced Flap with Connective Tissue Graft or Collagen Matrix for Treatment of Isolated Gingival Recessions. Int. J. Periodontics Restor. Dent. 2019, 39, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.J. Clinical evaluation of 3 techniques to augment keratinized tissue without root coverage. J. Periodontol. 2001, 72, 932–938. [Google Scholar] [CrossRef]
- Cieślik-Wegemund, M.; Wierucka-Młynarczyk, B.; Tanasiewicz, M.; Gilowski, Ł. Tunnel Technique with Collagen Matrix Compared With Connective Tissue Graft for Treatment of Periodontal Recession: A Randomized Clinical Trial. J. Periodontol. 2016, 87, 1436–1443. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aragoneses, J.; Suárez, A.; Rodríguez, C.; Aragoneses, J.M. Clinical and Histological Differences between Guided Tissue Regeneration with Acellular Dermal Matrix of Porcine Origin and Autologous Connective Tissue: An Animal Study. Materials 2021, 14, 272. https://doi.org/10.3390/ma14020272
Aragoneses J, Suárez A, Rodríguez C, Aragoneses JM. Clinical and Histological Differences between Guided Tissue Regeneration with Acellular Dermal Matrix of Porcine Origin and Autologous Connective Tissue: An Animal Study. Materials. 2021; 14(2):272. https://doi.org/10.3390/ma14020272
Chicago/Turabian StyleAragoneses, Javier, Ana Suárez, Cinthia Rodríguez, and Juan Manuel Aragoneses. 2021. "Clinical and Histological Differences between Guided Tissue Regeneration with Acellular Dermal Matrix of Porcine Origin and Autologous Connective Tissue: An Animal Study" Materials 14, no. 2: 272. https://doi.org/10.3390/ma14020272
APA StyleAragoneses, J., Suárez, A., Rodríguez, C., & Aragoneses, J. M. (2021). Clinical and Histological Differences between Guided Tissue Regeneration with Acellular Dermal Matrix of Porcine Origin and Autologous Connective Tissue: An Animal Study. Materials, 14(2), 272. https://doi.org/10.3390/ma14020272






