Effect of Radiation-Induced Cross-Linking on Thermal Aging Properties of Ethylene-Tetrafluoroethylene for Aircraft Cable Materials
Abstract
1. Introduction
2. Experimental Procedures
2.1. Materials
2.2. Sample Preparation
2.3. Irradiation
2.4. Ageing Experiments
2.5. Measurements
3. Results and Discussion
3.1. Investigations into Thermal Stability
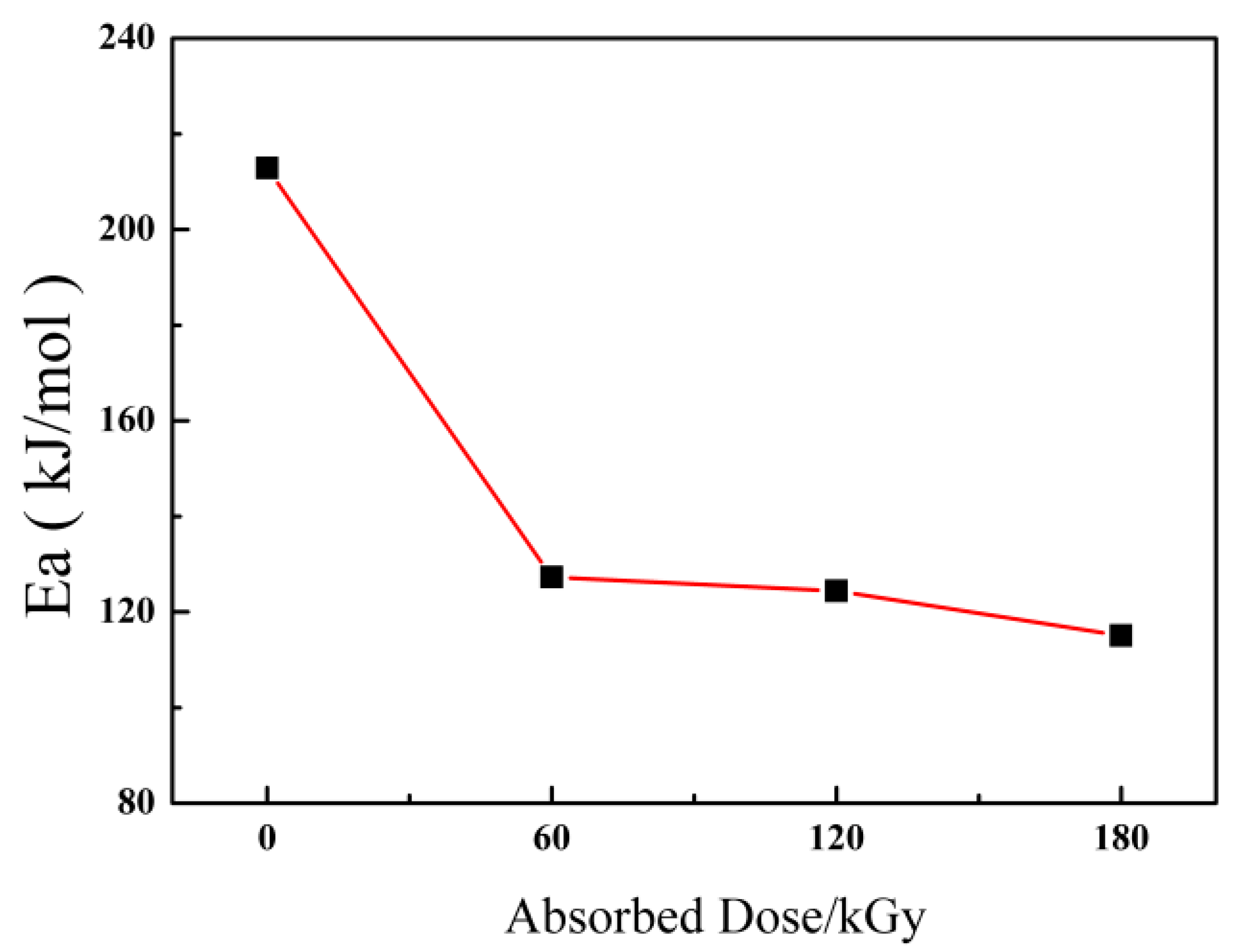
3.2. Kinetic Analysis of DSC
3.3. Mechanical Properties
3.4. Fracture Morphology
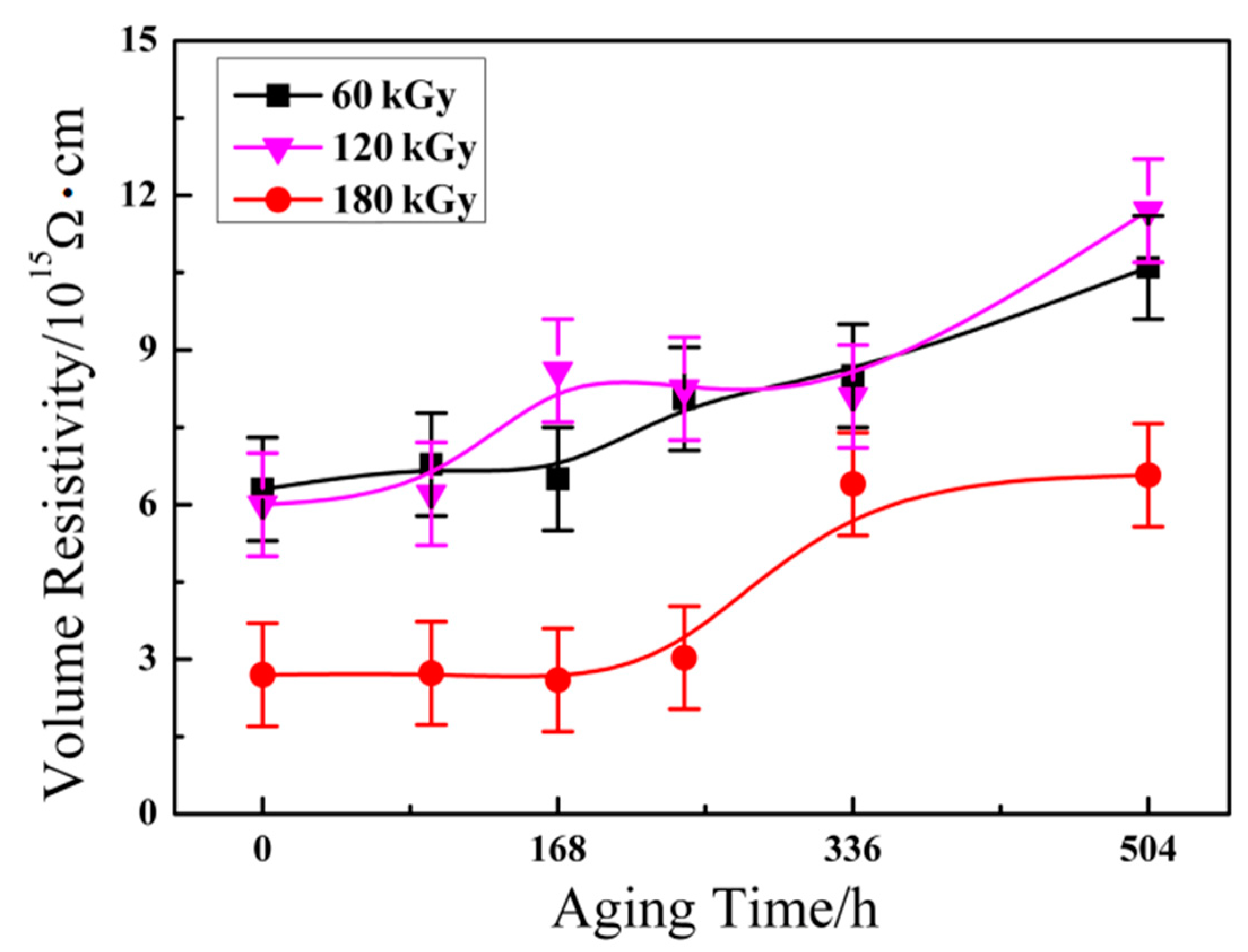
3.5. Volume Resistivity
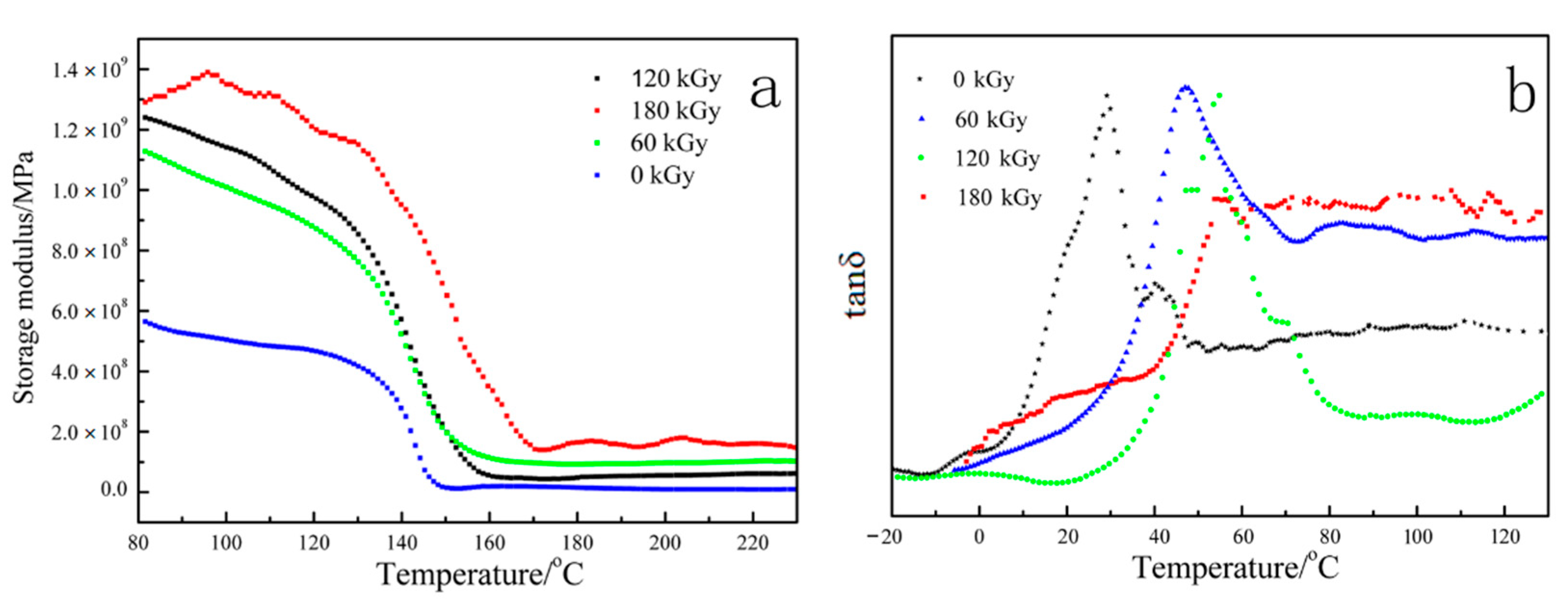
3.6. Dynamic Mechanical Thermal Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nasef, M.M.; Saidi, H.; Dahlanc, K.Z.M. Investigation of electron irradiation induced-changes in polyvinylidene fluoride) films. Polym. Degrad. Stabil. 2002, 1, 85–92. [Google Scholar] [CrossRef]
- Chen, J.H.; Asano, M.; Yamaki, T.; Yoshida, M. Chemical and radiation crosslinked polymer electrolyte membranes prepared from radiation-grafted ETFE films for DMFC applications. J. Power Sources 2006, 1, 69–77. [Google Scholar] [CrossRef]
- Forsythe, J.S.; Hill, D.J.T. The radiation chemistry of fluoropolymers. Prog. Polym. Sci. 2000, 1, 101–136. [Google Scholar] [CrossRef]
- Zen, H.A.; Ribeiro, G.; Geraldes, A.N.; Souza, C.P.; Parra, D.F.; Lugao, A.B. Effect of radiation induced cross-linked and degradation of ETFE films. Radiat. Phys. Chem. 2013, 84, 136–139. [Google Scholar] [CrossRef]
- Ponce-Gonzalez, J.; Ouachan, I.; Varcoe, J.R. Radition-induced grafting of a bultyl-spacer styrenic monomer onto ETFEF: The synthesis of the most alkali stable radiation-grafted anion-exchange membrane to date. J. Mater. Chem. A 2018, 6, 823–827. [Google Scholar] [CrossRef]
- Gautam, D.; Gupta, B.; Ikram, S. Radiation-induced graft copolymerization of a-methyl styrene and butyl acrylate mixture into polyetheretherketone films. J. Appl. Polym. Sci. 2013, 128, 1854–1860. [Google Scholar] [CrossRef]
- Yamaki, T.; Kobayashi, K.; Asano, M.; Kubota, H.; Yoshida, M. Preparation of proton exchange membranes based on crosslinked polytetrafluoroethylene for fuel cell applications. Polymer 2004, 19, 6569–6573. [Google Scholar] [CrossRef]
- Chen, J.H.; Masaharu, A.; Yasunari, M. Preparation of ETFE-based fuel cell membranes using UV-induced photografting and electron beam-induced crosslinking techniques. J. Membr. Sci. 2006, 1–2, 373–379. [Google Scholar] [CrossRef]
- Hu, J.; Chen, W.; Zhao, B.; Yang, D. Buildings with ETFE foils: A review on material properties, architectural performance and structural behavior. Constr. Build Mater. 2017, 131, 411–422. [Google Scholar] [CrossRef]
- Osorio, A.F.; Mizutani, K.; Fernandez-Pello, C.; Fujita, O. Microgravity flammability limits of ETFE insulated wires exposed to external radiation. Proc. Combust Inst. 2015, 35, 2683–2689. [Google Scholar] [CrossRef]
- Abdolzadeh, M.; Sadeqkhani, M.; Ahmadi, A. Computational modeling of a BIPV/T ethylene tetrafluoroethylen ETFE cushion structure roof. Energy 2017, 133, 998–1012. [Google Scholar] [CrossRef]
- Youcef, H.B.; Gürsel, S.A.; Wokaun, A.; Scherer, G.G. The influence of crosslinker on the properties of radiation-grafted films and membranes based on ETFE. J. Membr. Sci. 2008, 1–2, 208–215. [Google Scholar]
- Kotera, S.; Yamaguchi, M. Flow property at capillary extrusion for ethylene-tetrafluoroethylene copolymer. J. Fluor. Chem. 2015, 176, 20–25. [Google Scholar] [CrossRef]
- Hossain, U.H.; Ensinger, W. Experimental simulation of radiation damage of polymers in space applications by cosmic-ray-type high energy heavy ions and the resulting changes in optical properties. Nucl. Instrum. Meth. B 2015, 365, 230–234. [Google Scholar] [CrossRef]
- Dreveton, A. Overview of the fluorochemicals industrial sectors. Procedia Eng. 2016, 138, 240–247. [Google Scholar] [CrossRef]
- Kotera, S.; Yamaguchi, M. Rheological characterization on thermal degradation of ethylene-tetrafluoroethylene copolymer. J. Fluorine. Chem. 2014, 166, 117–121. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, W.; Hu, J.; Chen, J.; Qiu, Z.; Zhou, J.; Gao, C. Mechanical properties of ETFE foils in form-developing of inflated cushion through flat-patterning. Constr. Build Mater. 2016, 111, 580–589. [Google Scholar] [CrossRef]
- Oshima, A.; Udagawa, A.; Morita, Y. Application of radiation-crosslinked polytetrafluoroethylene to fiber-reinforced composite materials. Radiat. Phys. Chem. 2001, 60, 467–471. [Google Scholar] [CrossRef]
- Galante, A.M.S.; Galante, O.L.; Campos, L.L. Study on application of PTFE, FEP and PFA fluoropolymers on radiation dosimetry. Nucl. Instrum. Meth. A 2010, 619, 177–180. [Google Scholar] [CrossRef]
- Zhong, X.; Yu, L.; Sun, J.; Zhang, Y. XPS studies on radiation-induced structural changes in copolymer of tetrafluoroethylene and ethylene. J. Appl. Polym. Sci. 1993, 47, 21–24. [Google Scholar] [CrossRef]
- Avrami, M.J. Kinetics of phase change. II. Transformation-time relations for random distribution of nuclei. J. Chem. Phys. 1940, 8, 212–224. [Google Scholar] [CrossRef]

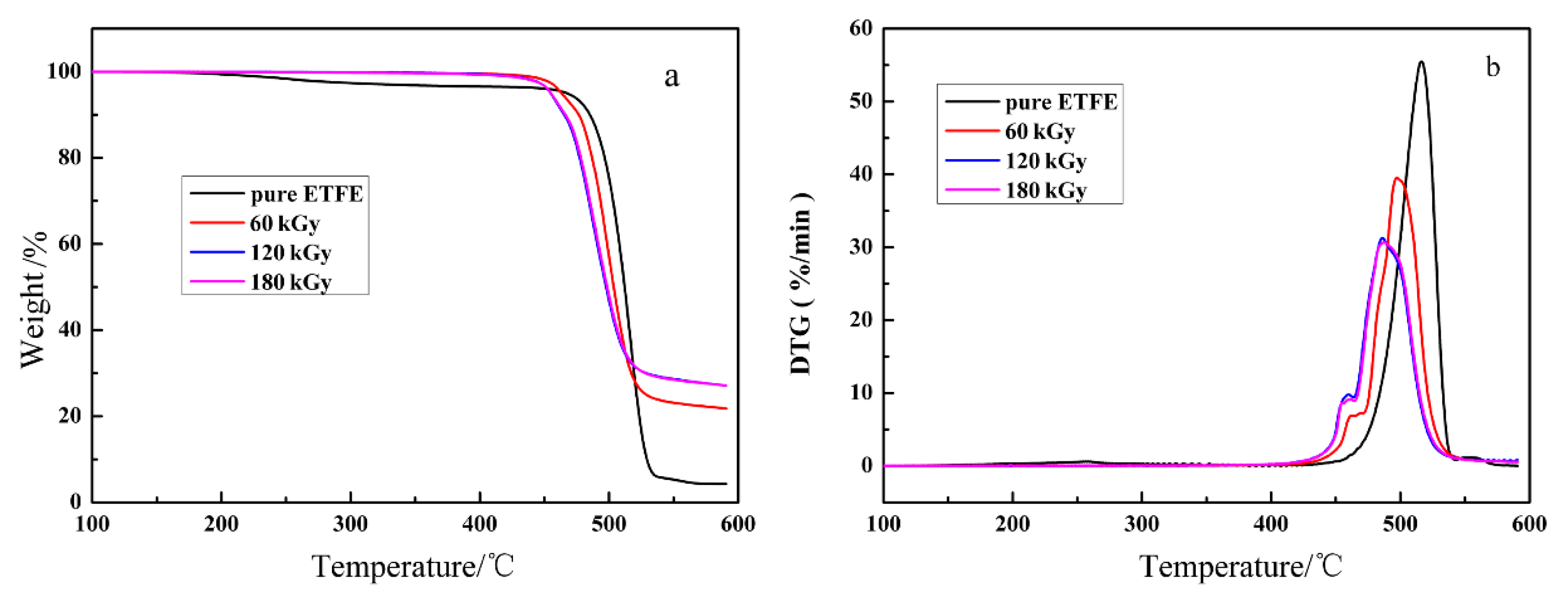

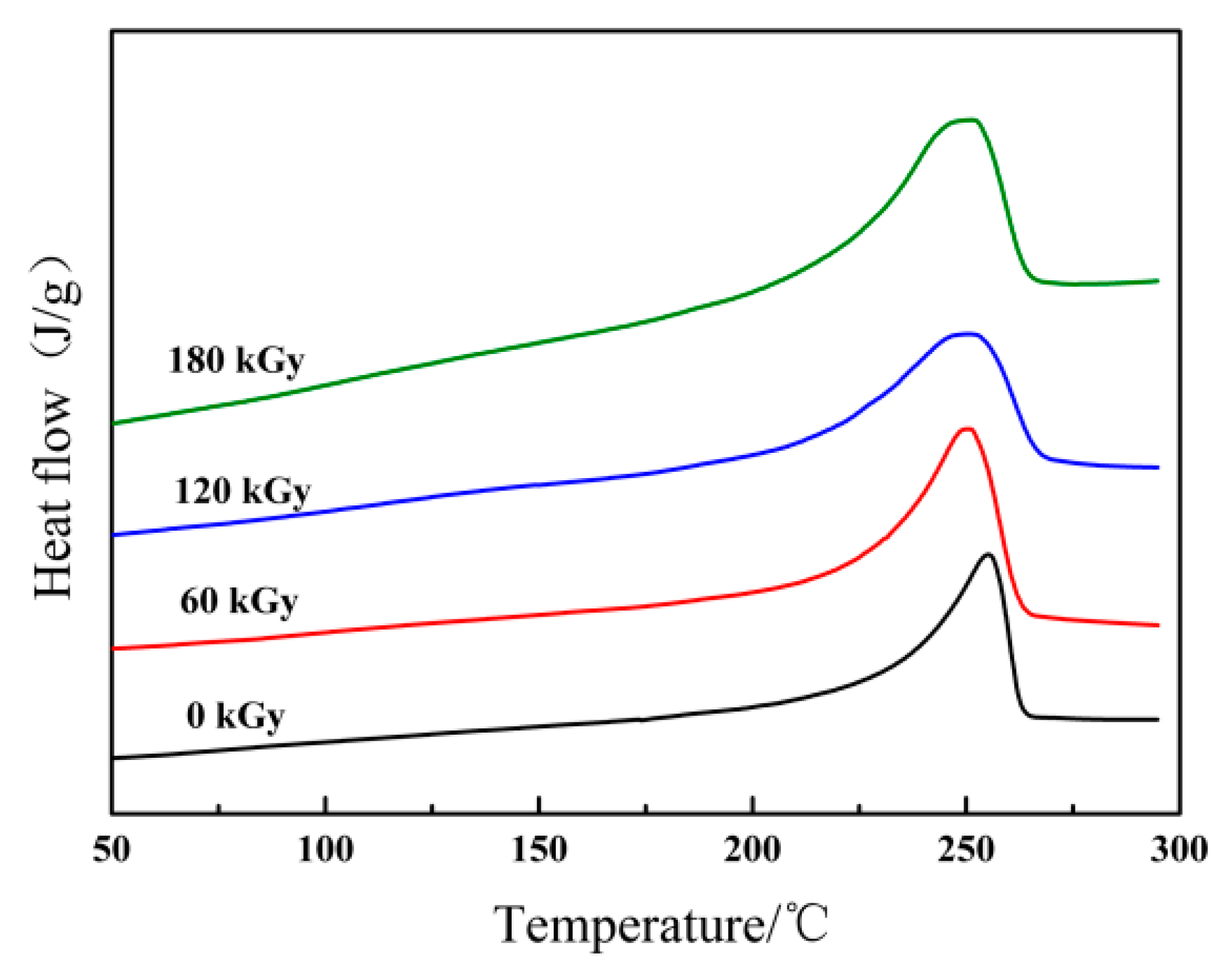
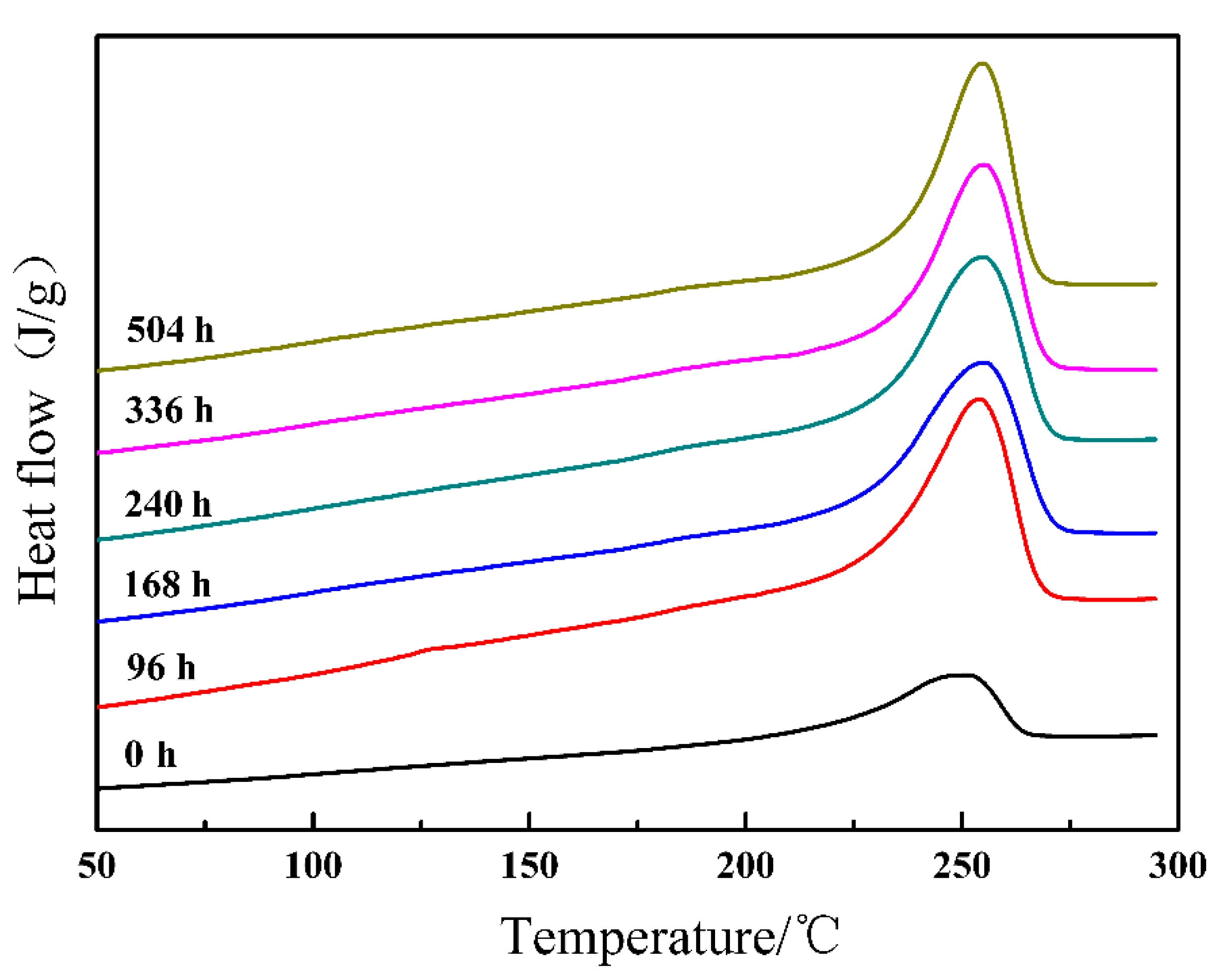


| Absorbed Dose (kGy) | Onset (°C) | Peak (°C) | Tc (°C) | ΔH (J/g) | Xc (%) |
|---|---|---|---|---|---|
| 0 | 232.8 | 255.1 | 237.9 | 41.1 | 36.3 |
| 60 | 225.2 | 250.5 | 237.0 | 34.4 | 30.3 |
| 120 | 214.4 | 250.7 | 238.0 | 31.0 | 27.4 |
| 180 | 221.6 | 251.1 | 238.2 | 24.1 | 21.3 |
| Aging Time (h) | Onset (°C) | Peak (°C) | Tc (°C) | ΔH (J/g) | Xc (%) |
|---|---|---|---|---|---|
| 0 | 221.6 | 251.1 | 238.2 | 24.1 | 21.3 |
| 96 | 229.1 | 254.0 | 241.5 | 27.9 | 24.6 |
| 168 | 226.3 | 255.2 | 241.0 | 29.9 | 26.4 |
| 240 | 229.6 | 255.0 | 241.7 | 30.4 | 26.8 |
| 336 | 232.5 | 255.1 | 241.5 | 32.4 | 28.6 |
| 504 | 234.7 | 254.9 | 241.7 | 31.6 | 27.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Chen, F.; Su, Z.; Xie, T. Effect of Radiation-Induced Cross-Linking on Thermal Aging Properties of Ethylene-Tetrafluoroethylene for Aircraft Cable Materials. Materials 2021, 14, 257. https://doi.org/10.3390/ma14020257
Zhang X, Chen F, Su Z, Xie T. Effect of Radiation-Induced Cross-Linking on Thermal Aging Properties of Ethylene-Tetrafluoroethylene for Aircraft Cable Materials. Materials. 2021; 14(2):257. https://doi.org/10.3390/ma14020257
Chicago/Turabian StyleZhang, Xiaodong, Fei Chen, Zhimin Su, and Taiping Xie. 2021. "Effect of Radiation-Induced Cross-Linking on Thermal Aging Properties of Ethylene-Tetrafluoroethylene for Aircraft Cable Materials" Materials 14, no. 2: 257. https://doi.org/10.3390/ma14020257
APA StyleZhang, X., Chen, F., Su, Z., & Xie, T. (2021). Effect of Radiation-Induced Cross-Linking on Thermal Aging Properties of Ethylene-Tetrafluoroethylene for Aircraft Cable Materials. Materials, 14(2), 257. https://doi.org/10.3390/ma14020257




