Antibacterial Textile Based on Hydrolyzed Milk Casein
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Casein Hydrolysis
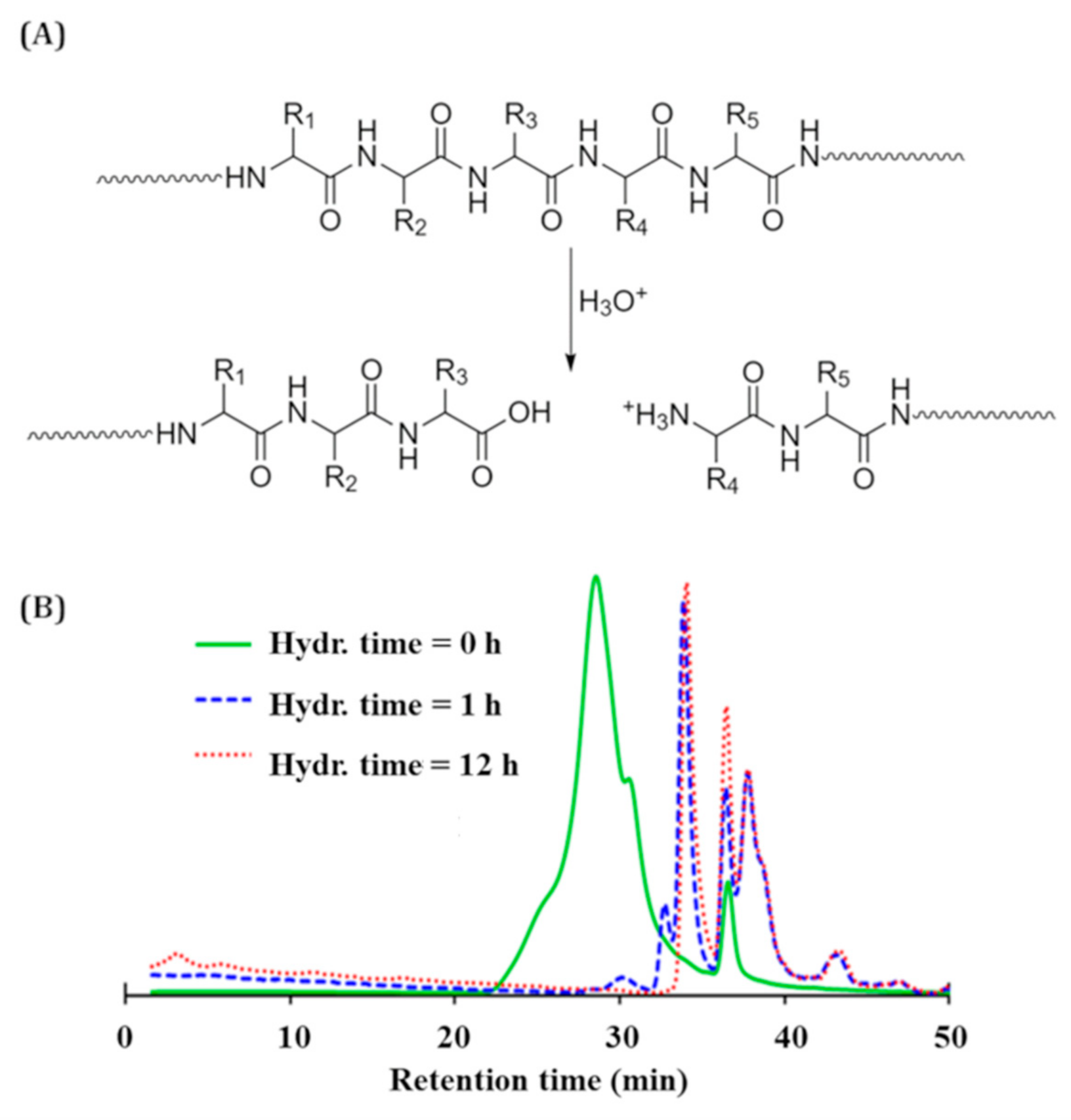
2.3. Size Exclusion Chromatography
2.4. Extrusion
2.5. Melt Spinning
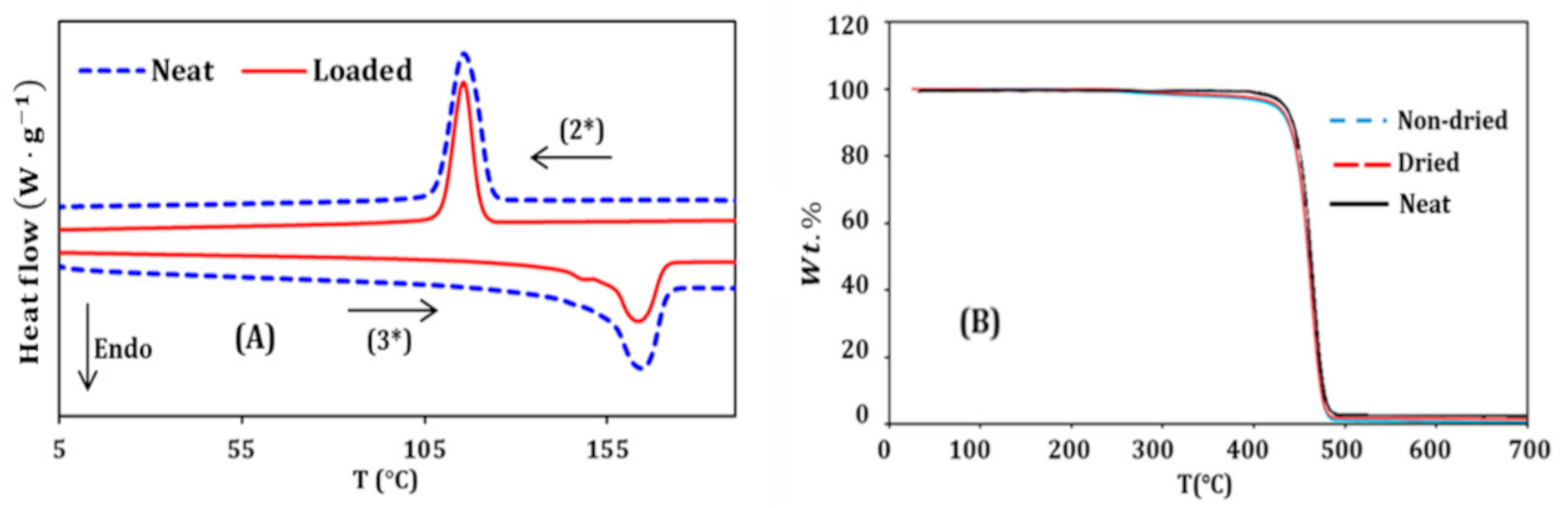
2.6. Thermal Gravimetric Analysis (TGA)
2.7. Deferential Scanning Calorimetry (DSC)
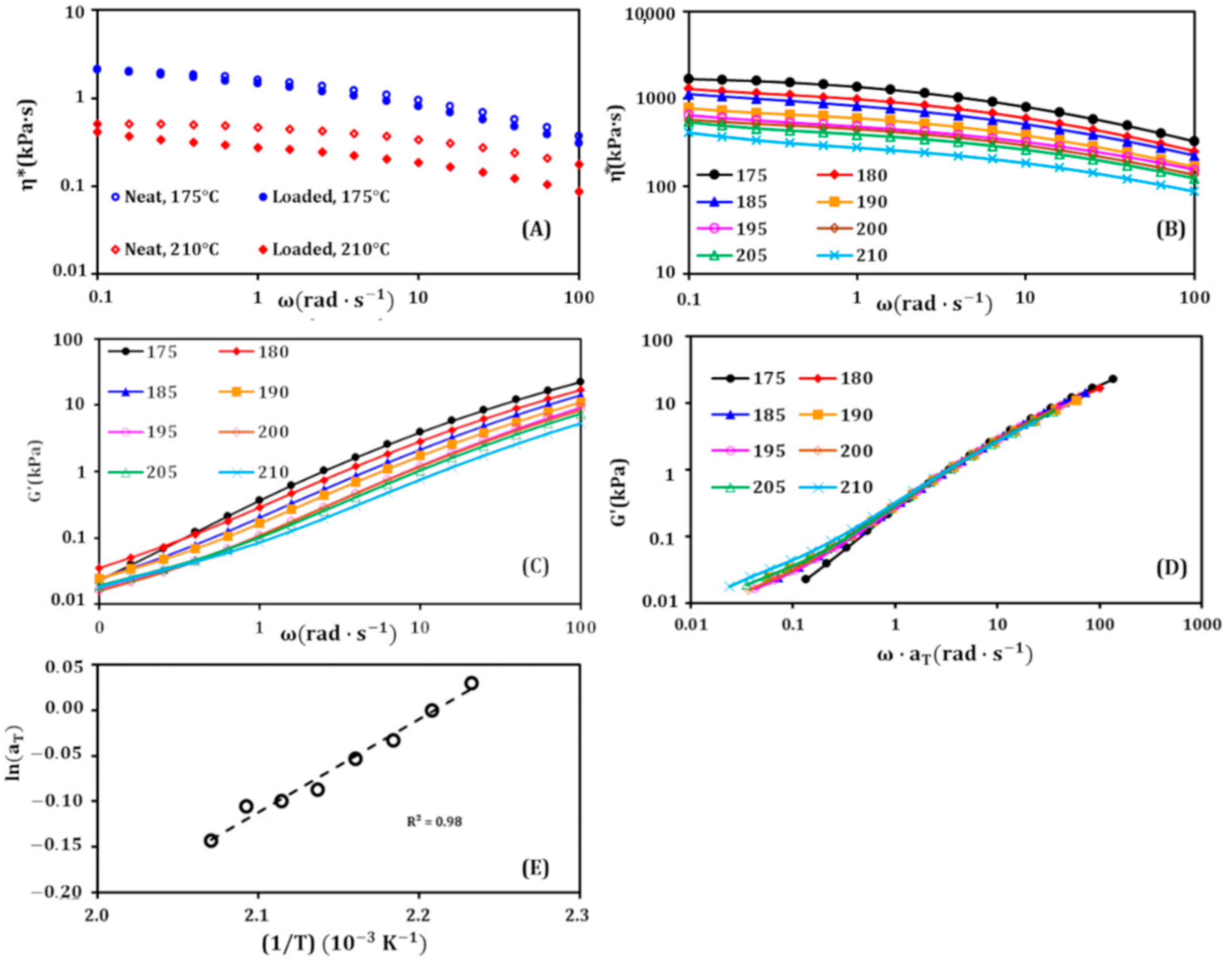
2.8. Dynamic Rheology
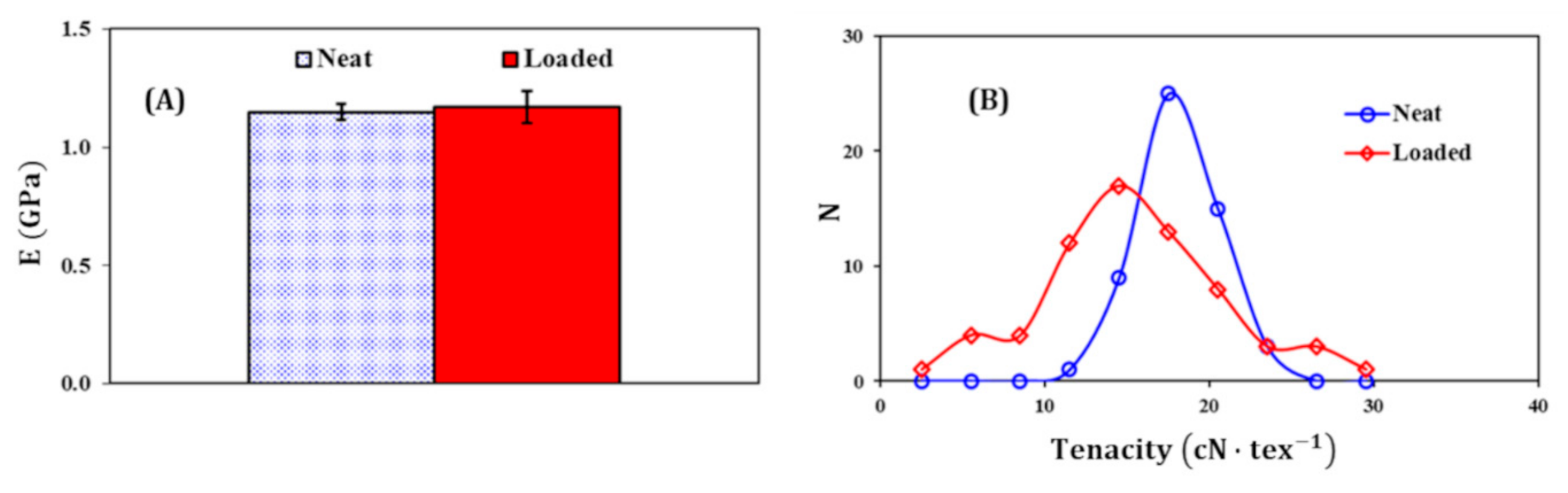
2.9. Tensile Tests
2.10. Antimicrobial Actsivity
3. Results and Discussion
3.1. Casein Hydrolysis
3.2. Rheological Study
3.3. Thermal Study
3.4. Mechanical Properties
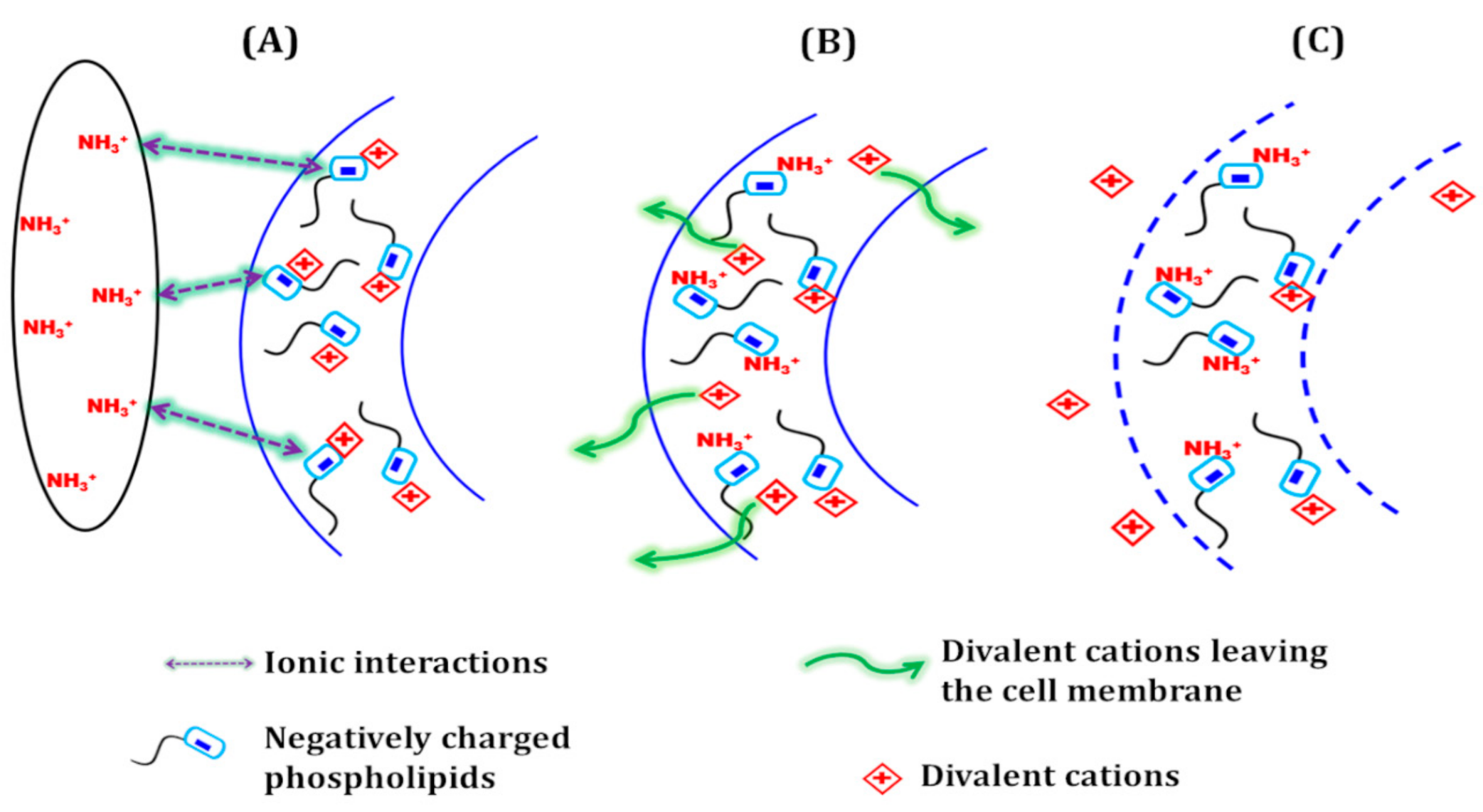
3.5. Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doumbia, A.; Vezin, H.; Ferreira, M.; Campagne, C.; Devaux, E. Studies of polylactide/zinc oxide nanocomposites: Influence of surface treatment on zinc oxide antibacterial activities in textile nanocomposites. J. Appl. Polym. Sci. 2015, 132, 41776–41785. [Google Scholar] [CrossRef]
- Gressier, P.; De Smet, D.; Behary, N.; Campagne, C.; Vanneste, M. Antibacterial polyester fabrics via diffusion process using active bio-based agents from essential oils. Ind. Crop. Prod. 2019, 136, 11–20. [Google Scholar] [CrossRef]
- Ashraf, M.; Dumont, F.; Campagne, C.; Champagne, P.; Perwuelz, A.; Leriche, A.; Chihib, N.E. Development of Antibacterial Polyester Fabric by Growth of ZnO Nanorods. J. Eng. Fibers Fabr. 2014, 9, 15–22. [Google Scholar] [CrossRef]
- Qin, Y. 7-Antimicrobial textile dressings to manage wound infection. In Advanced Textiles for Wound Care, 2nd ed.; Rajendran, S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 193–210. ISBN 978-0-08-102192-7. [Google Scholar]
- Rajendran, S.; Anand, S.C. 14-Woven textiles for medical applications. In Woven Textiles; Gandhi, K.L., Ed.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2012; pp. 414–441. ISBN 978-1-84569-930-7. [Google Scholar]
- Gupta, D.; Bhaumik, S. Antimicrobial treatments for textiles. Indian J. Fibre Text. Res. 2007, 32, 254–263. [Google Scholar]
- Minet, J.; Cayla, A.; Campagne, C. Lignin as Sustainable Antimicrobial Fillers to Develop PET Multifilaments by Melting Process. Org. Polym. 2019. [Google Scholar] [CrossRef]
- Tan, L.-Y.; Sin, L.T.; Bee, S.-T.; Ratnam, C.T.; Woo, K.-K.; Tee, T.-T.; Rahmat, A.R. A review of antimicrobial fabric containing nanostructures metal-based compound. J. Vinyl Addit. Technol. 2019, 25, E3–E27. [Google Scholar] [CrossRef]
- Morais, D.S.; Guedes, R.M.; Lopes, M.A. Antimicrobial Approaches for Textiles: From Research to Market. Materials 2016, 9, 498. [Google Scholar] [CrossRef]
- Gao, G.; Cranston, R. Recent Advances in Antimicrobial Treatments of Textiles. Text. Res. J. 2008, 78, 60–72. [Google Scholar] [CrossRef]
- Ballottin, D.; Fulaz, S.; Cabrini, F.; Tsukamoto, J.; Durán, N.; Alves, O.L.; Tasic, L. Antimicrobial textiles: Biogenic silver nanoparticles against Candida and Xanthomonas. Mater. Sci. Eng. C 2017, 75, 582–589. [Google Scholar] [CrossRef]
- Mirjalili, M.; Yaghmaei, N.; Mirjalili, M. Antibacterial properties of nano silver finish cellulose fabric. J. Nanostruct. Chem. 2013, 3, 43. [Google Scholar] [CrossRef]
- Zhang, F.; Wu, X.; Chen, Y. Application of Silver Nanoparticles to Cotton Fabric as an Antibacterial Textile Finish. Fibers Polym. 2009, 10, 496–501. [Google Scholar] [CrossRef]
- Simončič, B.; Klemenčič, D. Preparation and performance of silver as an antimicrobial agent for textiles: A review. Text. Res. J. 2015, 86, 210–223. [Google Scholar] [CrossRef]
- Yıldız, A.; Değirmencioğlu, M. Synthesis of Silver Abietate as an Antibacterial Agent for Textile Applications. Bioinorg. Chem. Appl. 2015, 2015, 1–5. [Google Scholar] [CrossRef]
- Borkow, G.; Felix, A.; Gabbay, J. Copper-Impregnated Antimicrobial Textiles; an Innovative Weapon to Fight Infection. In Medical and Healthcare, Textiles; Anand, S.C., Kennedy, J.F., Miraftab, M., Rajendran, S., Eds.; Woodhead Publishing Series in Textiles; Woodhead Publishing: Sawston, UK, 2010; pp. 14–22. ISBN 978-1-84569-224-7. [Google Scholar]
- Hasan, R. Production of Antimicrobial Textiles by Using Copper Oxide Nanoparticles. Int. J. Contemp. Res. Rev. 2018, 9, 20195–20202. [Google Scholar] [CrossRef]
- Teli, M.D.; Sheikh, J. Modified bamboo rayon–copper nanoparticle composites as antibacterial textiles. Int. J. Biol. Macromol. 2013, 61, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Selvam, S.; Rajiv Gandhi, R.; Suresh, J.; Gowri, S.; Ravikumar, S.; Sundrarajan, M. Antibacterial effect of novel synthesized sulfated β-cyclodextrin crosslinked cotton fabric and its improved antibacterial activities with ZnO, TiO2 and Ag nanoparticles coating. Int. J. Pharm. 2012, 434, 366–374. [Google Scholar] [CrossRef]
- Staneva, D.; Vasileva-Tonkova, E.; Grabchev, I. A New Bioactive Complex between Zn(II) and a Fluorescent Symmetrical Benzanthrone Tripod for an Antibacterial Textile. Materials 2019, 12, 3473. [Google Scholar] [CrossRef] [PubMed]
- Montazer, M.; Pakdel, E.; Behzadnia, A. Novel feature of nano-titanium dioxide on textiles: Antifelting and antibacterial wool. J. Appl. Polym. Sci. 2011, 121, 3407–3413. [Google Scholar] [CrossRef]
- Rastgoo, M.; Montazer, M.; Malek, R.M.A.; Harifi, T.; Mahmoudi Rad, M. Ultrasound mediation for one-pot sonosynthesis and deposition of magnetite nanoparticles on cotton/polyester fabric as a novel magnetic, photocatalytic, sonocatalytic, antibacterial and antifungal textile. Ultrason. Sonochem. 2016, 31, 257–266. [Google Scholar] [CrossRef]
- Uddin, F. Environmental concerns in antimicrobial finishing of textiles. Int. J. Text. Sci. 2014, 3, 15–20. [Google Scholar] [CrossRef]
- Windler, L.; Height, M.; Nowack, B. Comparative evaluation of antimicrobials for textile applications. Environ. Int. 2013, 53, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Guggenbichler, P.; Heldt, P.; Jünger, M.; Ladwig, A.; Thierbach, H.; Weber, U.; Daeschlein, G. Hygienic relevance and risk assessment of antimicrobial-impregnated textiles. Curr. Probl. Dermatol. 2006, 33, 78–109. [Google Scholar] [CrossRef]
- Trabold, T.; Babbitt, C.W. Sustainable Food Waste-to-Energy Systems; Academic Press: Cambridge, MA, USA, 2018; ISBN 978-0-12-811158-1. [Google Scholar]
- Malacarne, M.; Martuzzi, F.; Summer, A.; Mariani, P. Protein and fat composition of mare’s milk: Some nutritional remarks with reference to human and cow’s milk. Int. Dairy J. 2002, 12, 869–877. [Google Scholar] [CrossRef]
- Jahan-Mihan, A.; Luhovyy, B.L.; Khoury, D.E.; Anderson, G.H. Dietary Proteins as Determinants of Metabolic and Physiologic Functions of the Gastrointestinal Tract. Nutrients 2011, 3, 574–603. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.E.; Folmer Côrrea, A.P.; Mancilla Canales, M.; Joner Daroit, D.; Brandelli, A.; Risso, P. Biological and physicochemical properties of bovine sodium caseinate hydrolysates obtained by a bacterial protease preparation. Food Hydrocoll. 2015, 43, 510–520. [Google Scholar] [CrossRef]
- Tzvetkova, I.; Dalgalarrondo, M.; Danova, S.; Iliev, I.; Ivanova, I.; Chobert, J.-M.; Haertlé, T. Hydrolysis of Major Dairy Proteins by Lactic Acid Bacteria from Bulgarian Yogurts. J. Food Biochem. 2007, 31, 680–702. [Google Scholar] [CrossRef]
- Klibanov, A.M. Permanently microbicidal materials coatings. J. Mater. Chem. 2007, 17, 2479–2482. [Google Scholar] [CrossRef]
- Varesano, A.; Vineis, C.; Aluigi, A.; Rombaldoni, F.; Pella, C.G. Antimicrobial polymers for textile products. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011. [Google Scholar]
- Thamizharasi, S.; Vasantha, J.; Reddy, B.S.R. Synthesis, characterization and pharmacologically active sulfamethoxazole polymers. Eur. Polym. J. 2002, 38, 551–559. [Google Scholar] [CrossRef]
- Moon, W.-S.; Kim, J.C.; Chung, K.-H.; Park, E.-S.; Kim, M.-N.; Yoon, J.-S. Antimicrobial activity of a monomer and its polymer based on quinolone. J. Appl. Polym. Sci. 2003, 90, 1797–1801. [Google Scholar] [CrossRef]
- Hebeish, A.; El-Naggar, M.E.; Fouda, M.M.G.; Ramadan, M.A.; Al-Deyab, S.S.; El-Rafie, M.H. Highly effective antibacterial textiles containing green synthesized silver nanoparticles. Carbohydr. Polym. 2011, 86, 936–940. [Google Scholar] [CrossRef]
- Juknius, T.; Ružauskas, M.; Tamulevičius, T.; Šiugždinienė, R.; Juknienė, I.; Vasiliauskas, A.; Jurkevičiūtė, A.; Tamulevičius, S. Antimicrobial Properties of Diamond-Like Carbon/Silver Nanocomposite Thin Films Deposited on Textiles: Towards Smart Bandages. Materials 2016, 9, 371. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Karimi, L. Antibacterial dyeing of polyamide using turmeric as a natural dye. AUTEX Res. J. 2013, 13, 51–56. [Google Scholar] [CrossRef]
- Yeo, S.Y.; Lee, H.J.; Jeong, S.H. Preparation of nanocomposite fibers for permanent antibacterial effect. J. Mater. Sci. 2003, 38, 2143–2147. [Google Scholar] [CrossRef]
- Jeong, S.H.; Yeo, S.Y.; Yi, S.C. The effect of filler particle size on the antibacterial properties of compounded polymer/silver fibers. J. Mater. Sci. 2005, 40, 5407–5411. [Google Scholar] [CrossRef]
- Radheshkumar, C.; Münstedt, H. Morphology and mechanical properties of antimicrobial polyamide/silver composites. Mater. Lett. 2005, 59, 1949–1953. [Google Scholar] [CrossRef]
- Radheshkumar, C.; Münstedt, H. Antimicrobial polymers from polypropylene/silver composites—Ag+ release measured by anode stripping voltammetry. React. Funct. Polym. 2006, 66, 780–788. [Google Scholar] [CrossRef]
- Belkhir, K.; Jegat, C.; Prochazka, F.; Taha, M. Quaternary ammonium-functionalized polymers in biodegradable matrices: Physicochemical properties, morphology, and biodegradability. J. Appl. Polym. Sci. 2017, 134, 45261. [Google Scholar] [CrossRef]
- Kurbanova, M.; Maslennikova, S. Acid Hydrolysis of Casein. Foods Raw Mater. 2014, 2, 27–30. [Google Scholar] [CrossRef]
- Zink, J.; Wyrobnik, T.; Prinz, T.; Schmid, M. Physical, Chemical and Biochemical Modifications of Protein-Based Films and Coatings: An Extensive Review. Int. J. Mol. Sci. 2016, 17, 1376. [Google Scholar] [CrossRef]
- Bagrodia, S.; Wilkes, G.L.; Kennedy, J.P. New polyisobutylene-based model elastomeric ionomers: RheoLogical behavior. Polym. Eng. Sci. 1986, 26, 662–672. [Google Scholar] [CrossRef]
- Eisenberg, A.; King, M. Ion-Containing Polymers, Physical Properties and Structures, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1977; ISBN 9780323156752. [Google Scholar]
- Vanhoorne, P.; Register, R.A. Low-Shear Melt Rheology of Partially-Neutralized Ethylene−Methacrylic Acid Ionomers. Macromolecules 1996, 29, 598–604. [Google Scholar] [CrossRef]
- Han, S.-I.; Im, S.S.; Kim, D.K. Dynamic mechanical and melt rheological properties of sulfonated poly (butylene succinate) ionomers. Polymer 2003, 44, 7165–7173. [Google Scholar] [CrossRef]
- Greener, J.; Gillmor, J.R.; Daly, R.C. Melt rheology of a class of polyester ionomers. Macromolecules 1993, 26, 6416–6424. [Google Scholar] [CrossRef]
- Tobolsky, A.V.; Lyons, P.F.; Hata, N. Ionic Clusters in High-Strength Carboxylic Rubbers. Macromolecules 1968, 1, 515–519. [Google Scholar] [CrossRef]
- Tao, W.; Shen, J.; Chen, Y.; Liu, J.; Gao, Y.; Wu, Y.; Zhang, L.; Tsige, M. Strain rate and temperature dependence of the mechanical properties of polymers: A universal time-temperature superposition principle. J. Chem. Phys. 2018, 149, 044105. [Google Scholar] [CrossRef]
- Yusoff, N.I.M.; Chailleux, E.; Airey, G. A comparative study of the influence of shift factor equations on master curve construction. Int. J. Pavement Res. Technol. 2011, 4, 324–336. [Google Scholar]
- Fan, B.; Kazmer, D.O. Low-temperature modeling of the time-temperature shift factor for polycarbonate. Adv. Polym. Technol. 2005, 24, 278–287. [Google Scholar] [CrossRef]
- Matsuura, H. Secondary Stress Relaxation Mechanism in Ion-Containing Polymers I. A New Analytical Method. Polym. J. 1986, 18, 1027–1035. [Google Scholar] [CrossRef][Green Version]
- Broze, G.; Jérôme, R.; Teyssié, P.; Marco, C. Experimental Study of the Rheological Properties of Ion Containing Amorphous Polymers. In Rheology: Fluids; Astarita, G., Marrucci, G., Nicolais, L., Eds.; Springer: Boston, MA, USA, 1980; Volume 2, pp. 527–533. ISBN 978-1-4684-3743-0. [Google Scholar]
- Belkhir, K.; Majesté, J.-C.; Jegat, C.; Taha, M. Rheological Study of Quaternary Ammonium-containing Star-shaped Polylactic acid. J. Appl. Polym. Sci. 2019, 136, 48337–48347. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, Z.; Mai, K. Non-isothermal crystallization kinetics of UHMWPE composites filled by oligomer-modified CaCO3. J. Therm. Anal. Calorim. 2020, 139, 1111–1120. [Google Scholar] [CrossRef]
- Fernández, M.D.; Guzmán, D.J.; Ramos, J.R.; Fernández, M.J. Effect of Alkyl Chain Length in POSS Nanocage on Non-Isothermal Crystallization Behavior of PCL/Amino-POSS Nanocomposites. Polymers 2019, 11, 1719. [Google Scholar] [CrossRef]
- Mai, K.; Wang, K.; Zeng, H. Multiple melting behavior of nucleated polypropylene. J. Appl. Polym. Sci. 2003, 88, 1608–1611. [Google Scholar] [CrossRef]
- Oliver-Ortega, H.; Méndez, J.A.; Mutjé, P.; Tarrés, Q.; Espinach, F.X.; Ardanuy, M. Evaluation of Thermal and Thermomechanical Behaviour of Bio-Based Polyamide 11 Based Composites Reinforced with Lignocellulosic Fibres. Polymers 2017, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- Shigaki, M.; Niki, Y.; Nishizawa, K.; Ushioda, H.; Nakanishi, T.; Nakajima, T. Effect of hygroscopic fabrics on wear comfort in the fluctuating microclimate of clothing. J. Therm. Biol. 1993, 18, 429–434. [Google Scholar] [CrossRef]
- Nordon, P.; David, H.G. Coupled diffusion of moisture and heat in hygroscopic textile materials. Int. J. Heat Mass Transf. 1967, 10, 853–866. [Google Scholar] [CrossRef]
- Soomro, N. Effect of Drying Methods on Quality of Cotton Fibers Before Ginning. Eur. Sci. J. 2014, 10, 303–312. [Google Scholar] [CrossRef]
- Hegyi, A.; Dico, C.; Szilagyi, H. Sheep Wool Thermal Insulating Mattresses Behaviour in the Water Vapours Presence. Procedia Manuf. 2020, 46, 410–417. [Google Scholar] [CrossRef]
- Sadik, T.; Massardier, V.; Becquart, F.; Taha, M. Polyolefins/Poly(3-hydroxybutyrate-co-hydroxyvalerate) blends compatibilization: Morphology, rheological, and mechanical properties. J. Appl. Polym. Sci. 2013, 127, 1148–1156. [Google Scholar] [CrossRef]
- Tseng, F.-P.; Lin, J.-J.; Tseng, C.-R.; Chang, F.-C. Poly(oxypropylene)-amide grafted polypropylene as novel compatibilizer for PP and PA6 blends. Polymer 2001, 42, 713–725. [Google Scholar] [CrossRef]
- Dal Lago, E.; Boaretti, C.; Piovesan, F.; Roso, M.; Lorenzetti, A.; Modesti, M. The Effect of Different Compatibilizers on the Properties of a Post-Industrial PC/PET Blend. Materials 2019, 12, 49. [Google Scholar] [CrossRef]
- Hajibaba, A.; Masoomi, M.; Nazockdast, H. Compatibilization effectiveness of maleated polypropylene compared to organoclay in PBT/PP blends. Iran. Polym. J. 2016, 25, 157–167. [Google Scholar] [CrossRef]
- Qiu, W.; Endo, T.; Hirotsu, T. A novel technique for preparing of maleic anhydride grafted polyolefins. Eur. Polym. J. 2005, 41, 1979–1984. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial peptides of multicellular organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Belkhir, K.; Lacroix, M.; Jamshidian, M.; Salmieri, S.; Jegat, C.; Taha, M. Evaluation of antibacterial activity of branched quaternary ammonium grafted green polymers. Food Packag. Shelf Life 2017, 12, 28–41. [Google Scholar] [CrossRef]
- Kennedy, S.; Aiken, E.; Gerbin, K.; Hahn, A.; Kamin, S.; Hagness, S.; Booske, J.; Murphy, W. Cationic Peptide Exposure Enhances Pulsed-Electric-Field-Mediated Membrane Disruption. PLoS ONE 2014, 9, e92528. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bieser, A.M.; Tiller, J.C. Mechanistic Considerations on Contact-Active Antimicrobial Surfaces with Controlled Functional Group Densities. Macromol. Biosci. 2011, 11, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Asri, L.A.T.W.; Crismaru, M.; Roest, S.; Chen, Y.; Ivashenko, O.; Rudolf, P.; Tiller, J.C.; van der Mei, H.C.; Loontjens, T.J.A.; Busscher, H.J. A Shape-Adaptive, Antibacterial-Coating of Immobilized Quaternary-Ammonium Compounds Tethered on Hyperbranched Polyurea and its Mechanism of Action. Adv. Funct. Mater. 2014, 24, 346–355. [Google Scholar] [CrossRef]
- Hu, X.; Lin, X.; Zhao, H.; Chen, Z.; Yang, J.; Li, F.; Liu, C.; Tian, F. Surface Functionalization of Polyethersulfone Membrane with Quaternary Ammonium Salts for Contact-Active Antibacterial and Anti-Biofouling Properties. Materials 2016, 9, 376. [Google Scholar] [CrossRef] [PubMed]
- Owen, L.; Laird, K. Development of a silver-based dual-function antimicrobial laundry additive and textile coating for the decontamination of healthcare laundry. J. Appl. Microbiol. 2020. [Google Scholar] [CrossRef] [PubMed]
- d’Água, R.B.; Branquinho, R.; Duarte, M.P.; Maurício, E.; Fernando, A.L.; Martins, R.; Fortunato, E. Efficient coverage of ZnO nanoparticles on cotton fibres for antibacterial finishing using a rapid and low cost in situ synthesis. New J. Chem. 2018, 42, 1052–1060. [Google Scholar] [CrossRef]
| Extrusion Temperature Profile | |||||
|---|---|---|---|---|---|
| Zone | Z1 (feed) | Z2 | Z3 | Z4 | Z5 (die) |
| T (°C) | 160 | 170 | 180 | 190 | 200 |
| Temperature Profile in the Single-Screw Extruder | Parameters of the Rolls | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Roll 1 | Roll 2 | R | ||||||||
| z1 | z2 | z3 | z4 | z5 | z6 | T (°C) | S1 (rpm) | T (°C) | S2 (rpm) | 2.5 |
| 175 | 180 | 190 | 190 | 195 | 195 | 70 | 80 | 80 | 200 | |
| 0 Contact Time | After 24 h | |||
|---|---|---|---|---|
| Test towards Staphylococcus aureus | Neat PP | Trial 1 | 4.4 × 04 | 1.3 × 107 |
| Trial 2 | 4.4 × 104 | 9.2 × 106 | ||
| Average CFU | 4.4 × 104 | 1.1 × 107 | ||
| Log(average CFU) | 4.9 | 7.1 | ||
| Growth value F | 2.5 | |||
| 5 wt.% loaded | Trial 1 | 1.8 × 105 | <20 * | |
| Trial 2 | 1.3 × 105 | <20 * | ||
| Average CFU | 1.6 × 105 | <20 | ||
| Log(average CFU) | 5.2 | <1.3 | ||
| Growth value G | <−3.9 | |||
| >6.4 | ||||
| Tests towards Klebsiella pneumoniae | Neat PP | Trial 1 | 4.6 × 104 | 4.0 × 107 |
| Trial 2 | 1.1 × 105 | 2.8 × 107 | ||
| Average CFU | 7.8 × 104 | 3.4 × 107 | ||
| Log(average CFU) | 4.9 | 7.5 | ||
| Growth value F | 2.6 | |||
| 5 wt.% loaded | Trial 1 | 2.2 × 105 | <20 * | |
| Trial 2 | 5.0 × 104 | <20 * | ||
| Average CFU | 1.4 × 105 | <20 | ||
| Log(average CFU) | 5.1 | <1.3 | ||
| Growth value G | <−3.8 | |||
| >6.4 | ||||
| Efficacy of Antibacterial Property | Antibacterial Value A |
|---|---|
| Significant | 2 ≤ A < 3 |
| Strong | A ≥ 3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belkhir, K.; Pillon, C.; Cayla, A.; Campagne, C. Antibacterial Textile Based on Hydrolyzed Milk Casein. Materials 2021, 14, 251. https://doi.org/10.3390/ma14020251
Belkhir K, Pillon C, Cayla A, Campagne C. Antibacterial Textile Based on Hydrolyzed Milk Casein. Materials. 2021; 14(2):251. https://doi.org/10.3390/ma14020251
Chicago/Turabian StyleBelkhir, Kedafi, Caroline Pillon, Aurélie Cayla, and Christine Campagne. 2021. "Antibacterial Textile Based on Hydrolyzed Milk Casein" Materials 14, no. 2: 251. https://doi.org/10.3390/ma14020251
APA StyleBelkhir, K., Pillon, C., Cayla, A., & Campagne, C. (2021). Antibacterial Textile Based on Hydrolyzed Milk Casein. Materials, 14(2), 251. https://doi.org/10.3390/ma14020251







