Research on the Preparation Parameters and Basic Properties of Premelted Calcium Aluminate Slag Prepared from Secondary Aluminum Dross
Abstract
1. Introduction
2. Analysis of Secondary Aluminum Dross
3. Thermodynamic Analysis and Melting Point Measurement
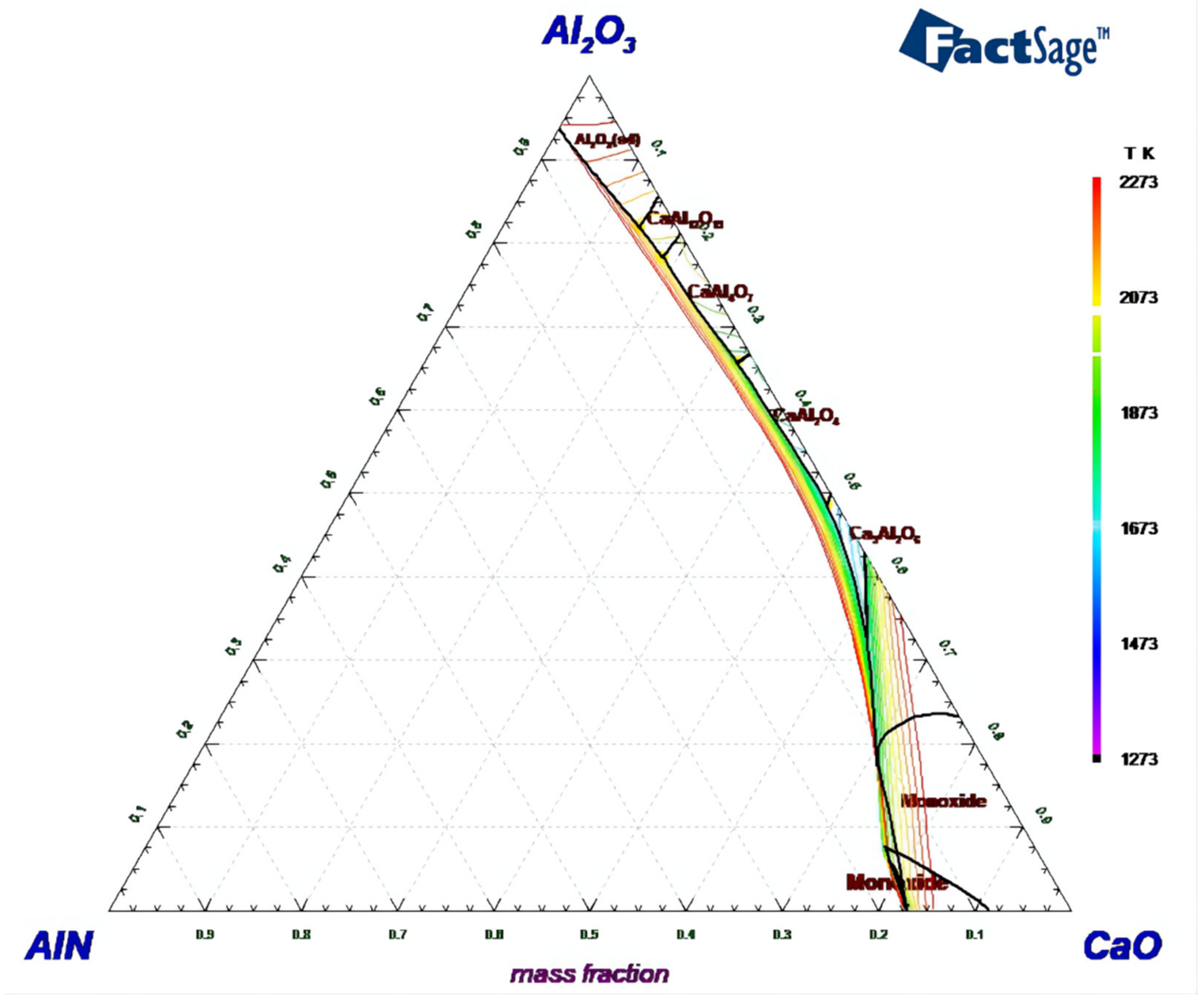
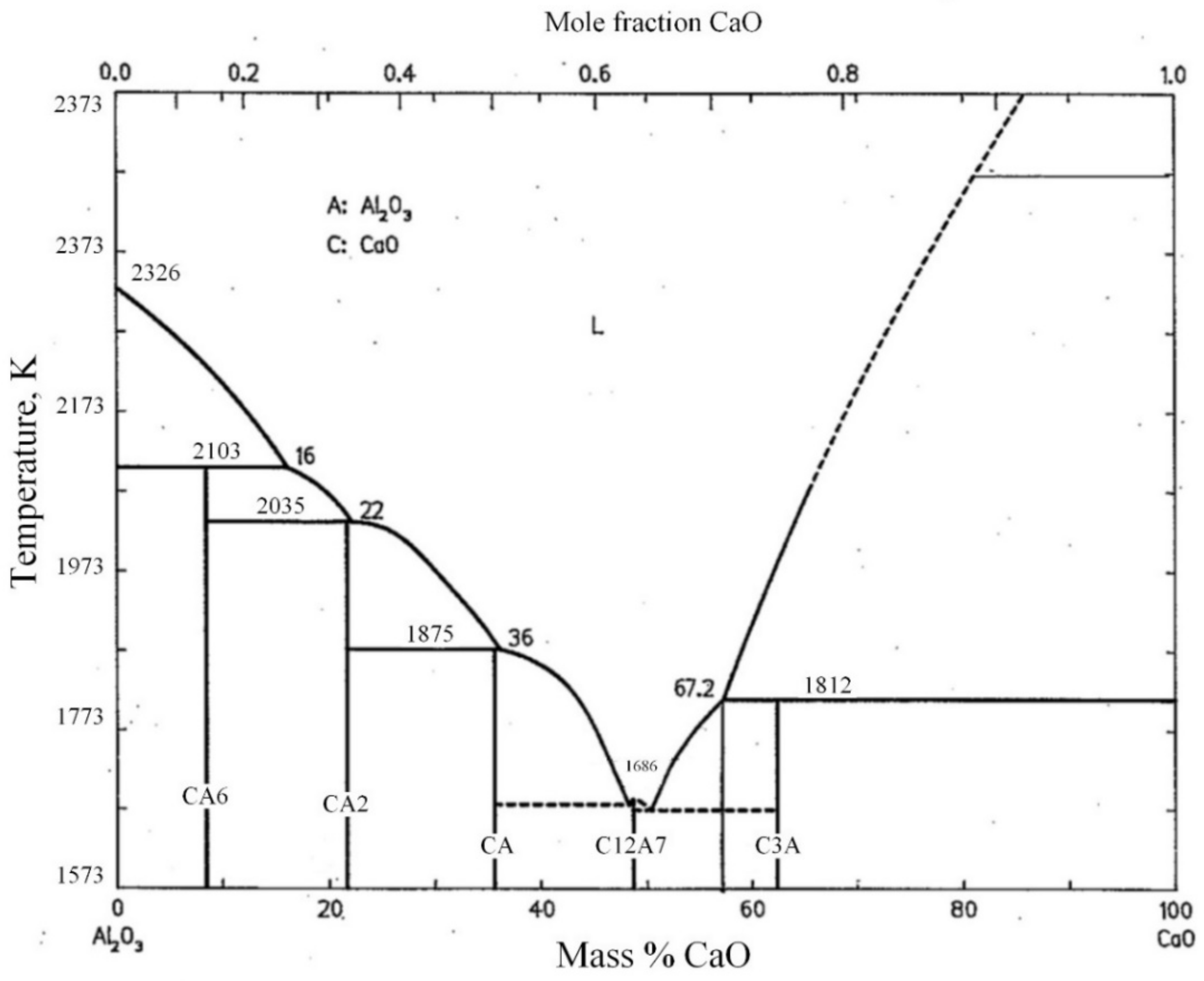
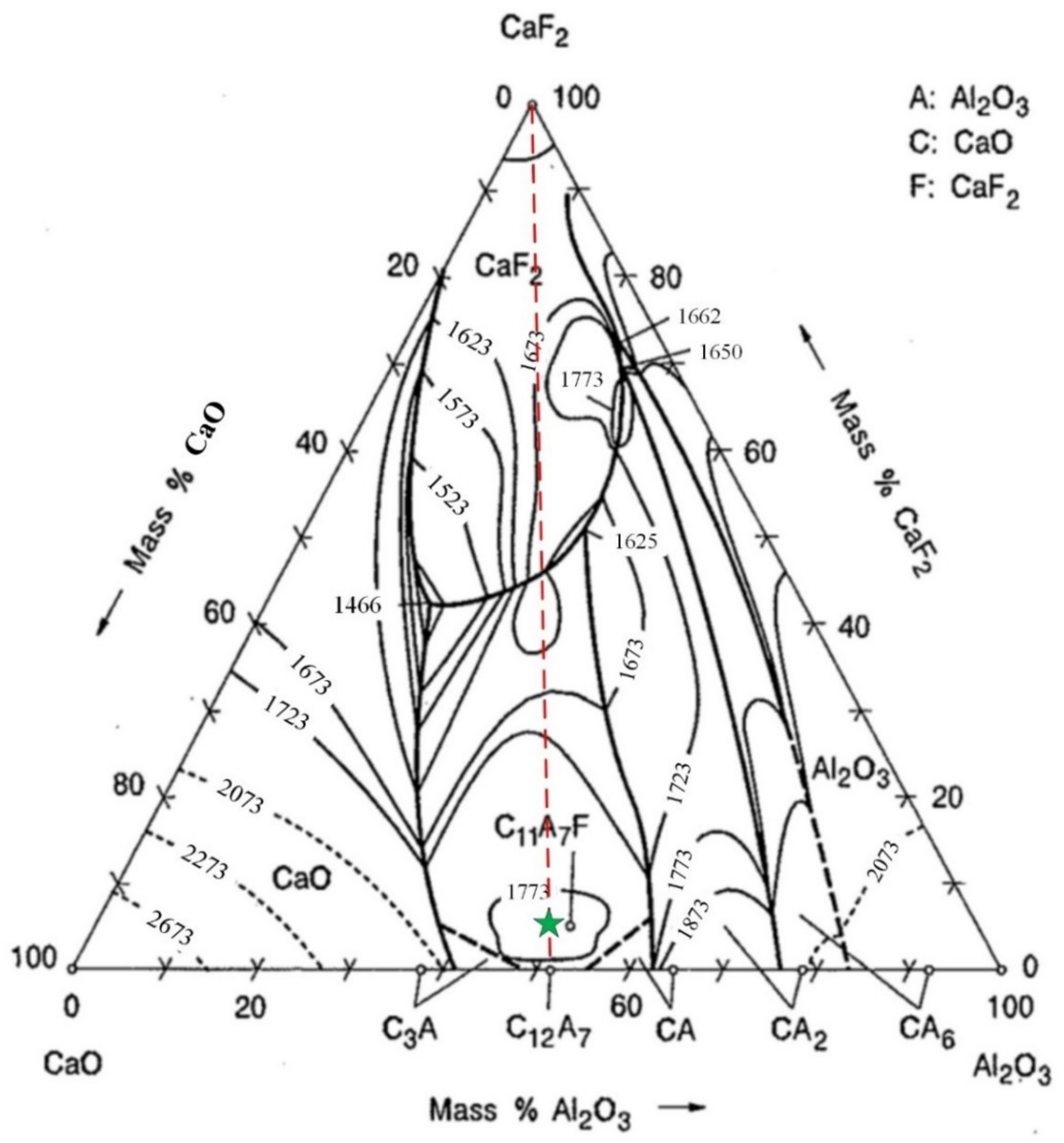
3.1. Phase Diagram Analysis
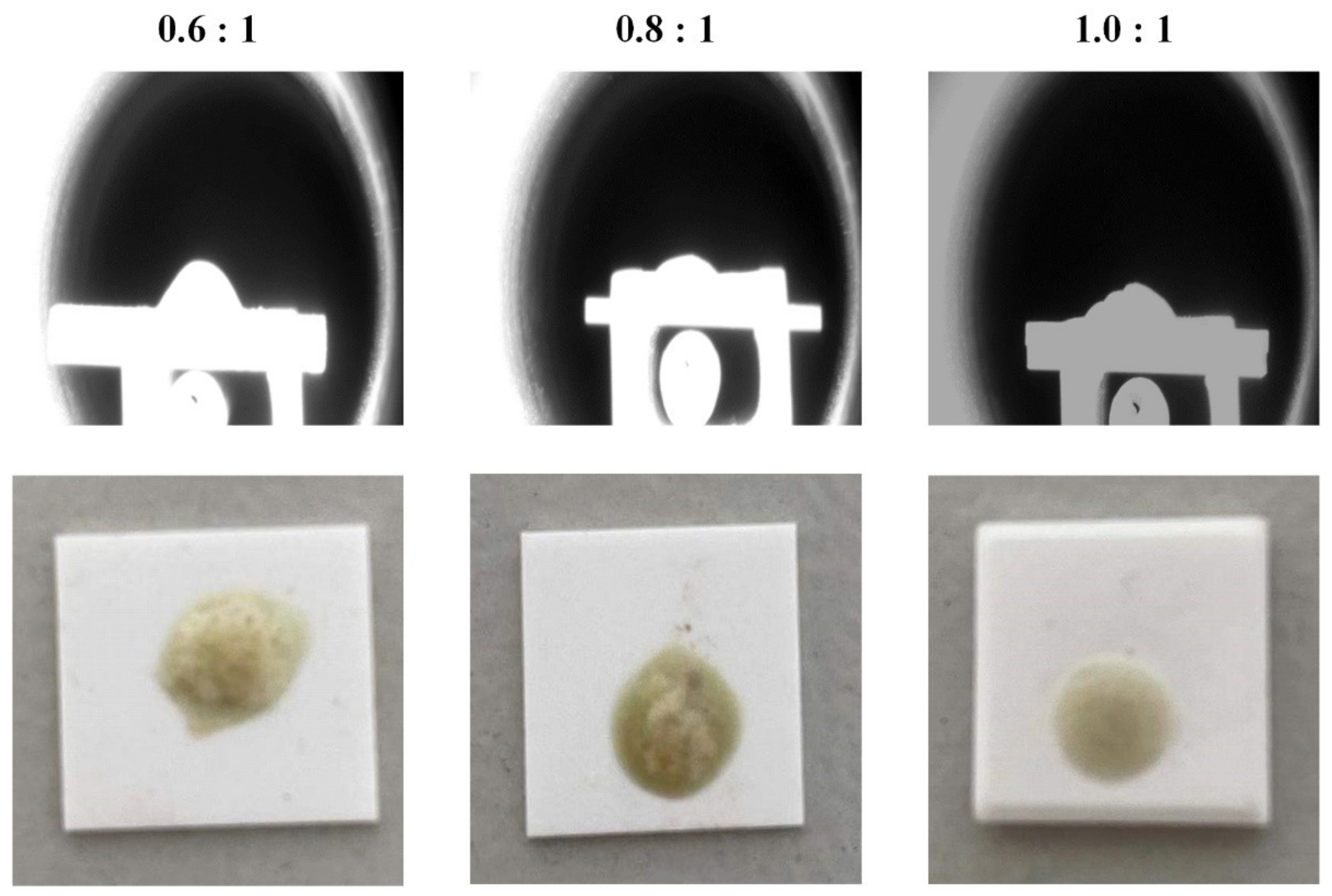
3.2. Melting Point Measurement Experiment
3.2.1. Experimental Scheme
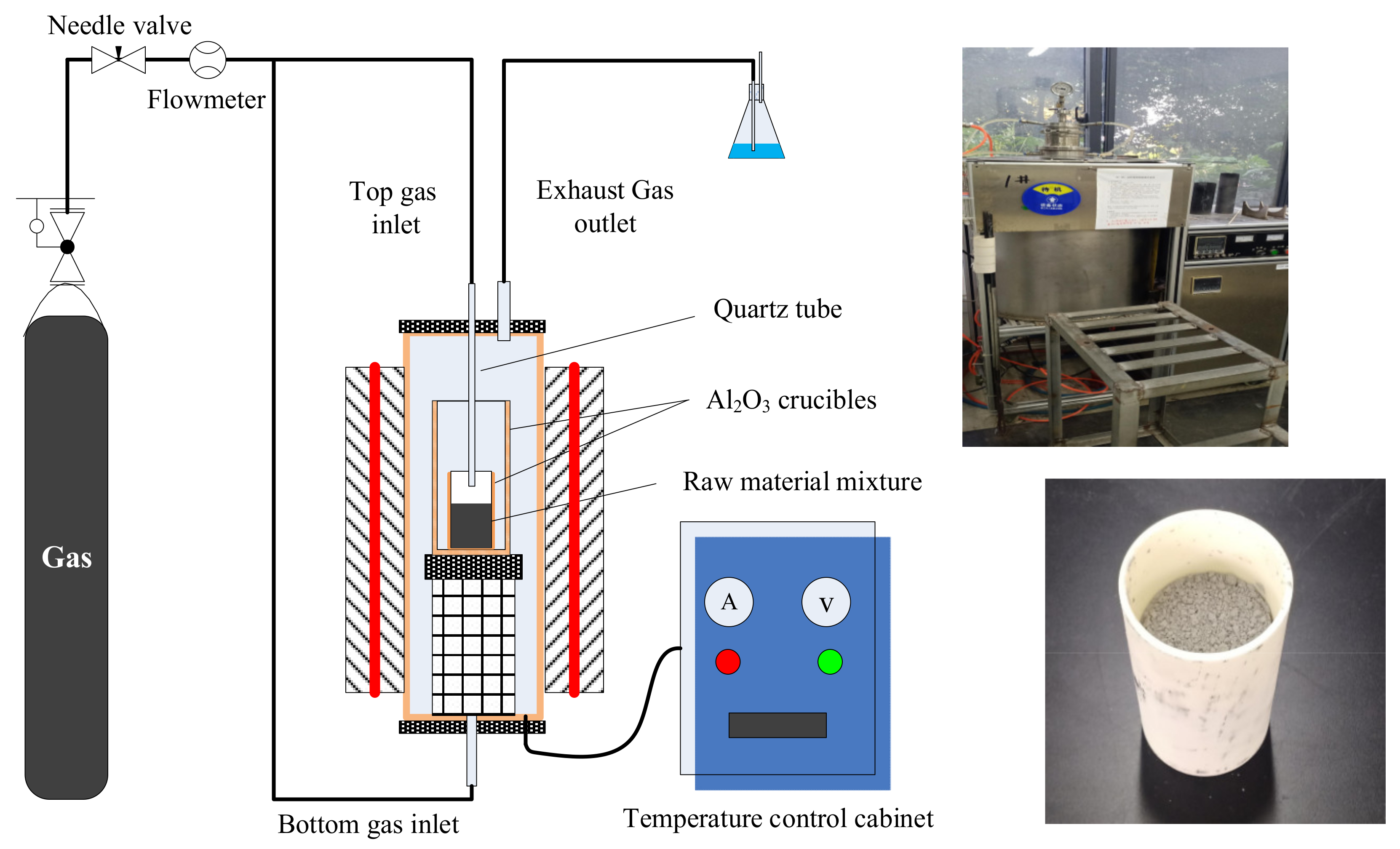
3.2.2. Experimental Procedures and Devices
3.2.3. Results and Discussion
3.3. Thermodynamic Calculation of the Calcination Product
4. Experiment of Premelted Slag Preparation
4.1. Experimental Scheme
4.2. Experimental Procedures
4.3. Results and Discussion
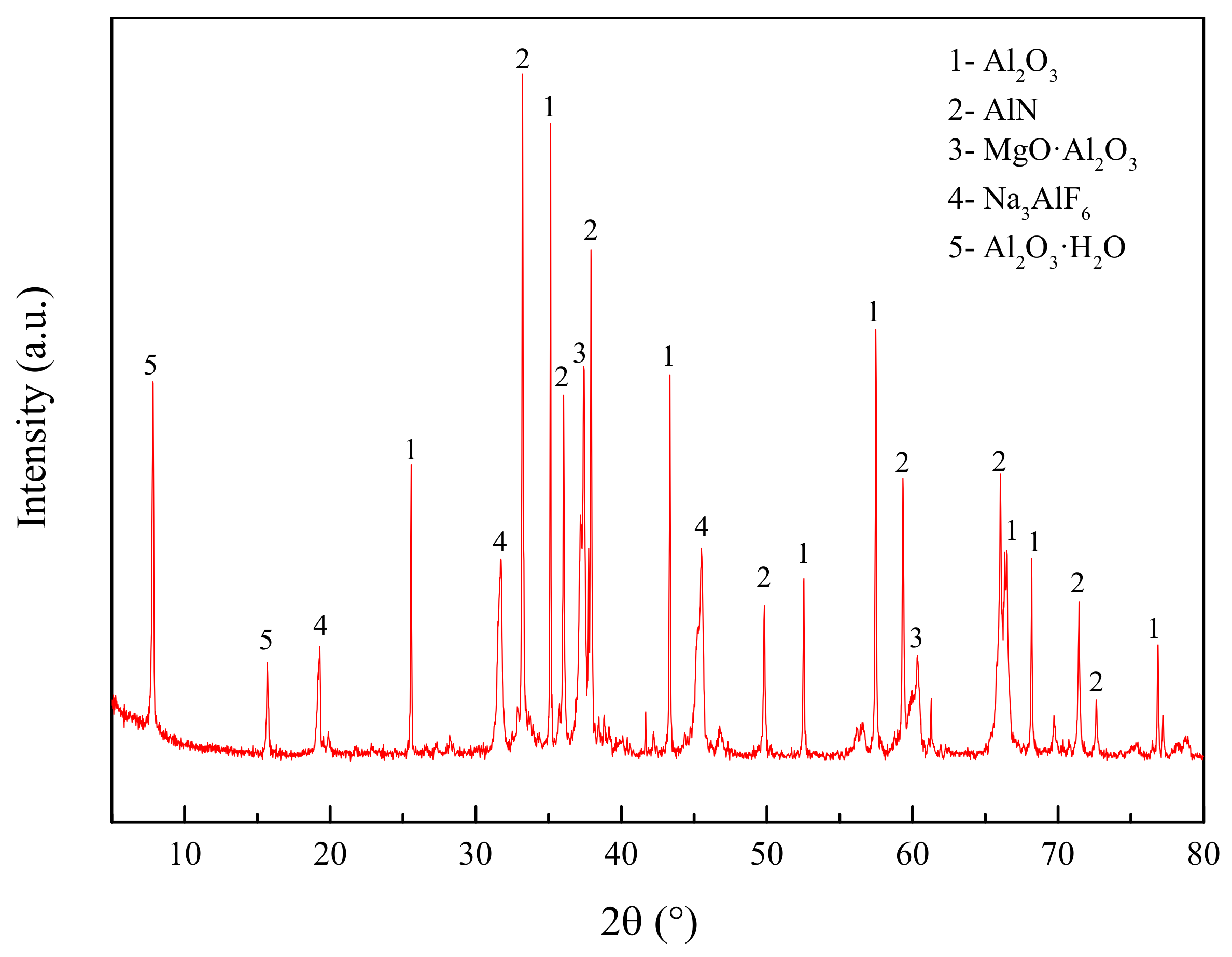
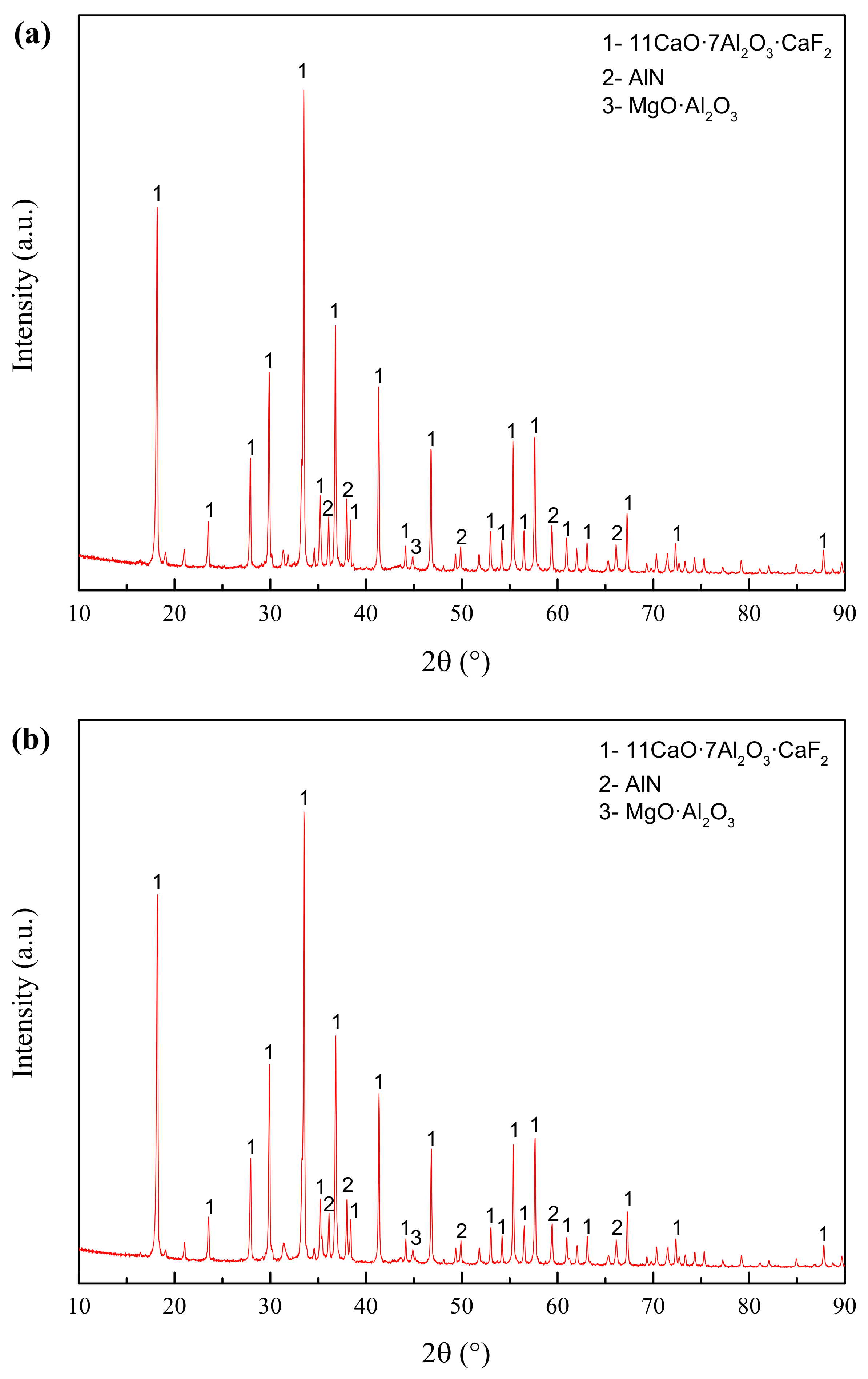
4.3.1. XRD Analysis
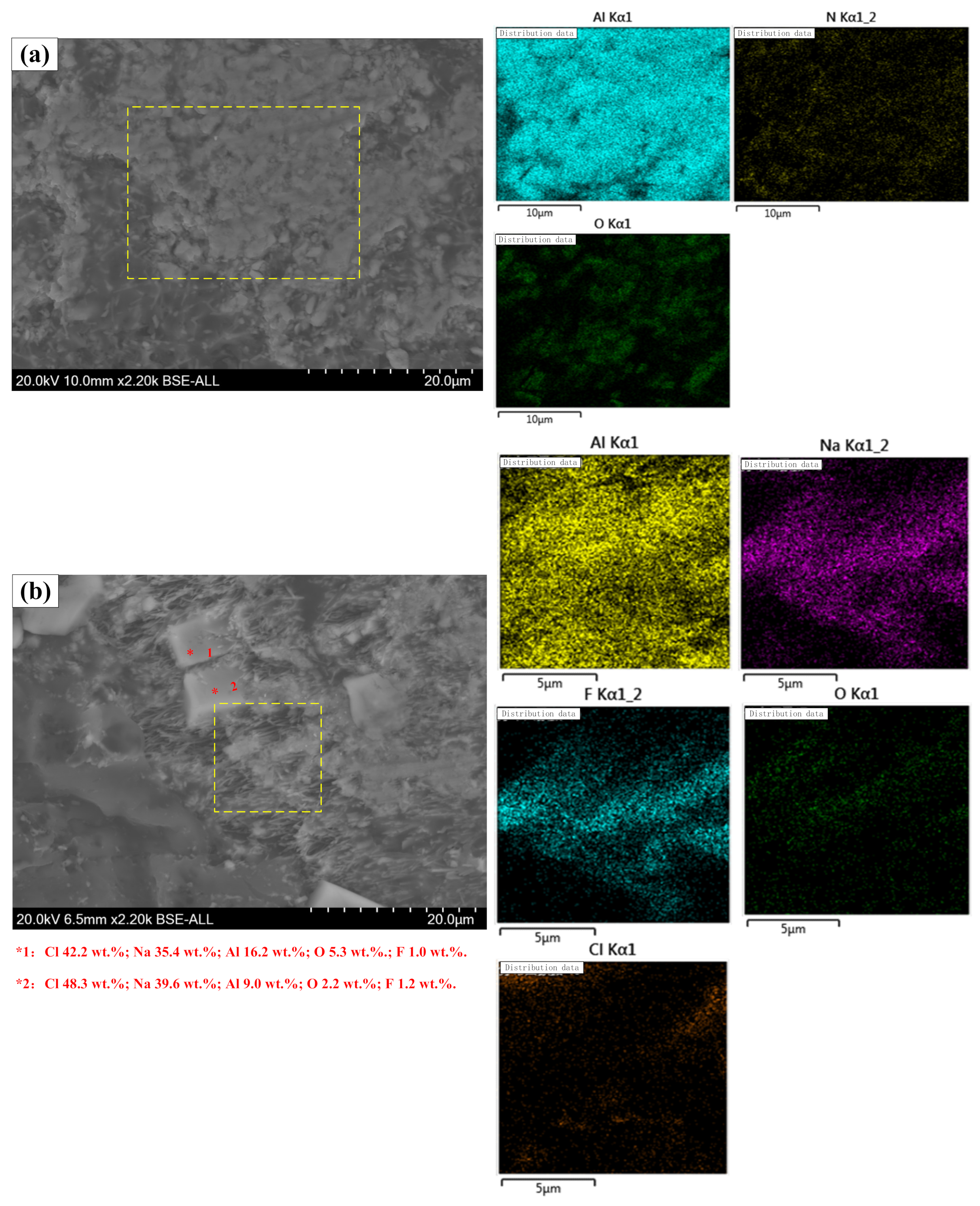
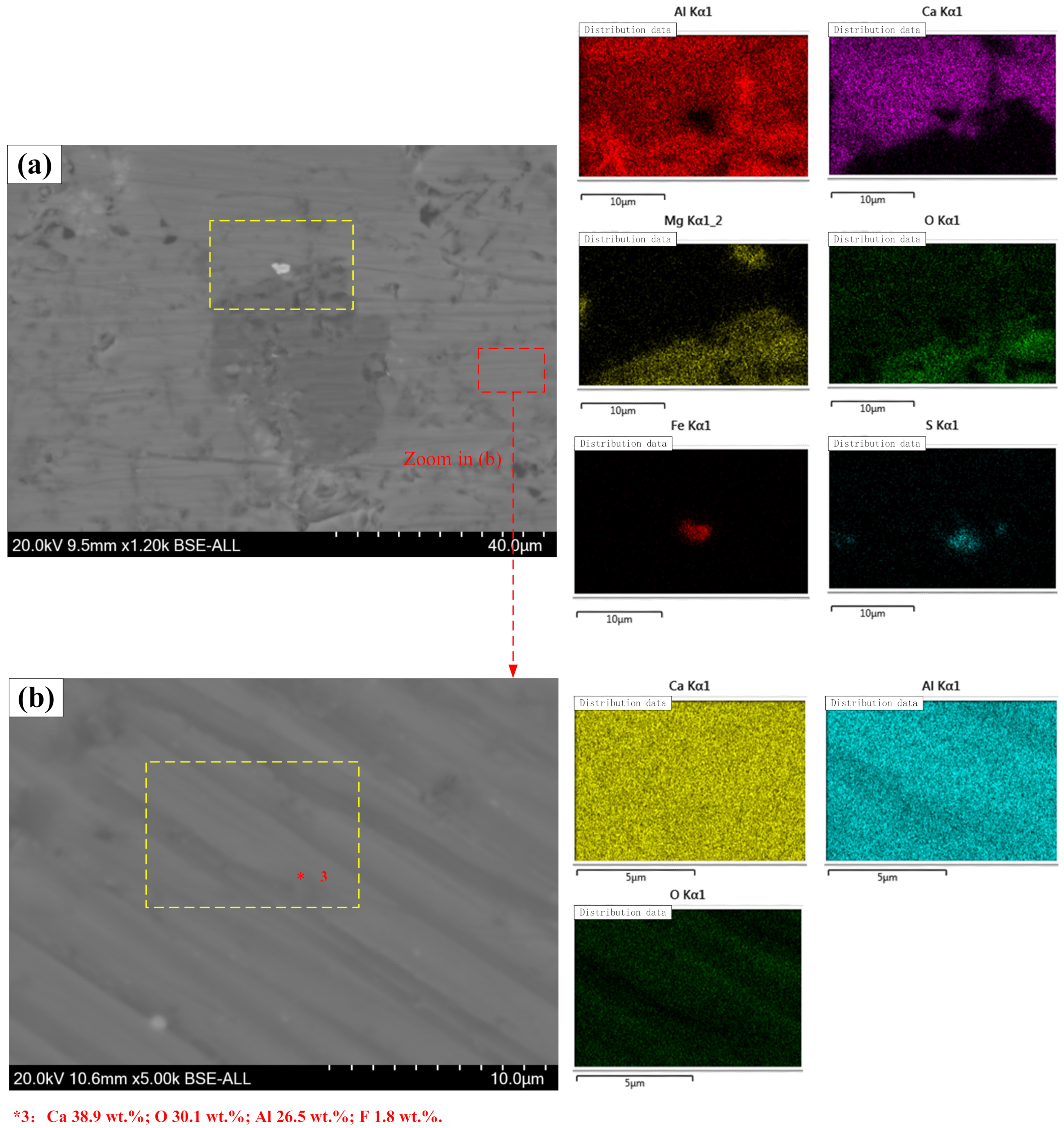
4.3.2. Micromorphology Analysis
4.3.3. Leaching Toxicity Assessment
5. Conclusions
- According to quantitative chemical analysis and XRD pattern analysis, specific phase components of the used secondary aluminum dross should be: 54.44 wt.% Al2O3, 29.05 wt.% AlN, 6.15 wt.% MgO·Al2O3, 4.81 wt.% Na3AlF6, 4.58 wt.% Al.
- Phase diagram analysis indicates that the AlN phase can hardly form a new phase with CaO and Al2O3, existing as an independent phase. The low-melting point composition of the CaO-Al2O3 binary system is near to the 12CaO·7Al2O3 phase. Adding CaF2 to the 12CaO·7Al2O3 phase can form a new phase of 11CaO·7Al2O3·CaF2.
- When the mass ratio of CaO to secondary aluminum dross varies in the range of 0.6:1 to 1.0:1, the mixture of CaO and secondary aluminum dross can melt within 1723 K. Moreover, the melting point decreases slightly with the increase in the mass ratio in the above range.
- Premelted calcium aluminate slag was obtained by calcinating the mixture of CaO and secondary aluminum dross with a mass ratio of 0.6:1 at 1723 K for 2 h. The premelted slag contains phases of 11CaO·7Al2O3·CaF2, AlN, and MgO·Al2O3, in which 11CaO·7Al2O3·CaF2 is the major phase. The original Na3AlF6 phase disappears completely, leading to undetectable water-soluble fluoride during the leaching toxicity detection. The experimental results agree well with the thermodynamic calculation results.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ma, Q.; Zhang, J.X. Analysis and development trend of the global primary aluminum market. Light Metals 2016, 11, 1–7. [Google Scholar]
- Redkin, A.; Apisarov, A.; Dedyukhin, A.; Kovrov, V. Recent Developments in Low-Temperature Electrolysis of Aluminum. ECS Trans. 2013, 50, 205–213. [Google Scholar] [CrossRef]
- Yu, B.Y.; Zhao, Z.H.; Zhang, S.; An, R.Y.; Chen, J.M.; Li, R.; Zhao, G.P. Technological development pathway for a low-carbon primary aluminum industry in China. Technol. Forecast. Soc. Chang. 2021, 173, 121052. [Google Scholar] [CrossRef]
- Yang, Y.C. Effect factors of sodium-cryolite electrolysis system in electrolytic aluminum process. Hydrometall. China 2012, 31, 89–91. [Google Scholar]
- Thonstad, J. Alternative electrolyte compositions for aluminum electrolysis. Miner. Process. Extr. Metall. 2005, 114, 188–191. [Google Scholar] [CrossRef]
- David, E.; Kopac, J. Aluminum recovery as a product with high added value using aluminum hazardous waste. J. Hazard. Mater. 2013, 261, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.M. The situation of generation, treatment and supervision of common industrial solid wastes in China. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 1–4. [Google Scholar] [CrossRef]
- Guo, R.; Liu, X.Z.; Li, Q.D.; Yi, X.M. Present situation of high value recycling technology of aluminum ash. Inorg. Chem. Ind. 2017, 49, 12–15, 25. [Google Scholar]
- Tsakiridis, P.E. Aluminum salt slag characterization and utilization: A review. J. Hazard. Mater. 2012, 217–218, 1–10. [Google Scholar] [CrossRef]
- Hwang, J.Y.; Huang, X.; Xu, Z. Recovery of metals from aluminum dross and salt cake. J. Miner. Mater. Charact. Eng. 2006, 5, 47–62. [Google Scholar]
- Du, Z.C.; Li, H.Q.; Bao, W.J.; Li, S.P.; Cai, W.Q. Reaction mechanism of desilification process of high aluminum fly ash by alkali solution. Chin. J. Process Eng. 2011, 11, 442–447. [Google Scholar]
- Xing, X.J.; Wu, Y.D. Review on development on the utilization of aluminum dross. Environ. Eng. 2021, 39, 148–152. [Google Scholar]
- Yang, H.; Shen, S.F.; Liu, H.Y.; Zheng, X.J.; Li, W.G.; Zhao, Q.C.; Wang, J.L.; Luo, Y.F. Study on mineralogical characteristics of secondary aluminum ash. Nonferrous Metals Eng. 2019, 9, 117–124. [Google Scholar]
- Jiang, L.; Qiu, M.F.; Ding, Y.D. Hydrolysis behavior of AlN in aluminum dross. Chin. J. Nonferrous Metals 2012, 22, 3555–3561. [Google Scholar]
- Shen, H.; Forssberg, E. An overview of recovery of metals form slags. Waste Manag. 2003, 23, 933–949. [Google Scholar] [CrossRef]
- Hong, J.P.; Wang, J. Process of aluminum dross recycling and life assessment for Al-Si alloys and brown fused alumi. Trans. Nonferrous Metal Soc. China 2010, 11, 2155–2161. [Google Scholar] [CrossRef]
- Navid, H.J.; Stark, T.D.; Roper, R. Classification and reactivity of secondary aluminum production waste. J. Hazard. Toxic Radioact. Waste 2014, 18, 1–11. [Google Scholar]
- Adeosun, S.O.; Akpan, E.I.; Dada, M.O. Refractory characteristics of aluminum dross-kaolin composite. JOM 2014, 66, 2253–2261. [Google Scholar] [CrossRef]
- Ewais, E.M.M.; Khalil, N.M.; Amin, M.S.; Ahmed, Y.M.Z.; Barakat, M.A. Utilization of aluminum sludge and aluminum slag (dross) for the manufacture of calcium aluminate cement. Ceram. Int. 2009, 35, 3381–3388. [Google Scholar] [CrossRef]
- Mailar, G.; Sujay, R.N.; Sreedhara, B.M.; Manu, D.S.; Parameshwar, H.; Jayakesh, K. Investigation of concrete produced using recycled aluminium dross for hot weather concreting conditions. Resour.-Effic. Technol. 2016, 2, 68–80. [Google Scholar] [CrossRef]
- Li, A.P.; Zhang, H.J.; Yang, H.M. Evaluation of aluminum dross as raw material for high-alumina refractory. Ceram. Int. 2014, 40, 12585–12590. [Google Scholar] [CrossRef]
- Li, F.T.; Jiang, J.Q.; Wu, S.J. Preparation and performance of a high purity poly-aluminum chloride. Chem. Eng. J. 2015, 156, 64–69. [Google Scholar] [CrossRef]
- Yang, Z.; Gao, B.; Yue, Q. Coagulation performance and residual aluminum speciation of Al2(SO4)3 and poly-aluminum chloride (PAC) in Yellow River water treatment. Chem. Eng. J. 2010, 165, 122–132. [Google Scholar] [CrossRef]
- Zarchi, I.; Friedler, E.; Rebhun, M. Poly-aluminum chloride as an alternative to alum for the direct filtration of drinking water. Environ. Technol. 2013, 34, 1199–1209. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.L.; Huang, Z.L.; Qin, Q.W.; Yuan, C.G.; Chen, J.Z.; Zhou, Y. Experiment study on the preparation of polyaluminum chloride with secondary aluminum dross. Metal Mine 2021, 50, 206–210. [Google Scholar]
- Du, K.F.; Lv, S.S.; Ni, H.J.; Wang, X.X.; Chen, L.F.; Li, Z.Y. Research on preparation of poly-aluminum chloride by aluminum ash and waste hydrochloric acid. J. Funct. Mater. 2020, 51, 1207–1213. [Google Scholar]
- Chao, X.; Zhang, T.A.; Zhang, Y.B.; Lv, G.Z.; Chen, Y. Study on the preparation of polyaluminum chloride by acid leaching of secondary aluminum dross. Nonferrous Metals Sci. Eng. 2021, 12, 1–6. [Google Scholar]
- Kang, W.T.; Li, J.J.; Li, X.Y.; Liu, Y.M. Study on the new process of manufacturing low-iron aluminum sulfate. J. Hebei Univ. Sci. Technol. 2001, 20, 65–67, 79. [Google Scholar]
- David, E.; Kopac, J. Hydrolysis of aluminum dross material to achieve zero hazardous waste. J. Hazard. Mater. 2012, 209, 501–509. [Google Scholar] [CrossRef]
- Yuasa, G.; Yajima, T.; Ukal, A. Refining practice and application of the Ladle Furnace (LF) Process in Japan. ISIJ Int. 1984, 24, 412–418. [Google Scholar] [CrossRef][Green Version]
- Li, Y.L.; Zhang, L.F.; Yang, W.; Wang, L.S. Development of ladle slag reducer using aluminum dross. Iron Steel 2014, 49, 17–23. [Google Scholar]
- Wang, D.Y.; Liu, C.J.; Min, Y.; Yu, F.Z.; Jiang, M.F. Effect of Al ash on desulfurization of pipe line steel. China Metall. 2007, 17, 14–16. [Google Scholar]
- Hassall, G.J.; Jackaman, D.P.; Hawkins, R.J. Phosphorus and sulfur removal from liquid steel in ladle steelmaking processes. Ironmak. Steelmak. 1991, 18, 359–369. [Google Scholar]
- Pezzin, R.O.; Berger, A.P.; Grillo, F.F.; Junca, E.; Furtado, H.S.; Oliveira, J.R. Analysis of the influence of the solid and liquid phases on steel desulfurization with slags from the CaO-Al2O3 systems using computational thermodynamics. J. Mater. Res. Technol. 2020, 9, 838–846. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Zhu, X.F.; Yang, J.W.; Zhao, Y.J.; Wang, S.N. Study on harmless application of industrial aluminum dross in steelmaking. Gansu Metall. 2020, 42, 115–117, 120. [Google Scholar]
- Li, S.; Liu, W.C.; Liu, Z.K.; Yan, K. Technical state and prospect on processing of aluminum dross. Nonferrous Metals (Extr. Metall.) 2018, 10, 25–30. [Google Scholar]
- Park, J.H.; Min, D.J. Thermodynamics of fluoride vaporisation from slags containing CaF2 at 1773 K. Steel Res. Int. 2004, 75, 807–811. [Google Scholar] [CrossRef]
- Li, X.Q.; Shen, S.F.; Wang, L.; Zheng, X.J. Study on roasting characteristics and removal of harmful elements of secondary aluminum dross. Nonferrous Metals (Extr. Metall.) 2020, 58, 69–74. [Google Scholar]
- Huan, S.X.; Wang, Y.W.; Di, Y.Z.; You, J.; Peng, J.P.; Hong, Y.M. Experimental study on alumina extraction by calcination of secondary aluminum ash. Conserv. Util. Miner. Resour. 2020, 40, 34–39. [Google Scholar]
- Verein Deutscher Eisenhüttenleute. Slag Atlas; Verlag Stahleisen: Dusseldorf, Germany, 1995. [Google Scholar]

| Element | Al | O | F | Na | Mg | Cl | Si | V | Ca | S | Ti | K | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content, wt.% | 50.89 | 23.4 | 3.6 | 2.73 | 1.34 | 1.19 | 0.725 | 0.529 | 0.491 | 0.363 | 0.322 | 0.302 | 0.229 |
| Element | T.Al | M.Al | F | N | Na |
|---|---|---|---|---|---|
| Content, wt.% | 55.49 | 4.58 | 2.61 | 9.92 | 2.25 |
| Mass Ratio of CaO to Secondary Aluminum Dross | 0:1 | 0.4:1 | 0.6:1 | 0.8:1 | 1.0:1 | 1.2:1 |
|---|---|---|---|---|---|---|
| Softening temperature, K | — | — | 1700 | 1672 | 1653 | — |
| Hemispherical temperature, K | — | — | 1709 | 1686 | 1667 | — |
| Flowing temperature, K | — | — | 1721 | 1705 | 1699 | — |
| Phase Components | Calculation Results in N2 Atmosphere | Calculation Results in Ar Atmosphere | ||
|---|---|---|---|---|
| Weight | Specific Composition | Weight | Composition | |
| Gas | 9.3122 g | 7.7302 g N2; 1.5469 g Na; 0.031709 g Mg; et al. | 12.657 g | 10 g Ar; 1.6393 g Na; 0.82349 g Mg; 0.13539 g Ca; et al. |
| Liq-Oxyfluoride | 41.473 g | 20.282 g CaO; 18.7433 g Al2O3; 1.3459 g MgO; 0.41321 g AlF3; 0.37792 g CaF2; 0.24021 g NaAlO2; et al. | 26.643 g | 13.409 g CaO; 13.039 g Al2O3; 0.071320 g AlF3; 0.061993 g CaF2; 0.050781 g MgO; et al. |
| 11CaO·7Al2O3·CaF2 | 82.573 g | — | 97.0039 g | — |
| AlN | 36.642 g | — | 30 g | — |
| Metallic Al | 0 | — | 3.6615 g | — |
| Sample | Standard Limit, mg/L | Detection Result, mg/L |
|---|---|---|
| Original secondary aluminum dross | 100 | 866 |
| Premelted slag calcinated in N2 atmosphere | ND | |
| Premelted slag calcinated in Ar atmosphere | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.; Wang, D.; Hou, D.; Zhao, W.; Li, X.; Qu, T.; Zhu, Q. Research on the Preparation Parameters and Basic Properties of Premelted Calcium Aluminate Slag Prepared from Secondary Aluminum Dross. Materials 2021, 14, 5855. https://doi.org/10.3390/ma14195855
Hu S, Wang D, Hou D, Zhao W, Li X, Qu T, Zhu Q. Research on the Preparation Parameters and Basic Properties of Premelted Calcium Aluminate Slag Prepared from Secondary Aluminum Dross. Materials. 2021; 14(19):5855. https://doi.org/10.3390/ma14195855
Chicago/Turabian StyleHu, Shaoyan, Deyong Wang, Dong Hou, Wei Zhao, Xianglong Li, Tianpeng Qu, and Qingde Zhu. 2021. "Research on the Preparation Parameters and Basic Properties of Premelted Calcium Aluminate Slag Prepared from Secondary Aluminum Dross" Materials 14, no. 19: 5855. https://doi.org/10.3390/ma14195855
APA StyleHu, S., Wang, D., Hou, D., Zhao, W., Li, X., Qu, T., & Zhu, Q. (2021). Research on the Preparation Parameters and Basic Properties of Premelted Calcium Aluminate Slag Prepared from Secondary Aluminum Dross. Materials, 14(19), 5855. https://doi.org/10.3390/ma14195855







