Preparation and Photocatalysis of CuO/Bentonite Based on Adsorption and Photocatalytic Activity
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of Na-Bentonite and CuO/Bentonite
2.3. Characterization
2.4. Sorption and Photocatalysis Processes
3. Results and Discussion
3.1. XRD Analysis
3.2. SEM Analysis
3.3. Photocatalytic Performance
3.3.1. Effect of H2O2 Dosageon Decolorization Efficiency of MB Solution
3.3.2. Effect of Calcination Temperature on Decolorization Efficiency of MB Solution
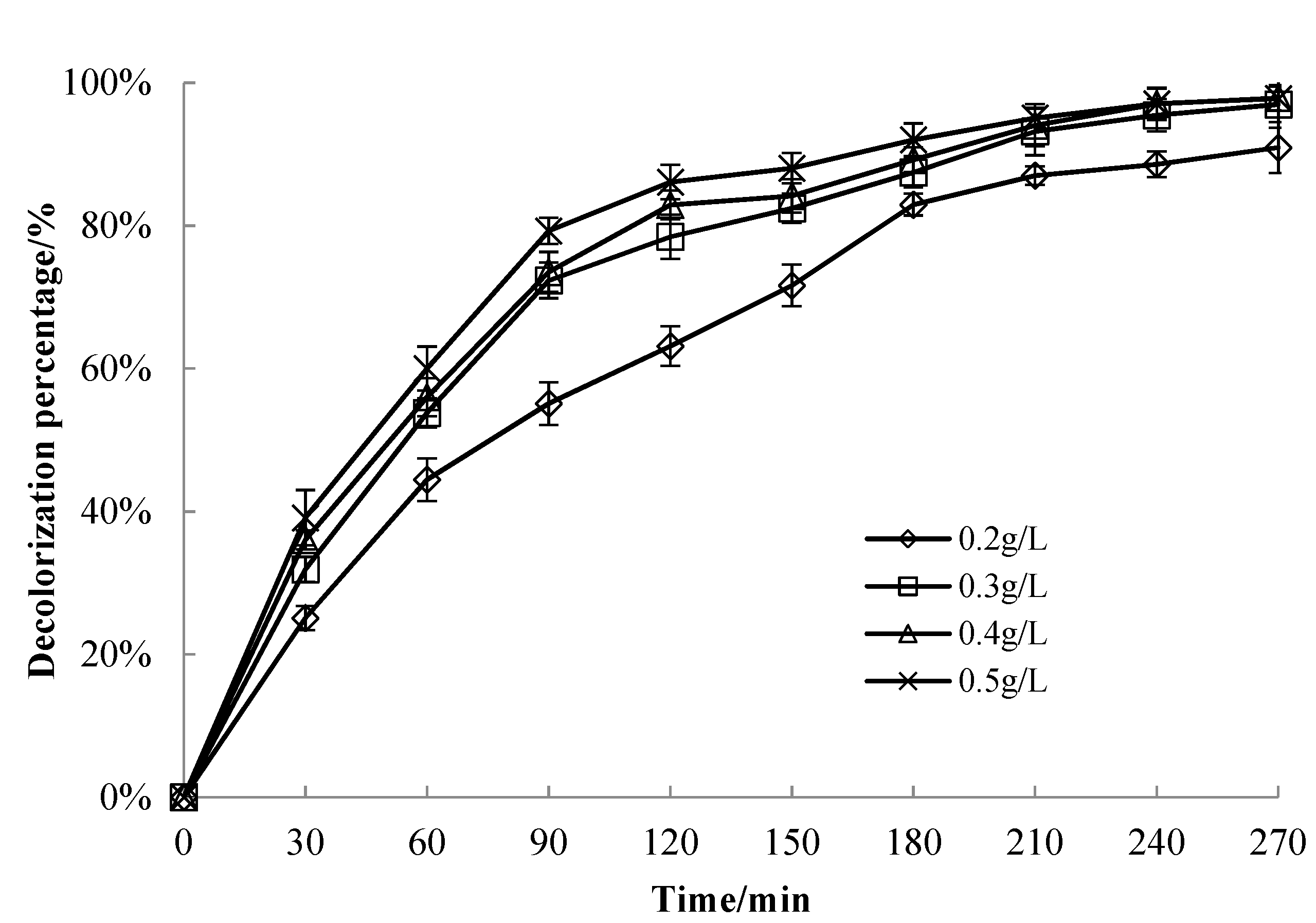
3.3.3. Effect of Catalyst Dosage on MB Solution Decolorization Performance
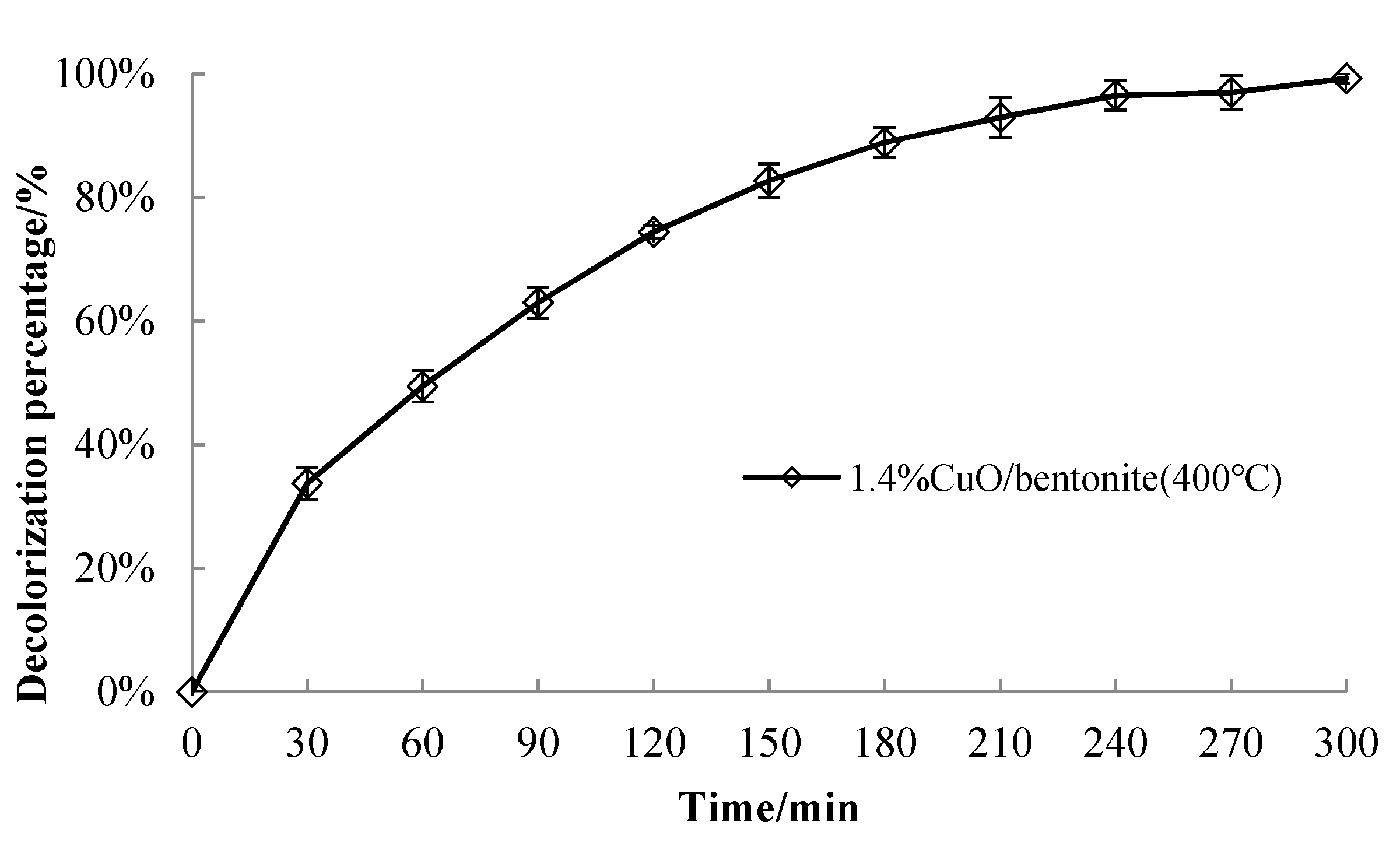
3.3.4. Effect of Irradiation Time on Catalyst Performance
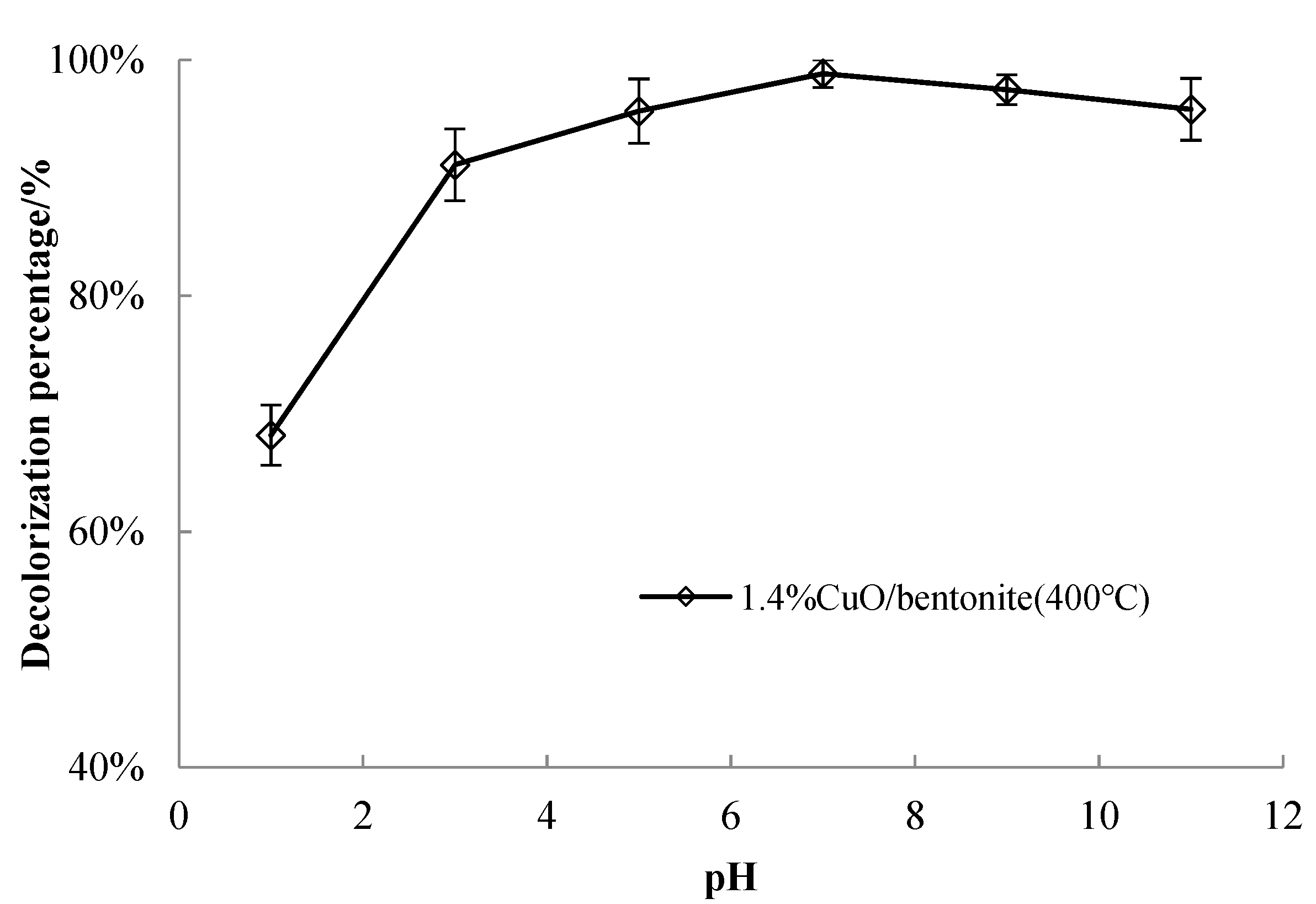
3.3.5. Effect of Solution pH on Catalyst Performance
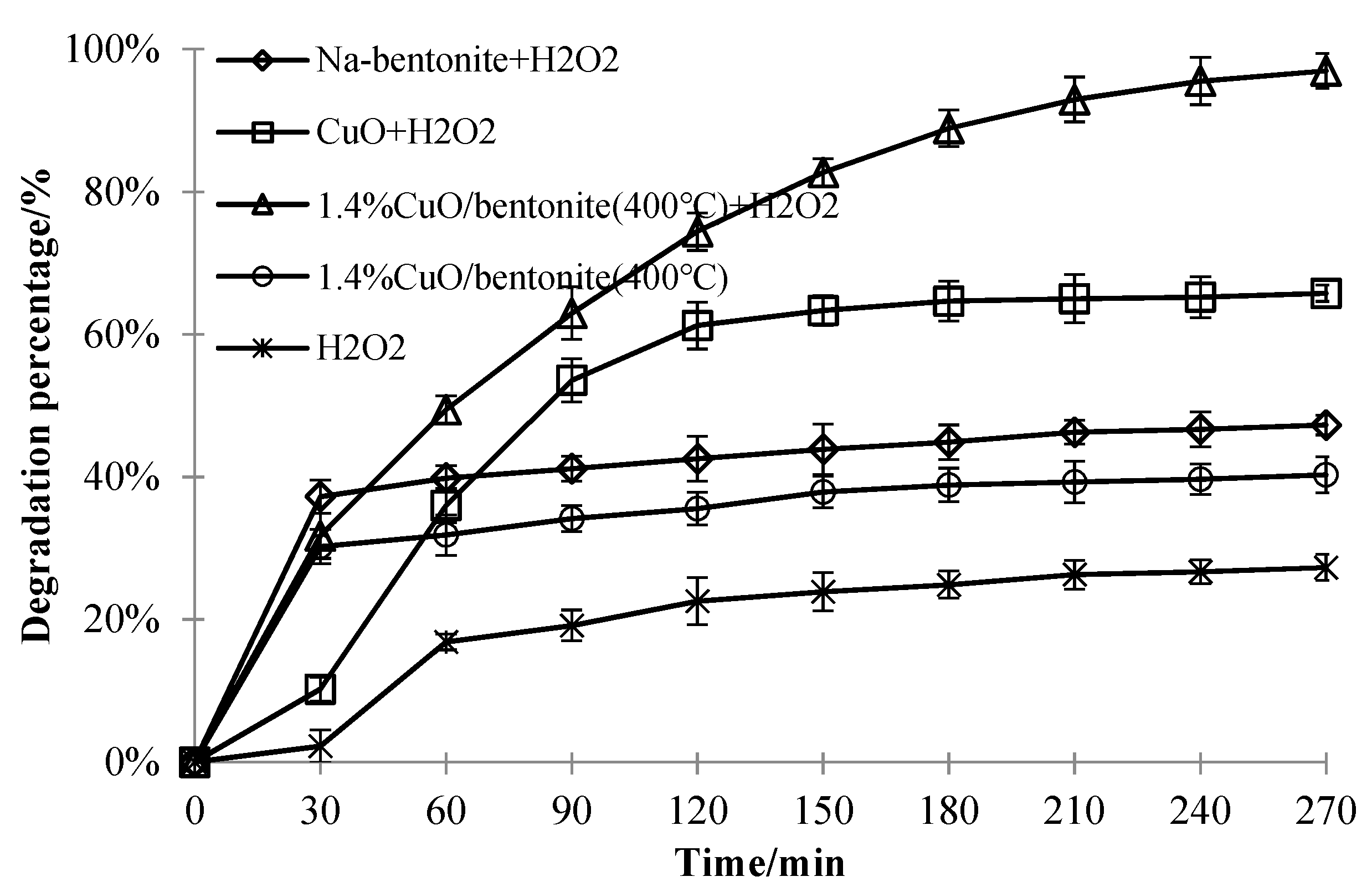
3.3.6. Effect of Different Materials on the Decolorization Efficiency ofMB
3.3.7. Adsorption Kinetics
3.3.8. Recycling of Catalyst
3.4. Photocatalytic Mechanism ofCuO/Bentonite Composite
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arora, S. Textile Dyes: It’s Impact on environment and its treatment. J. Bioremediat. Biodegrad. 2014, 5, e146. [Google Scholar] [CrossRef]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic Carbonnitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Cao, S.; Yu, J.G. g-C3N4-based photocalysts for hydrogen gener-ation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic Carbon nitride based nano-composites: A review. Nanoscale 2015, 7, 15–37. [Google Scholar] [CrossRef]
- Chala, S.; Wetchakun, K.; Phanichphant, S.; Inceesungvorn, B.; Wetchakun, N. Enhanced visible-light-response photocatalytic degradation of methylene blue on Fe-loaded BiVO4 photocatalyst. J. Alloy. Compd. 2014, 597, 129–135. [Google Scholar] [CrossRef]
- Ameen, S.; Akhtar, M.S.; Seo, H.K.; Shin, H.S. Solution-processed CeO2/TiO2 nanocomposite as potent visible light photocatalyst for the degradation of bromophenol dye. Chem. Eng. J. 2014, 247, 193–198. [Google Scholar] [CrossRef]
- Zhang, L.; He, Y.M.; Ye, P.; Wu, Y.; Wu, T.H. Visible light photocatalytic activities of ZnFe2O4 loaded by Ag3VO4 heterojunction composites. J. Alloy. Compd. 2013, 549, 105–113. [Google Scholar] [CrossRef]
- Zhao, D.; Yang, X.; Chen, C.; Wang, X. Enhanced photocatalytic degradation of methylene blue on multiwalled carbon nanotubes-TiO2. J. Colloid Interface Sci. 2013, 398, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Li, J.H.; Zhao, W.; Guo, Y.; Wei, Z.B.; Han, M.S.; He, H.; Yang, S.G.; Sun, C. Facile synthesis and high activity of novel BiVO4/FeVO4 heterojunction photocatalyst for degradation of metronidazole. Appl. Surf. Sci. 2015, 351, 270–279. [Google Scholar] [CrossRef]
- Wetchakun, N.; Chainet, S.; Phanichphant, S.; Wetchakun, K. Efficient photocatalytic degradation of methylene blue over BiVO4/TiO2 nanocomposites. Ceram. Int. 2015, 41, 5999–6004. [Google Scholar] [CrossRef]
- Lin, S.L.; Liu, L.; Hu, J.S.; Liang, Y.H.; Cui, W.Q. Nano Ag@ AgBr surface-sensitized Bi2WO6 photocatalyst: Oil-in-water synthesis and enhanced photocatalytic degradation. Appl. Surf. Sci. 2015, 324, 20–29. [Google Scholar] [CrossRef]
- Pal, S.; Maiti, S.; Maiti, U.N.; Chattopadhyay, K.K. Low temperature solution processed ZnO/CuO heterojunction photocatalyst for visible light induced photo-degradation of organic pollutants. CrystEngComm 2015, 17, 1464–1476. [Google Scholar] [CrossRef]
- Vadivel, S.; Vanitha, M.; Muthukrishnaraj, A.; Balasubramanian, N. Graphene Oxide-BiOBr composite material as highly efficient photocatalyst for degradation of methylene blue and rhodamine-B dyes. J. Water Process. Eng. 2014, 1, 17–26. [Google Scholar] [CrossRef]
- Cho, Y.M.; Choi, W.Y.; Lee, C.H.; Hyeon, T.W.; Lee, H.I. Visible light-induced degradation of carbon tetrachloride on dye-sensitized TiO2. Environ. Sci. Technol. 2001, 35, 966–970. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, I.P.S.; Srivastava, P.; Singh, G. Nanocrystalline transition metal oxides as catalysts in the thermal decomposition of ammonium perchlorate. Propellants Explos. Pyrotech. 2009, 34, 351–356. [Google Scholar] [CrossRef]
- Li, C.F.; Yin, Y.D.; Hou, H.G.; Fan, N.T.; Yuan, F.L.; Shi, Y.M.; Meng, Q.L. Preparation and characterization of Cu(OH)2 and CuO nanowires by the coupling route of microemulsion with homogenous precipitation. Solid State Commun. 2010, 150, 585–589. [Google Scholar] [CrossRef]
- Zhang, X.; Gu, A.; Wang, G.; Wei, Y.; Wang, W.; Wu, H.; Fang, B. Fabrication of CuO nanowalls on Cu substrate for a high performance enzyme-free glucose sensor. Crystengcomm 2010, 4, 1120–1126. [Google Scholar] [CrossRef]
- Tseng, H.H.; Wey, M.Y. Study of SO2 adsorption and thermal regeneration over activated carbon-supported copper oxide catalysts. Carbon 2004, 42, 2269–2278. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, C.; Wei, H.; Wang, Q.; Li, K. In situ synthesis and immobilization of a Cu(II)-pyridyl complex on silica microspheres as a novel Fenton-like catalyst for RhB degradation at near-neutral pH. RSC Adv. 2017, 37, 22825–22835. [Google Scholar] [CrossRef] [Green Version]
- Sangkhun, W.; Laokiat, L.; Tanboonchuy, V.; Khamdahsag, P.; Grisdanurak, N. Photocatalytic degradation of BTEX using W-doped TiO2 immobilized on fiberglass cloth under visible light. Superlattices Microstruct. 2012, 52, 632–642. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Zabihi-Mobarakeh, H. Heterogeneous photodecolorization of mixture of methylene blue and bromophenol blue using CuO-nano-clinoptilolite. J. Ind. Eng. Chem. 2014, 20, 1421–1431. [Google Scholar] [CrossRef]
- Pan, Z.; Stemmler, E.A.; Cho, H.J.; Fan, W.; LeBlanc, L.A.; Patterson, H.H.; Amirbahman, A. Photocatalytic degradation of 17α-ethinylestradiol (EE2) in the presence of TiO2-doped zeolite. J. Hazard. Mater. 2014, 279, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Szostak, K.; Banach, M. Sorption and photocatalytic degradation of methylene blue on bentonite-ZnO-CuO nanocomposite. J. Mol. Liq. 2019, 286, 110859. [Google Scholar] [CrossRef]
- Li, W.; Zhu, S.; Song, T. Preparation of copper-loaded activated carbon catalyst and catalytic oxidation of dyeing wastewater. For. Prod. Chem. Ind. 2006, 26, 26–30. [Google Scholar]
- Nezamzadeh-Ejhieh, A.; Hushmandrad, H. Solar photodecolorization of methylene blue by CuO/X zeolite as a heterogeneous catalyst. Appl. Catal. A Gen. 2010, 388, 149–159. [Google Scholar] [CrossRef]
- Liu, Z.C.; Liu, Z.F.; Cui, T.; Dong, L.X.; Hang, J.; Han, L.; Li, G.M.; Liu, C.P. Photocatalyst from one-dimensional TiO2 nanowires/synthetic zeolite composites. Mater. Express 2014, 4, 465–474. [Google Scholar] [CrossRef]
- Cui, T.; Liu, Z.F.; Zheng, X.R.; Liu, Z.C.; Li, Y.B.; Li, W.; Wang, B.; Guo, K.Y.; Han, J.H. Zeolite-based CuO nanotubes catalysts: Investigating the characterization, mechanism, and decolouration process of methylene blue. J. Nanopart. Res. 2014, 16, 2608–2618. [Google Scholar] [CrossRef]
- Ramakrishna, K.R.; Viraraghavan, T. Dye removal using low cost adsorbents. Water Sci. Technol. 1997, 36, 189–196. [Google Scholar] [CrossRef]
- Luckham, P.F.; Rossi, S. The colloidal and rheological properties of bentonite suspensions. Adv. Colloid Interface Sci. 1999, 82, 43–92. [Google Scholar] [CrossRef] [Green Version]
- Eren, E.; Afsin, B. An investigation of Cu(II) adsorption by raw and acidactivated bentonite: A combined potentiometric, thermodynamic, XRD, IR, DTA study. J. Hazard. Mater. 2008, 151, 682–691. [Google Scholar] [CrossRef]
- Ghiaci, M.; Sedaghat, M.E.; Aghaei, H.; Gil, A. Synthesis of CdS- and ZnS-modified bentonite nanoparticles and their applications to the degradation of eosin B. J. Chem. Technol. Biotechnol. 2009, 84, 1908–1915. [Google Scholar] [CrossRef]
- Sun, J.; Yuan, Y.; Qiu, L.; Jiang, X.; Xie, A.; Shen, Y.; Zhu, J. Fabrication of composite photocatalyst g-C3N4-ZnO and enhancement of photocatalytic activity under visible light. Dalton Trans. 2012, 41, 6756–6763. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.J.; Liu, L.C.; Chen, D.P. Synthesis of CdS/bentonite nanocomposite powders for H-2 production by photocatalytic decomposition of water. Powder Technol. 2013, 241, 7–11. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Q.; Zhu, L.; Zou, J.; Wang, K.; Yang, M.; Komarneni, S. Visible light photocatalytic activity enhancement of Ag3PO4 dispersed on exfoliated bentonite for degradation of rhodamine B. Appl. Catal. 2016, 182, 26–32. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, T.; Hu, R. Preparation of mussel biomimetic three-dimensional graphene and its adsorption on methylene blue. Environ. Chem. 2018, 37, 2540–2547. [Google Scholar]
- Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Bakar, A.; Noor, F.; Him, N.; Raikhan, N.; Lau, W.J. Adsorption kinetics of methylene blue dyes onto magnetic graphene oxide. J. Environ. Chem. Eng. 2018, 6, 2803–2811. [Google Scholar] [CrossRef]
- Andreola, F.; Siligardi, C.; Manfredini, T.; Carbonchi, C. Rheological behaviour and mechanical properties of porcelain stoneware bodies containing Italian clay added with bentonites. Ceram. Int. 2009, 35, 1159–1164. [Google Scholar] [CrossRef]
- Zhirong, I.; Uddin, M.A.; Zhanxue, S. FT-IR and Xrd Analysisof natural Na-bentonite and Cu(II)-loaded Na-bentonite. Spectrochim. Acta A 2011, 79, 1013–1016. [Google Scholar] [CrossRef]
- Eren, E.; Tabak, A.; Eren, B. Performance of magnesium oxidecoated bentonite in removal process of copper ions from aqueous solution. Desalination 2010, 257, 163–169. [Google Scholar] [CrossRef]
- Sumetha, S.; Pongsaton, A.; Apinya, S. Dependence of optical properties on doping metal, crystallite size and defectconcentration of M-doped ZnO nanopowders (M = Al, Mg, Ti). Ceram. Int. 2011, 37, 1359–1365. [Google Scholar]
- Cho, Y.S.; Huh, Y.D. Synthesis of ultralong copper nanowires by reduction of copper–amine complexes. Mater. Lett. 2008, 63, 227–229. [Google Scholar] [CrossRef]
- Hu, F.; Chen, L.Q.; Tao, X. Preparation of bentonite supported catalyst and its application in heterogeneous Fenton degradation of methylene blue. Text. Aux. 2019, 36, 17–20. [Google Scholar]
- Zhu, P.; Liu, M.; Zhagn, J. Preparation of Cu-ZnO/bentonite and its pho-tocatalytic function for degrading methylorange. J. Saf. Environ. 2014, 14, 148–152. [Google Scholar]
- Dariani, R.S.; Esmaeili, A.; Mortezaali, A.; Dehghanpour, S. Photocatalytic reaction and degradation of meth-ylene blue on TiO2 nano-sized particles. Optik 2016, 127, 7143–7154. [Google Scholar] [CrossRef]
- Guo, H.X.; Wu, C.G.; Cui, S.P.; Li, F.; Wang, Z.M.; Ma, X.Y. Preparation and properties of Ce-doped ZnO/Bentonite composite photocatalyst. Nanotechnol. Precis. Eng. 2014, 12, 313–319. [Google Scholar]
- Mahmoodi, N.M.; Arami, M.; Limaee, N.Y.; Tabrizi, N.S. Kinetics of heterogeneous photocatalytic degradation of reactive dyes in an immobilized TiO2 photocatalytic reactor. J. Colloid Interface Sci. 2006, 295, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, N.; Salari, D.; Khataee, A.R. Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A 2004, 162, 317–322. [Google Scholar] [CrossRef]
- Zhou, X.F.; Li, X.; Gao, Q.Z.; Yuan, J.L.; Wen, J.Q.; Fang, Y.P.; Liu, W.; Zhang, S.S.; Liu, Y.J. Metal-free carbon nanotube-SiC nanowire heterostructures with enhanced photocatalytic H2 evolution under visible light irradiation. Catal. Sci. Technol. 2015, 5, 2798–2806. [Google Scholar] [CrossRef]





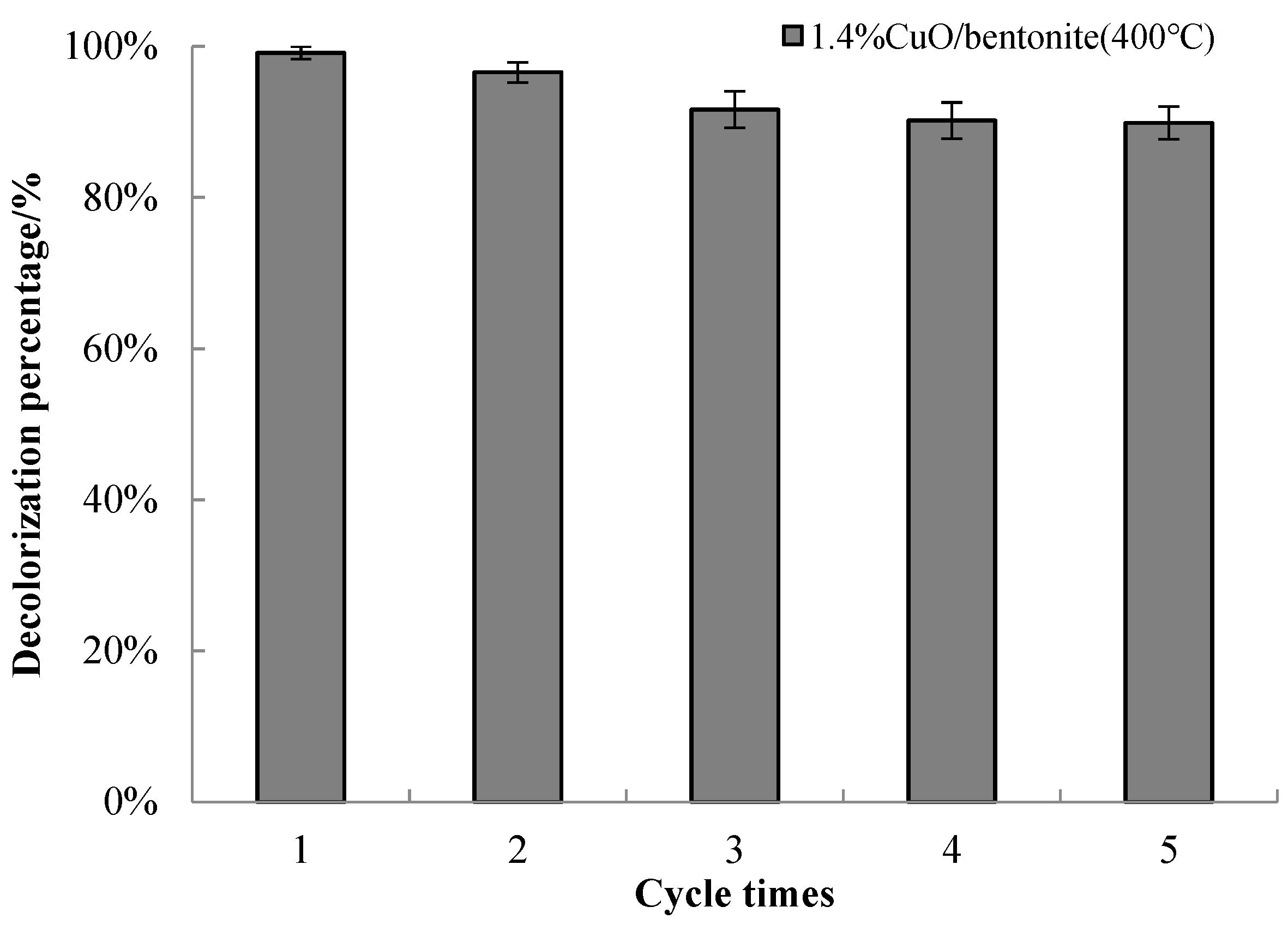
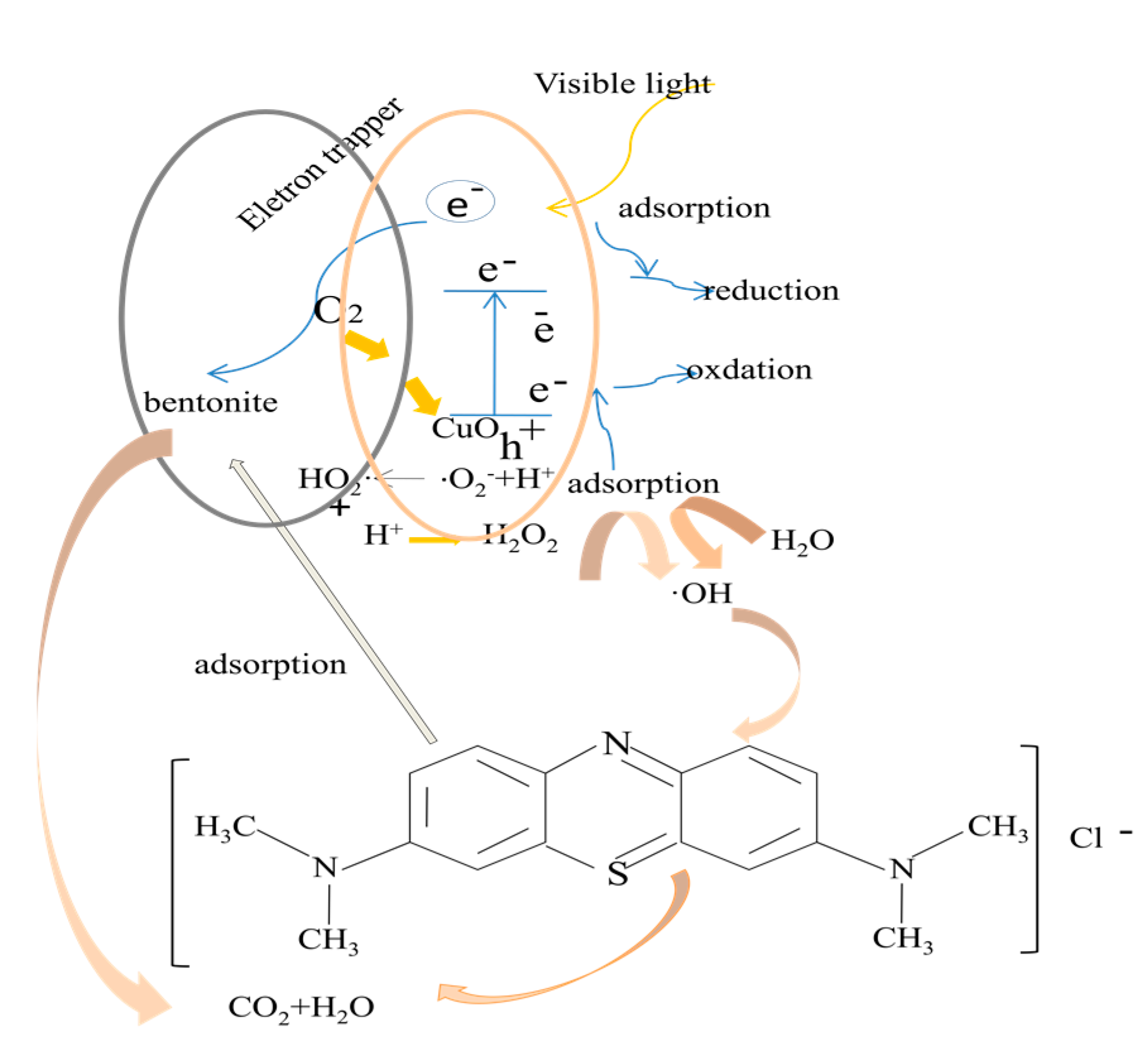
| MBConcentration (mg/L) | Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|---|
| qe (mg/g) | k1 (min−1) | R2 | qe (mg/g) | k2 (g·mg−1·min−1) | R2 | |
| 20 | 68.03 | 0.119 | 0.998 | 66.67 | 0.015 | 0.999 |
| 30 | 84.85 | 0.047 | 0.992 | 111.11 | 0.009 | 0.999 |
| 40 | 89.74 | 0.014 | 0.957 | 142.86 | 0.007 | 0.991 |
| 50 | 132.42 | 0.043 | 0.994 | 200.00 | 0.005 | 0.998 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Xu, H.; Shi, J.; Liu, Z.; Zhao, L. Preparation and Photocatalysis of CuO/Bentonite Based on Adsorption and Photocatalytic Activity. Materials 2021, 14, 5803. https://doi.org/10.3390/ma14195803
Yang C, Xu H, Shi J, Liu Z, Zhao L. Preparation and Photocatalysis of CuO/Bentonite Based on Adsorption and Photocatalytic Activity. Materials. 2021; 14(19):5803. https://doi.org/10.3390/ma14195803
Chicago/Turabian StyleYang, Cuina, Hongfa Xu, Jicun Shi, Zhifeng Liu, and Lei Zhao. 2021. "Preparation and Photocatalysis of CuO/Bentonite Based on Adsorption and Photocatalytic Activity" Materials 14, no. 19: 5803. https://doi.org/10.3390/ma14195803
APA StyleYang, C., Xu, H., Shi, J., Liu, Z., & Zhao, L. (2021). Preparation and Photocatalysis of CuO/Bentonite Based on Adsorption and Photocatalytic Activity. Materials, 14(19), 5803. https://doi.org/10.3390/ma14195803







