Adsorption of Organosilanes on the Surface of Aluminium and the Formation of Organosilane Films to Protect It from Corrosion
Abstract
:1. Introduction
2. Materials
2.1. Material for Adsorption Studies
- Vinyltrimethoxysilane (VS) C5H12O3Si
- Aminoethylaminopropyltrimethoxysilane—diaminesilane (DAS) C8H22N2O3Si
2.2. Material for Electrochemical and Corrosion Studies
Application of Organosilane Films to Aluminium Foil
2.3. Piesoquartz Nanobalance
2.4. Infrared Spectroscopy
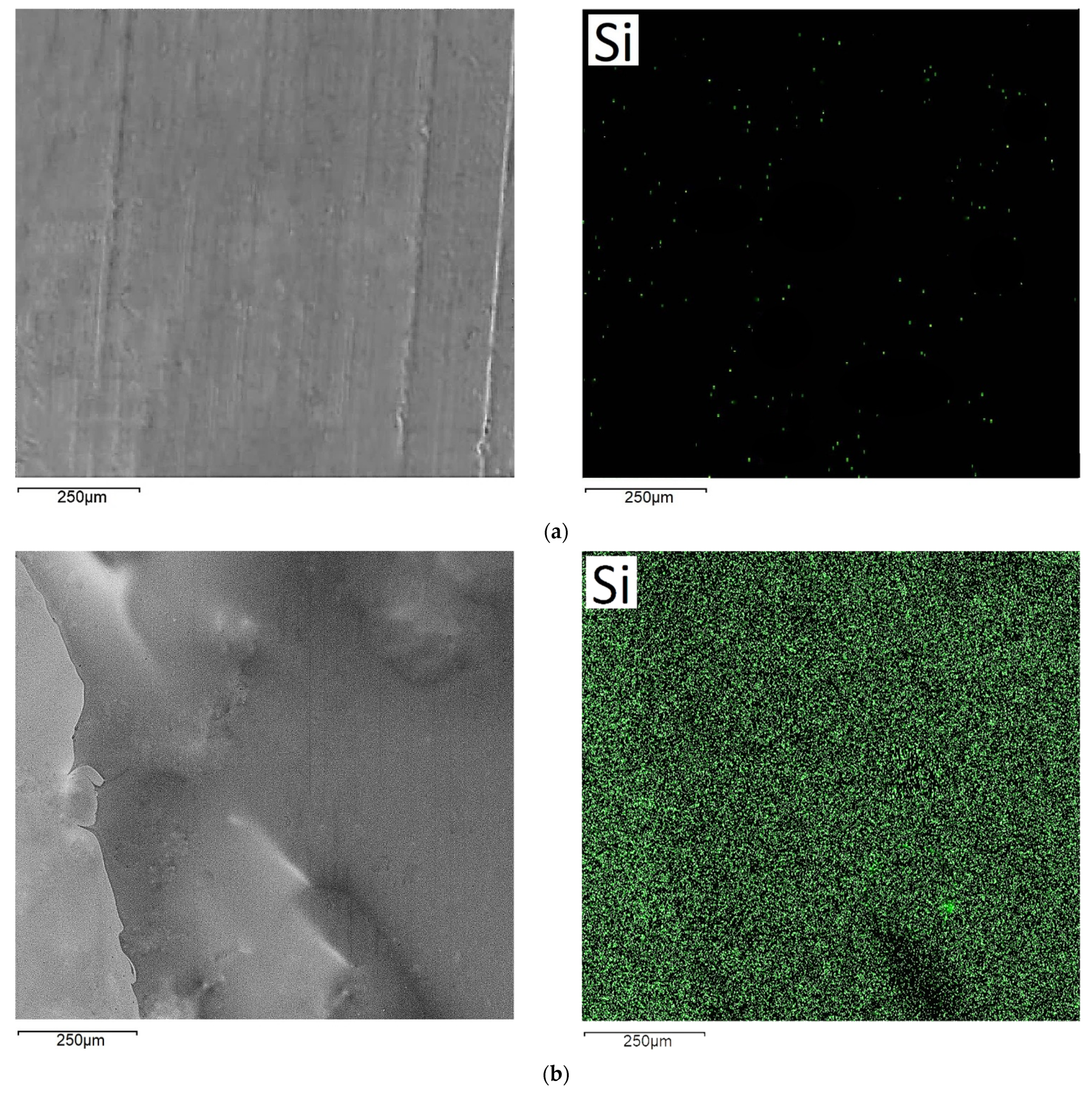
2.5. Scanning Electron Microscopy (SEM)
2.6. Corrosion–Electrochemical Studies
2.6.1. Electrochemical Tests
2.6.2. Corrosion Tests
3. Results
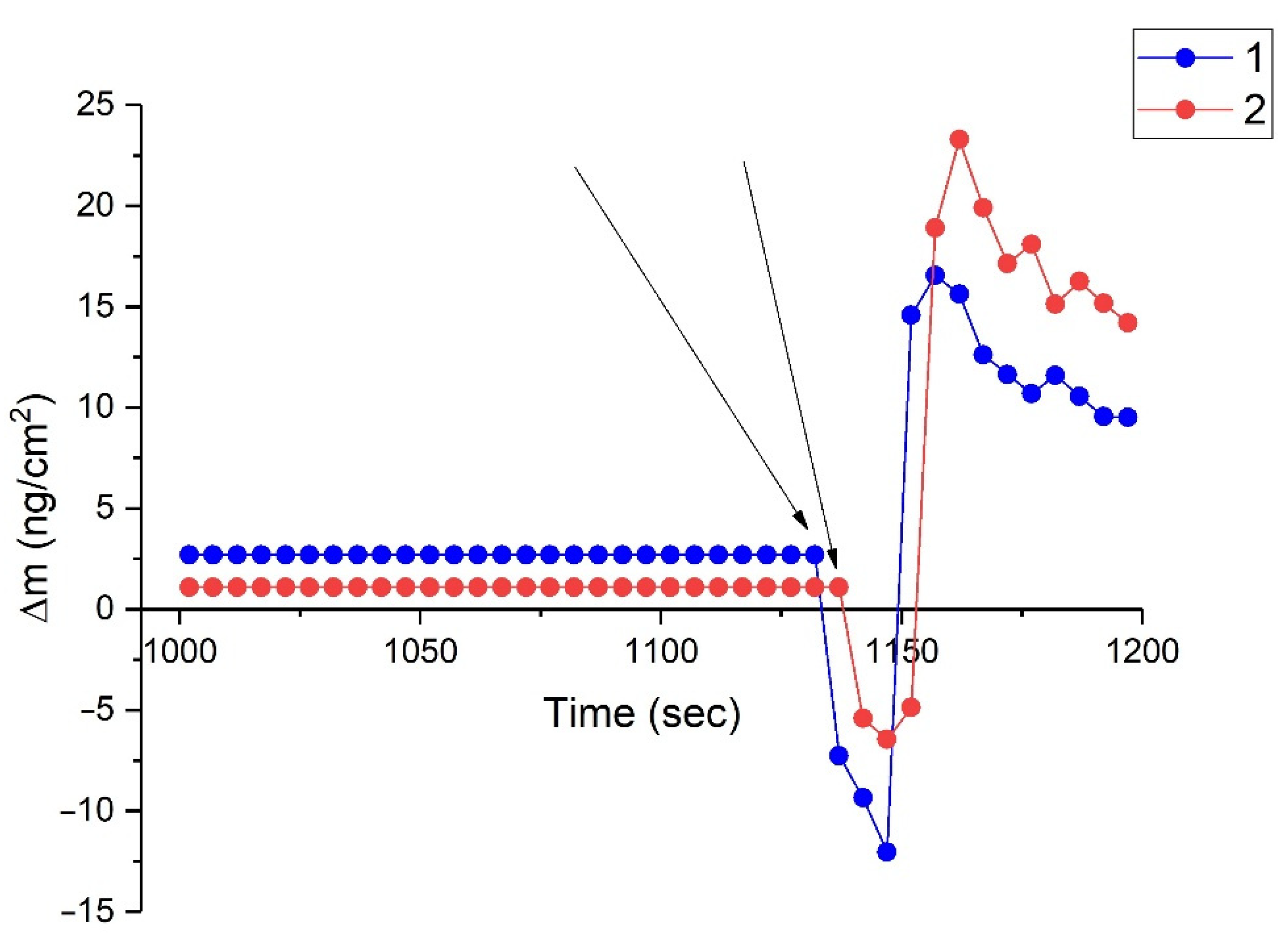
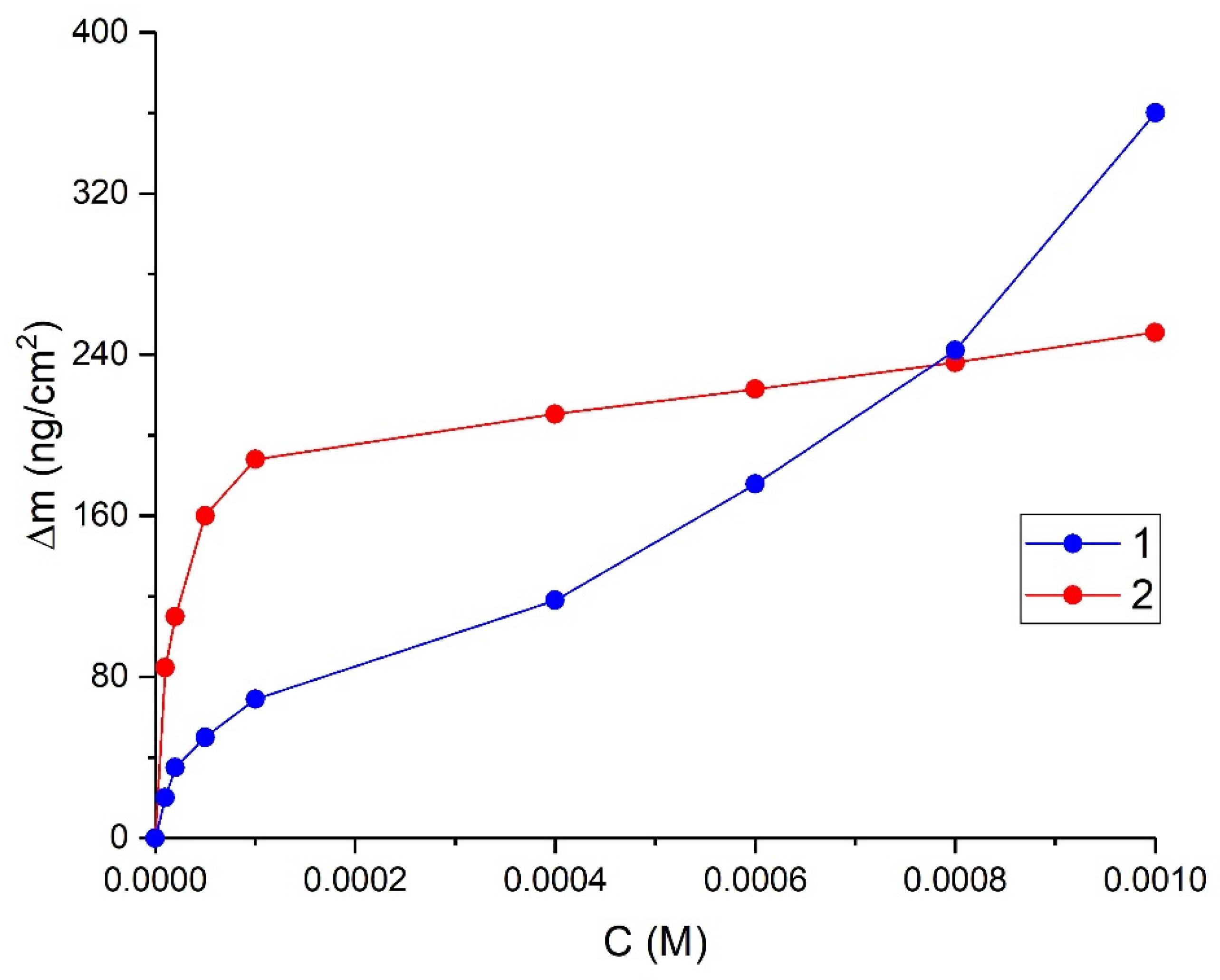
3.1. Adsorption of Organosilanes
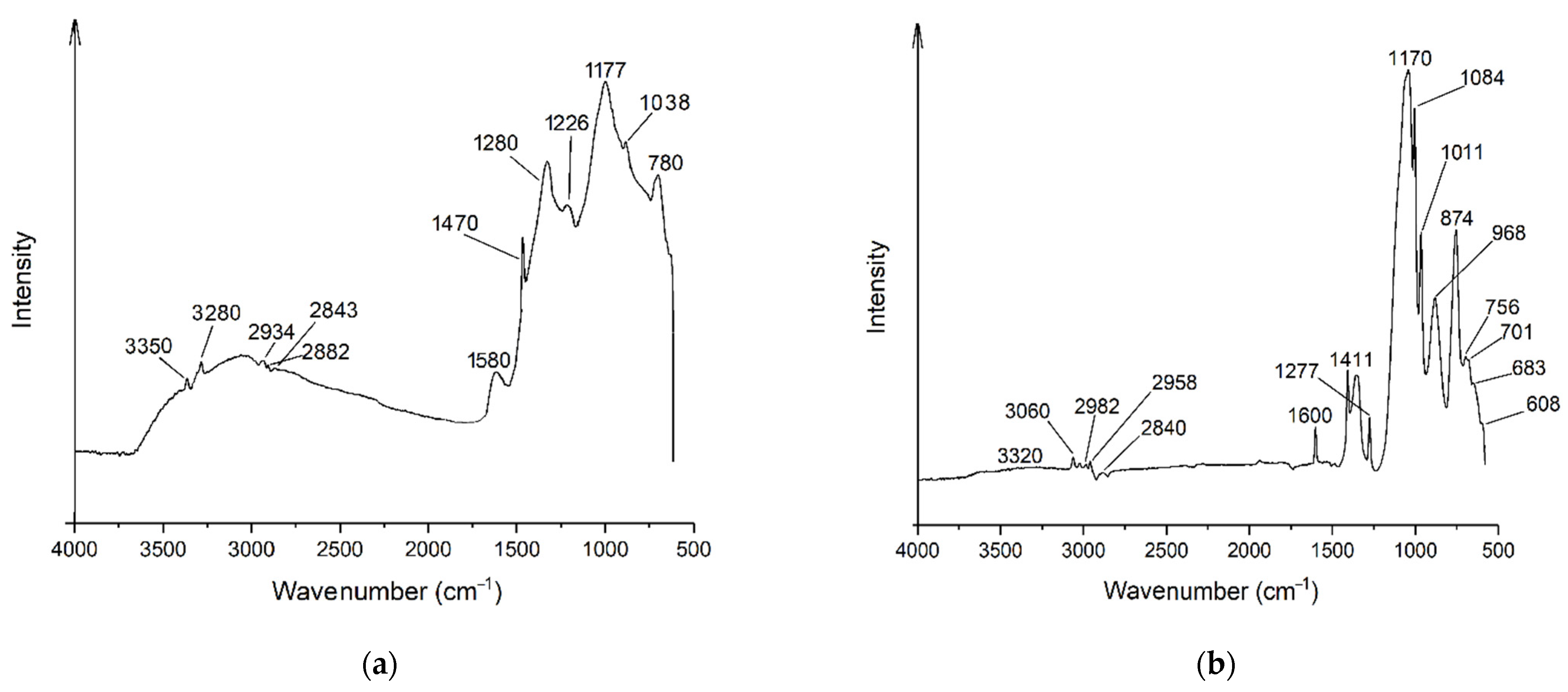
3.2. FTIR Spectroscopy
3.3. Structural Properties of Films
3.4. Corrosion–Electrochemical Behavior of Organosilane Films on the Surface of Al
4. Discussion of Results
4.1. Absorption of DAS and VS on the Surface of Aluminium
4.2. Properties of Organosilane Films Formed on Al
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flamini, D.O.; Trueba, M.; Trasatti, S.P. Aniline-based silane as a primer for corrosion inhibition of aluminium. Prog. Org. Coat. 2012, 74, 302–310. [Google Scholar] [CrossRef]
- Soltis, J. Passivity breakdown, pit initiation and propagation of pits in metallic materials—Review. Corros. Sci. 2015, 90, 5–22. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Gladkikh, N.A.; Maleeva, M.A.; Maksaeva, L.B.; Kostina, Y.V.; Shapagin, A.V.; Yurasova, T.A.; Kotenev, V.A.; Tsivadze, A.Y. The formation of self-organizing organosilicone layers on a carbon steel surface and their effect on the electrochemical and corrosion behavior of the metal. Prot. Met. Phys. Chem. Surf. 2019, 55, 895–902. [Google Scholar] [CrossRef]
- Hepel, M.; Cateforis, E. Studies of copper corrosion inhibition using electrochemical quartz crystal nanobalance and quartz crystal immittance techniques. Electrochim. Acta 2001, 46, 3801–3815. [Google Scholar] [CrossRef]
- Horcas, I.; Fernandez, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 8. [Google Scholar] [CrossRef]
- Kotenev, V.A.; Petrunin, M.A.; Maksaeva, L.B.; Tsivadze, A.Y. 3D visualization of the dissolution products of a metal in the near-electrode layer at the metal-solution interface. Prot. Met. 2005, 41, 507–520. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Yurasova, T.A.; Terekhova, E.V.; Maleeva, M.A.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. Formation of organosilicon self-organizing nanolayers on an iron surface from vapor phase and their effect on corrosion behavior of metal. Prot. Met. Phys. Chem. Surf. 2015, 51, 1010–1017. [Google Scholar] [CrossRef]
- Gladkikh, N.; Makarychev, Y.; Chirkunov, A.; Shapagin, A.; Petrunin, M.; Maksaeva, L.; Maleeva, M.; Yurasova, T.; Marshakov, A. Formation of polymer-like anticorrosive films based on organosilanes with benzotriazole, carboxylic and phosphonic acids. Protection of copper and steel against atmospheric corrosion. Prog. Org. Coat. 2020, 141, 10. [Google Scholar] [CrossRef]
- Petrunin, M.; Maksaeva, L.; Gladkikh, N.; Makarychev, Y.; Maleeva, M.; Yurasova, T.; Nazarov, A. Thin benzotriazole films for inhibition of carbon steel corrosion in neutral electrolytes. Coatings 2020, 10, 362. [Google Scholar] [CrossRef] [Green Version]
- Kuznetsov, Y.I. Organic Inhibitors of Corrosion of Metals; Springer Science+Business Media: Boston, MA, USA, 1996; p. 283. [Google Scholar]
- Makarychev, Y.; Gladkikh, N.; Arkhipushkin, I.; Kuznetsov, Y. Corrosion Inhibition of Low-Carbon Steel by Hydrophobic Organosilicon Dispersions. Metals 2021, 11, 1269. [Google Scholar] [CrossRef]
- Maleeva, M.A.; Ignatenko, V.E.; Shapagin, A.V.; Sherbina, A.A.; Maksaeva, L.B.; Marshakov, A.I.; Petrunin, M.A. Modification of bituminous coatings to prevent stress corrosion cracking of carbon steel. Int. J. Corros. Scale Inhib. 2015, 4, 226–234. [Google Scholar] [CrossRef]
- Andreev, N.N.; Kuznetsov, Y.I. Volatile inhibitors of atmospheric corrosion. III. Principles and methods of efficiency estimation. Int. J. Corros. Scale Inhib. 2013, 2, 39–52. [Google Scholar] [CrossRef]
- Corrosion Inhibitors: Principles, Mechanisms and Applications; Hart, E. (Ed.) Nova Science Publishers Incorporated: Hauppauge, NY, USA, 2016; p. 173. [Google Scholar]
- Petrunin, M.A.; Gladkikh, N.A.; Maleeva, M.A.; Maksaeva, L.B.; Yurasova, T.A. The use of organosilanes to inhibit metal corrosion. A review. Int. J. Corros. Scale Inhib. 2019, 8, 882–907. [Google Scholar]
- Petrunin, M.A.; Gladkikh, N.A.; Maleeva, M.A.; Rybkina, A.A.; Terekhova, E.V.; Yurasova, T.A.; Ignatenko, V.E.; Maksaeva, L.B.; Kotenev, V.A.; Tsivadze, A.Y. Improving the anticorrosion characteristics of polymer coatings in the case of their modification with compositions based on organosilanes. Prot. Met. Phys. Chem. Surf. 2021, 57, 374–388. [Google Scholar] [CrossRef]
- Plueddemann, P.E. Silane Coupling Agents; Springer Science+Business Media: New York, NY, USA, 1982; p. 238. [Google Scholar]
- Bažant, V.; Chvalovský, V.; Rathouský, J. Organosilicon Compounds; Publishing House of the Czechoslovak Academy of Sciences: New York, NY, USA; Academic Press: Prague, Czech Republic, 1965; p. 587. [Google Scholar]
- Moriguchi, K.; Utagava, S. Silane: Chemistry, Applications, and Performance; Nova Science Publishers Incorporated: New York, NY, USA, 2013; p. 176. [Google Scholar]
- Osterholtz, F.D.; Pohl, E.R. Kinetics of the hydrolysis and condensation of organofunctional alkoxysilanes—A review. J. Adhes. Sci. Technol. 1992, 6, 127–149. [Google Scholar] [CrossRef]
- Oyola-Reynoso, S.; Tevis, I.D.; Chen, J.; Chang, B.S.; Cinar, S.; Bloch, J.F.; Thuo, M.M. Recruiting physisorbed water in surface polymerization for bio-inspired materials of tunable hydrophobicity. J. Mater. Chem. A 2016, 4, 14729–14738. [Google Scholar] [CrossRef] [Green Version]
- Naik, V.V.; Crobu, M.; Venkataraman, N.V.; Spencer, N.D. Multiple Transmission-reflection IR spectroscopy shows that surface hydroxyls play only a minor role in alkylsilane monolayer formation on silica. J. Phys. Chem. Lett. 2013, 4, 2745–2751. [Google Scholar] [CrossRef]
- Gladkikh, N.; Makarychev, Y.; Petrunin, M.; Maleeva, M.; Maksaeva, L.; Marshakov, A. Synergistic effect of silanes and azole for enhanced corrosion protection of carbon steel by polymeric coatings. Prog. Org. Coat. 2020, 138, 10. [Google Scholar] [CrossRef]
- Subramanian, V.; van Ooij, W.J. Effect of the amine functional group on corrosion rate of iron coated with films of organofunctional silanes. Corrosion 1998, 54, 204–215. [Google Scholar] [CrossRef]
- Fedel, M.; Olivier, M.; Poelman, M.; Deflorian, F.; Rossi, S.; Druart, M.E. Corrosion protection properties of silane pre-treated powder coated galvanized steel. Prog. Org. Coat. 2009, 66, 118–128. [Google Scholar] [CrossRef]
- Gladkikh, N.; Makarychev, Y.; Maleeva, M.; Petrunin, M.; Maksaeva, L.; Rybkina, A.; Marshakov, A.; Kuznetsov, Y. Synthesis of thin organic layers containing silane coupling agents and azole on the surface of mild steel. Synergism of inhibitors for corrosion protection of underground pipelines. Prog. Org. Coat. 2019, 132, 481–489. [Google Scholar] [CrossRef]
- Elawady, A.A.; Abdelnabey, B.A.; Aziz, S.G. Kinetic-thermodynamic and adsorption-isotherms analyses for the inhibition of the acid corrosion of steel by cyclic and open-chain amines. J. Electrochem. Soc. 1992, 139, 2149–2154. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Gladkikh, N.A.; Maleeva, M.A.; Yurasova, T.A.; Terekhova, E.V.; Maksaeva, L.B. Application of the quartz crystal microbalance technique in corrosion studies. A review. Int. J. Corros. Scale Inhib. 2020, 9, 92–117. [Google Scholar]
- GOST 5272–68. Corrosion of metals. Terms. In Term 8. Application: Zh; IPK Publishing Standards: Moscow, Russia, 1999; p. 15. [Google Scholar]
- ASTM D 610–08. Standard Test Method for Evaluating Degree of Rusting on Painted STEEL Surfaces; ASTM International: West Conshohocken, PA, USA, 2019; p. 7. [Google Scholar]
- Kern, P.; Landolt, D. Adsorption of an organic corrosion inhibitor on iron and gold studied with a rotating EQCM. J. Electrochem. Soc. 2001, 148, B228–B235. [Google Scholar] [CrossRef]
- Nuzzo, R.G.; Fusco, F.A.; Allara, D.L. Spontaneously organized molecular assemblies: Preparation and properties of solution adsorbed monolayers of organic disulfides on gold surfaces. J. Am. Chem. Soc. 1987, 109, 2358–2368. [Google Scholar] [CrossRef]
- Kern, P.; Landolt, D. Adsorption of organic corrosion inhibitors on iron in the active and passive state. A replacement reaction between inhibitor and water studied with the rotating quartz crystal microbalance. Electrochim. Acta 2001, 47, 589–598. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Gladkikh, N.A.; Narkevich, E.N.; Yurasova, T.A.; Rybkin, A.A.; Terekhova, E.V.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. The effect of vinyl-siloxane nanolayers on the corrosion behavior of zinc. Prot. Met. Phys. Chem. Surf. 2018, 54, 795–803. [Google Scholar] [CrossRef]
- Petrunin, M.A.; Maksaeva, L.B.; Yurasova, T.A.; Gladkikh, N.A.; Terekhova, E.V.; Kotenev, V.A.; Kablov, E.N.; Tsivadze, A.Y. Adsorption of vinyl trimethoxysilane and formation of vinyl siloxane nanolayers on zinc surface from aqueous solution. Prot. Met. Phys. Chem. Surf. 2016, 52, 964–971. [Google Scholar] [CrossRef]

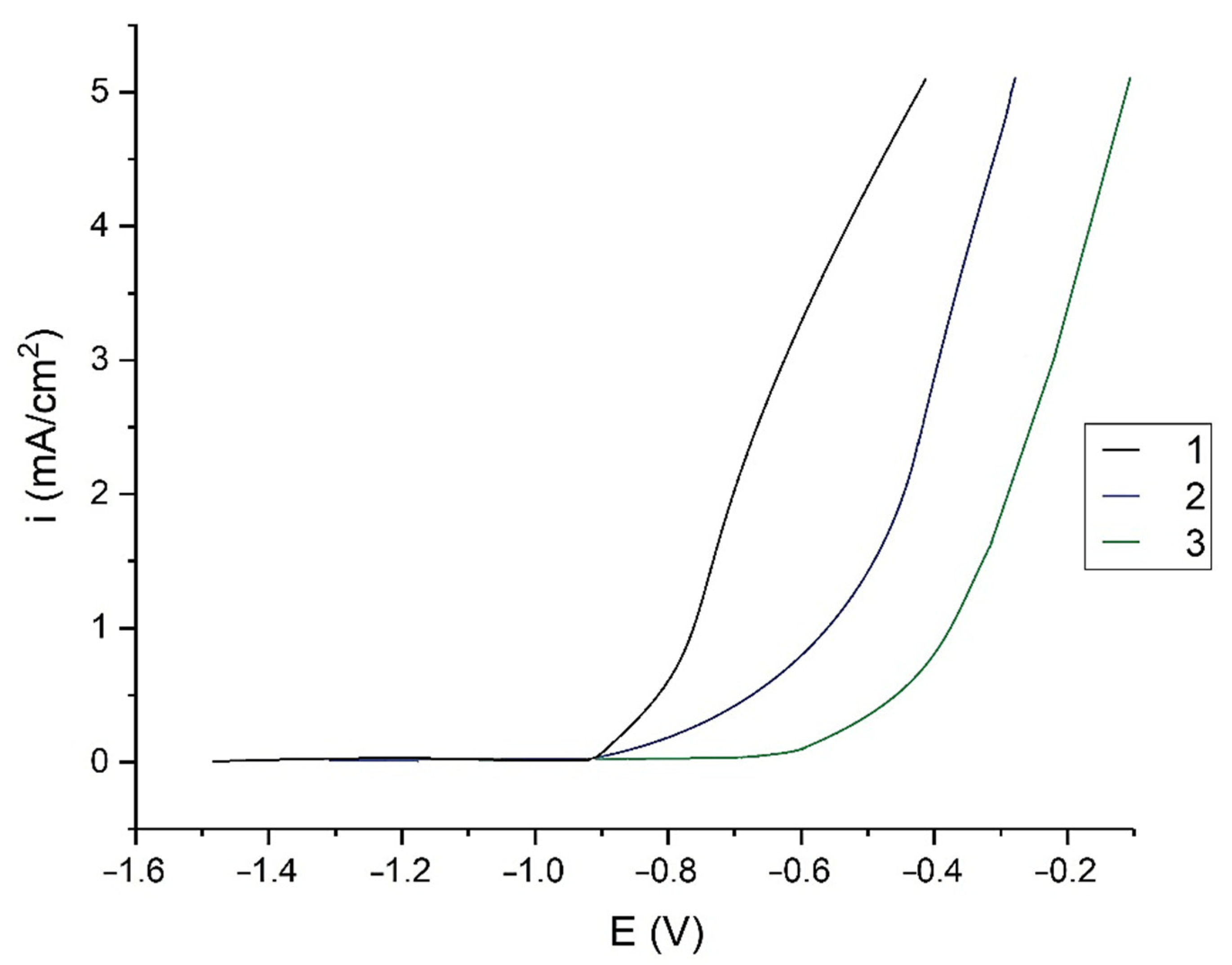

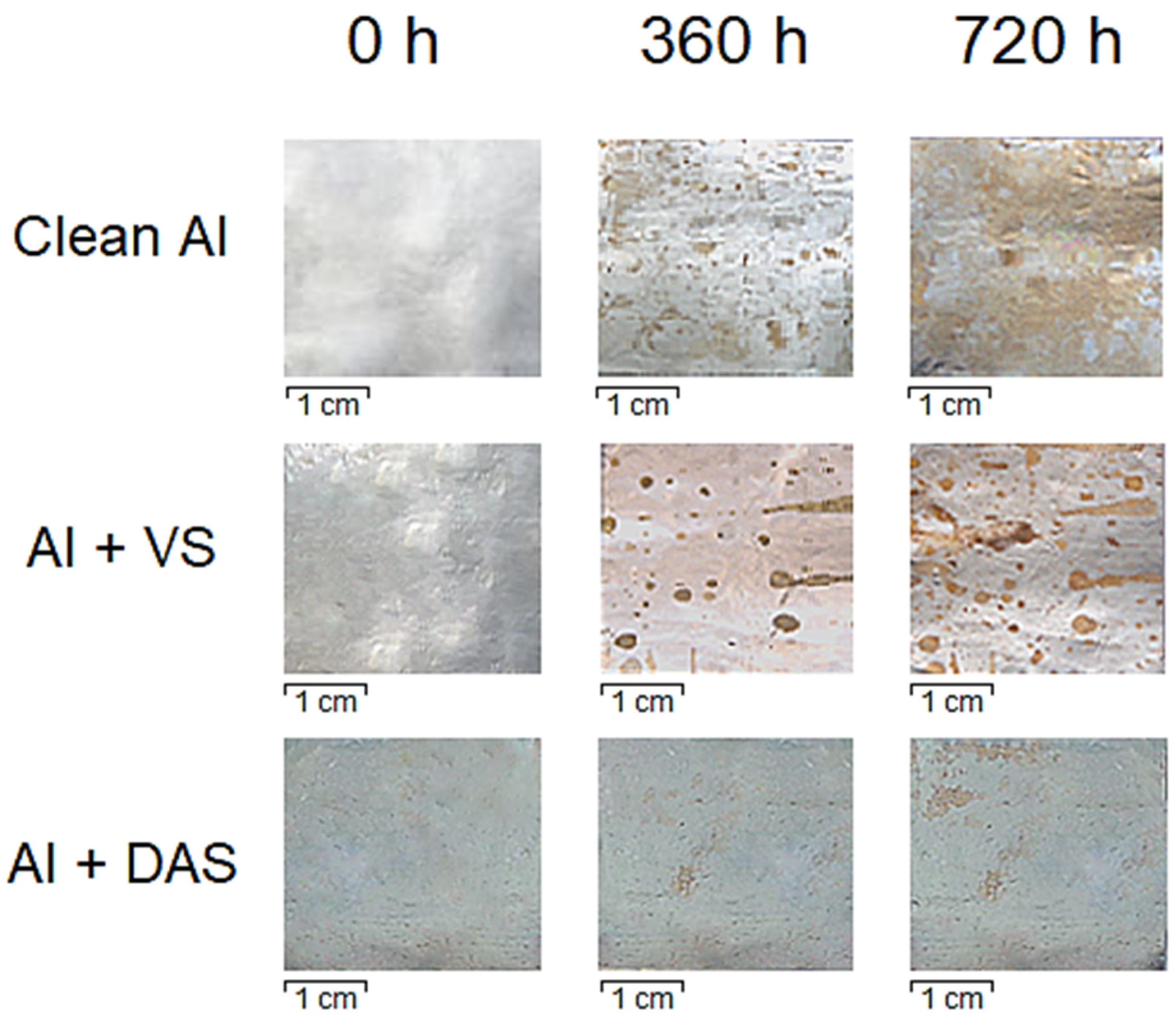
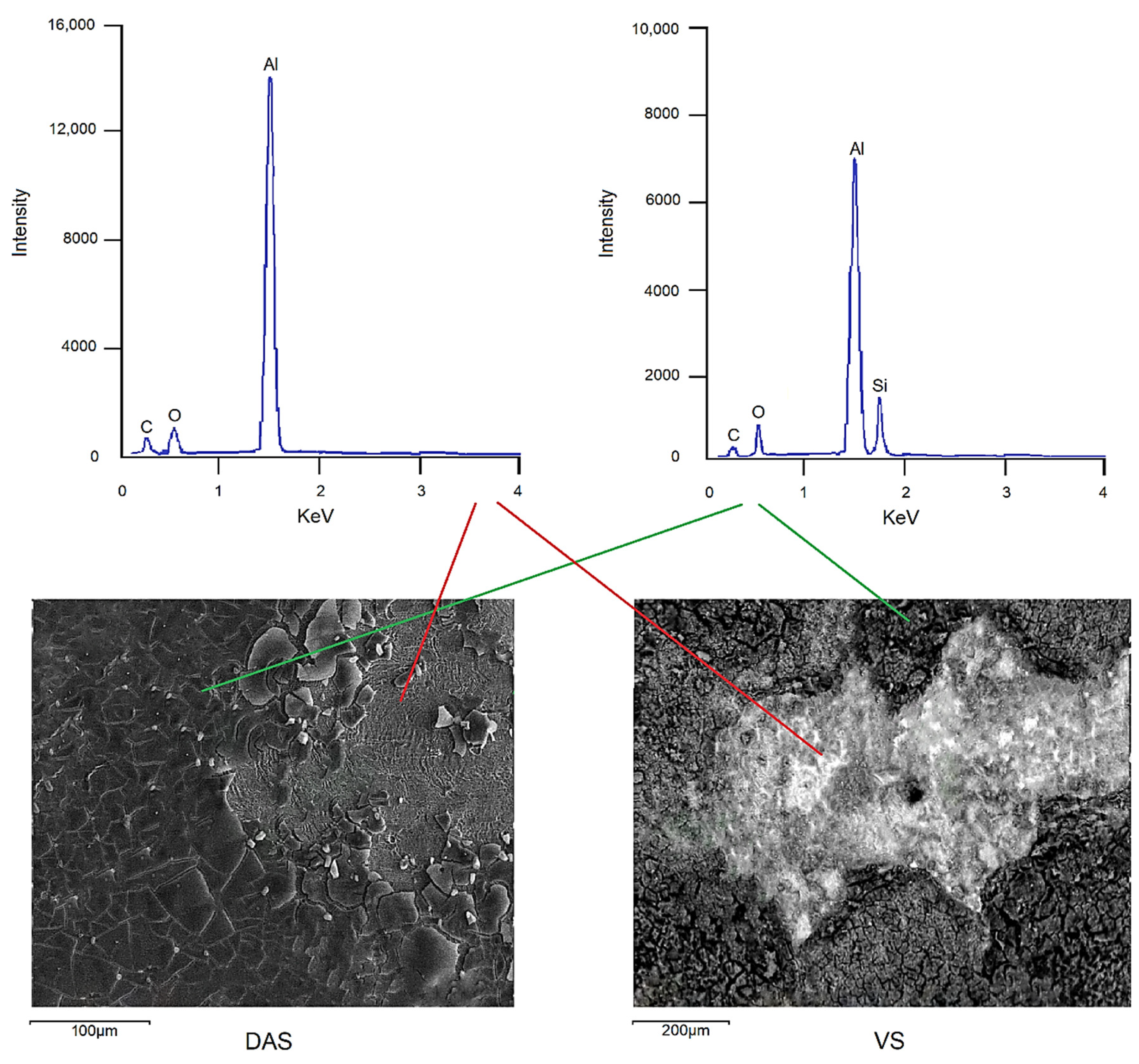

| System | Ecor, V | Epit, V |
|---|---|---|
| Clean Al | −1.50 | −0.94 |
| Al + VS | −1.39 | −0.87 |
| Al + DAS | −1.15 | −0.76 |
| System | τ, h | Q, % | ||
|---|---|---|---|---|
| Exposure Time, h | ||||
| 0 | 360 | 720 | ||
| Clean Al | 156 | - | 16.0 | 50.0 |
| Al + VS | 218 | - | 3.0 | 10.0 |
| Al + DAS | 242 | - | 0.3 | 3.0 |
| No. | Adsorption Isotherm | DAS | VS | ||
|---|---|---|---|---|---|
| Correlation, R2 | Correlation, R2 | ||||
| 1 | Langmür | 0.990 | - | 0.991 | - |
| 2 | BET | 1.000 | −27.56 | 1.000 | −25.48 |
| 3 | Temkin | 0.995 | −47.32 | 0.923 | −46.61 |
| 4 | Langmür Multicenter | 0.999 | −45.15 | 0.999 | −44.54 |
| 5 | Flory–Huggins | 0.999 | −43.29 | 0.998 | −40.73 |
| 6 | Frumkin | 0.994 | −38.26 | 0.984 | −37.96 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gladkikh, N.; Petrunin, M.; Maksaeva, L.; Yurasova, T. Adsorption of Organosilanes on the Surface of Aluminium and the Formation of Organosilane Films to Protect It from Corrosion. Materials 2021, 14, 5757. https://doi.org/10.3390/ma14195757
Gladkikh N, Petrunin M, Maksaeva L, Yurasova T. Adsorption of Organosilanes on the Surface of Aluminium and the Formation of Organosilane Films to Protect It from Corrosion. Materials. 2021; 14(19):5757. https://doi.org/10.3390/ma14195757
Chicago/Turabian StyleGladkikh, Natalia, Maxim Petrunin, Ludmila Maksaeva, and Tatyana Yurasova. 2021. "Adsorption of Organosilanes on the Surface of Aluminium and the Formation of Organosilane Films to Protect It from Corrosion" Materials 14, no. 19: 5757. https://doi.org/10.3390/ma14195757
APA StyleGladkikh, N., Petrunin, M., Maksaeva, L., & Yurasova, T. (2021). Adsorption of Organosilanes on the Surface of Aluminium and the Formation of Organosilane Films to Protect It from Corrosion. Materials, 14(19), 5757. https://doi.org/10.3390/ma14195757







