Impact of γ-rays Irradiation on Hybrid TiO2-SiO2 Sol-Gel Films Doped with RHODAMINE 6G
Abstract
:1. Introduction
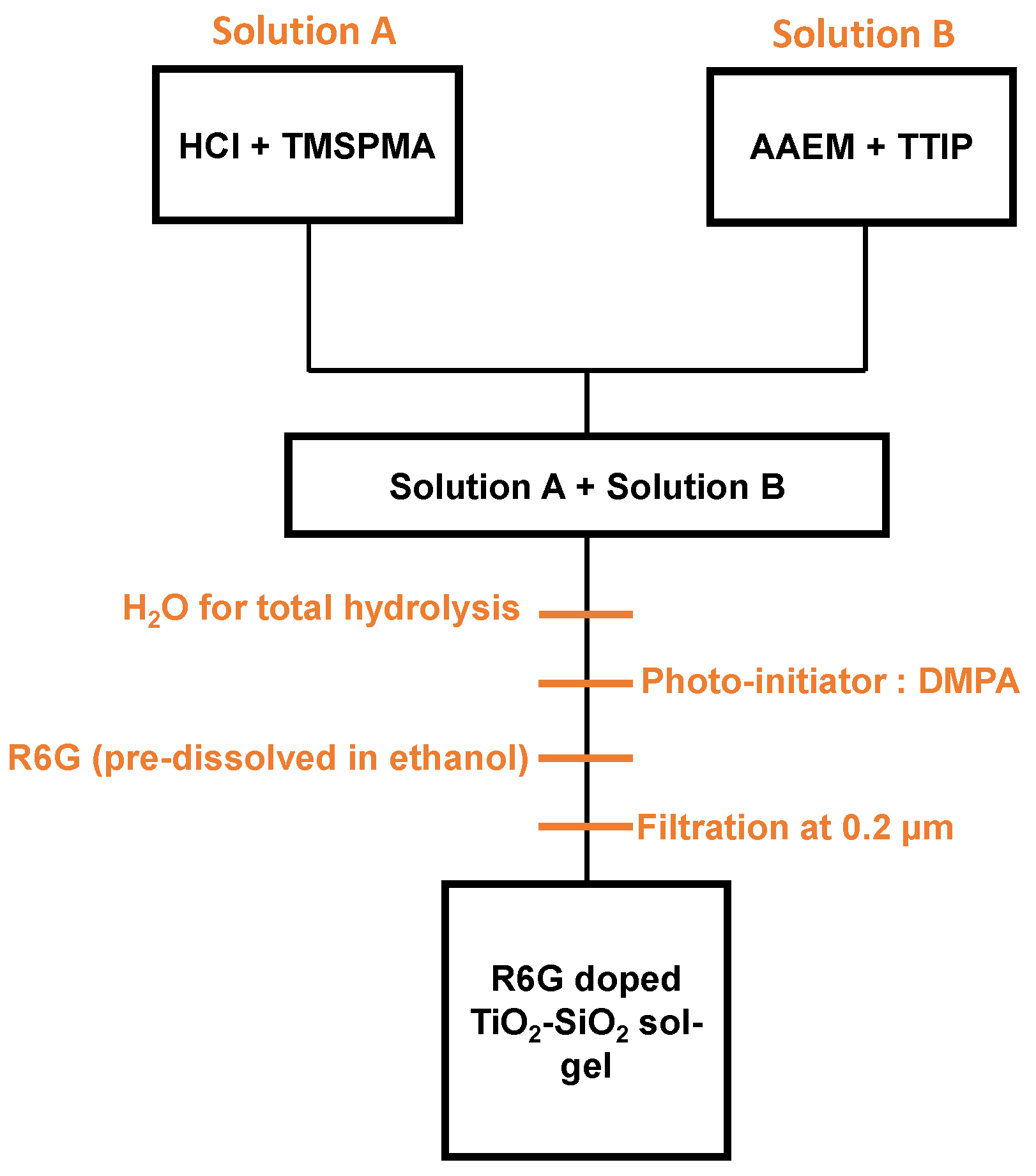
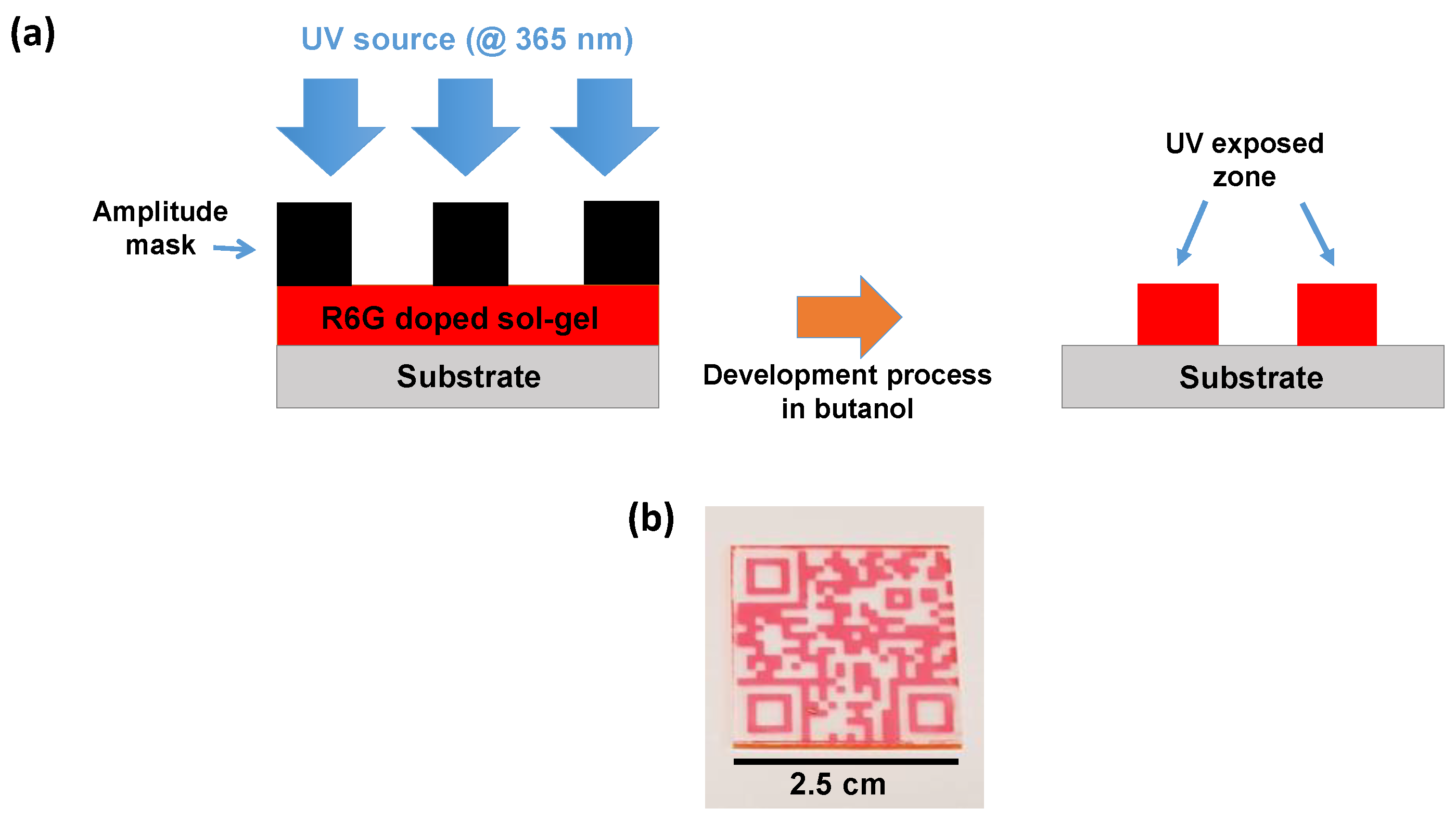
2. Materials and Methods
3. Results
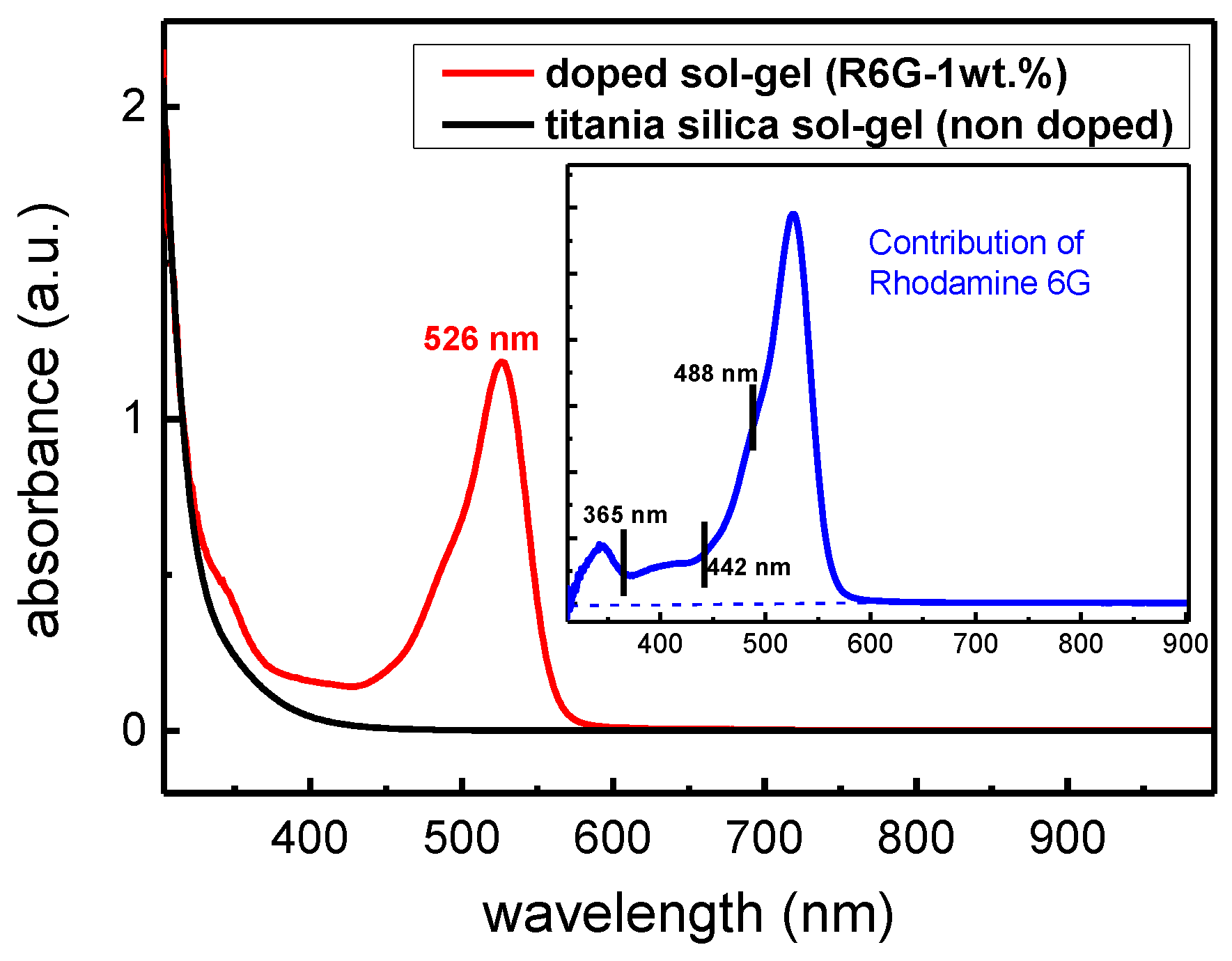
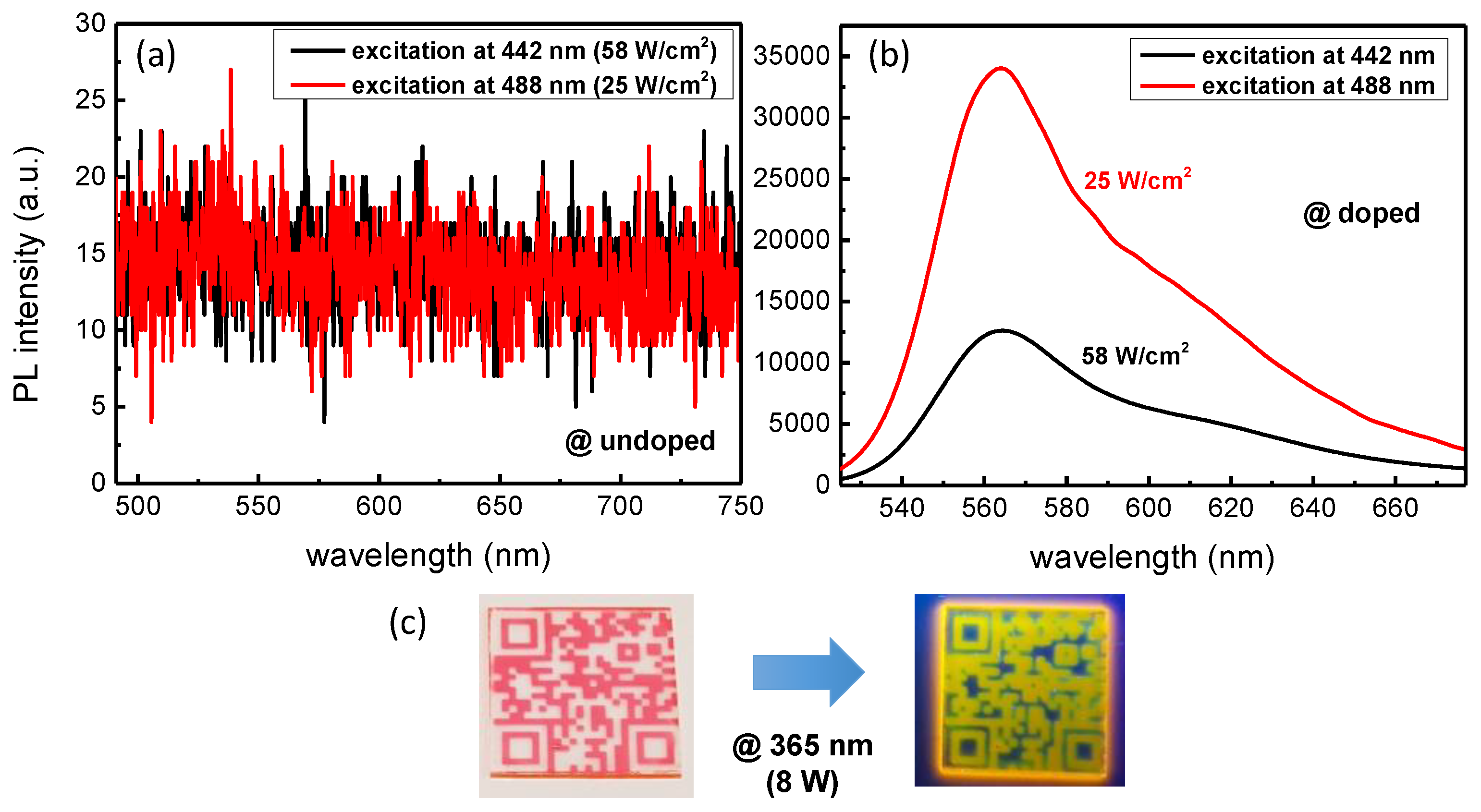
3.1. Absorption and PL Characterization of R6G Doped Sol-Gel Films
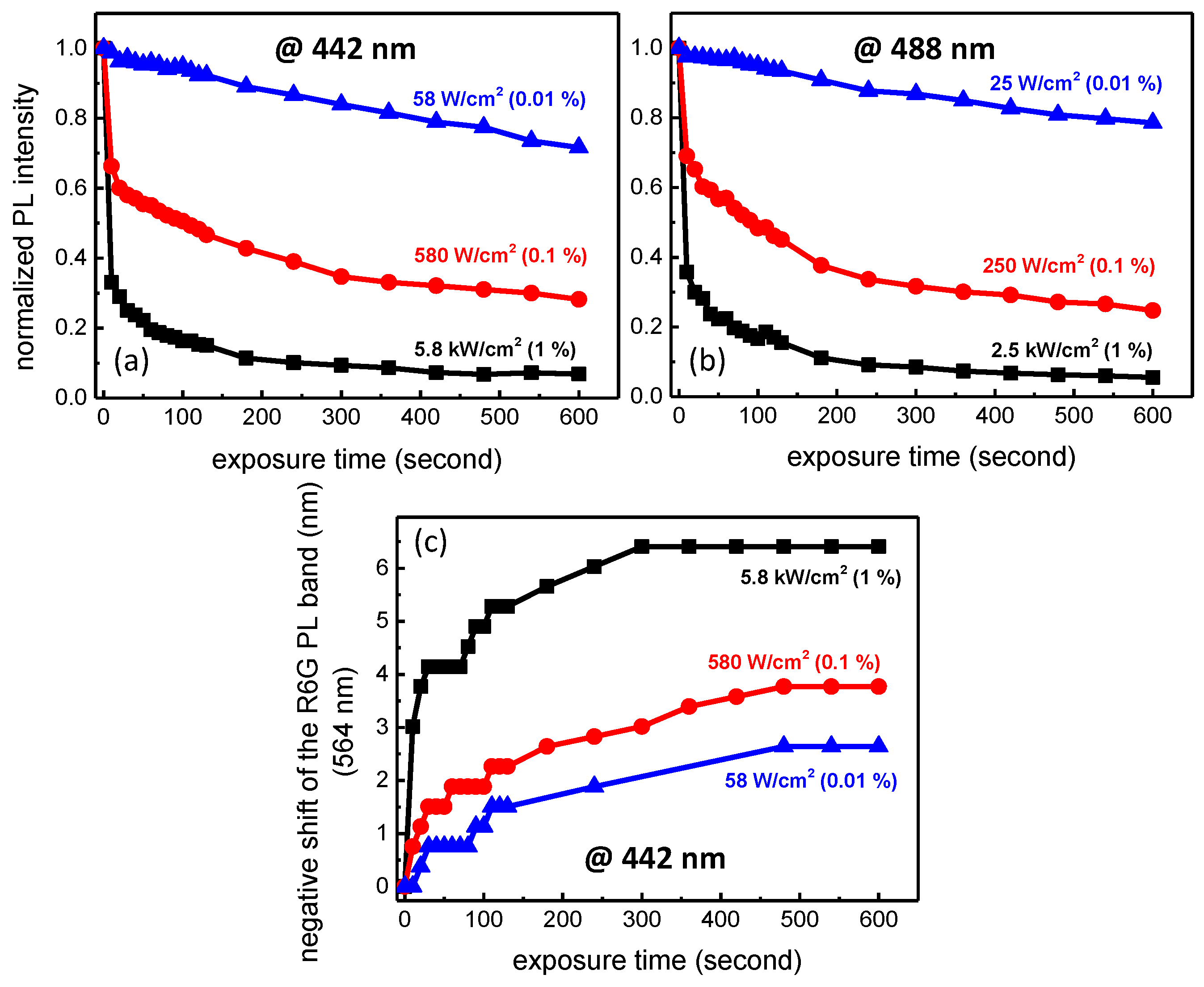
3.2. Photobleaching under Visible Light Exposure
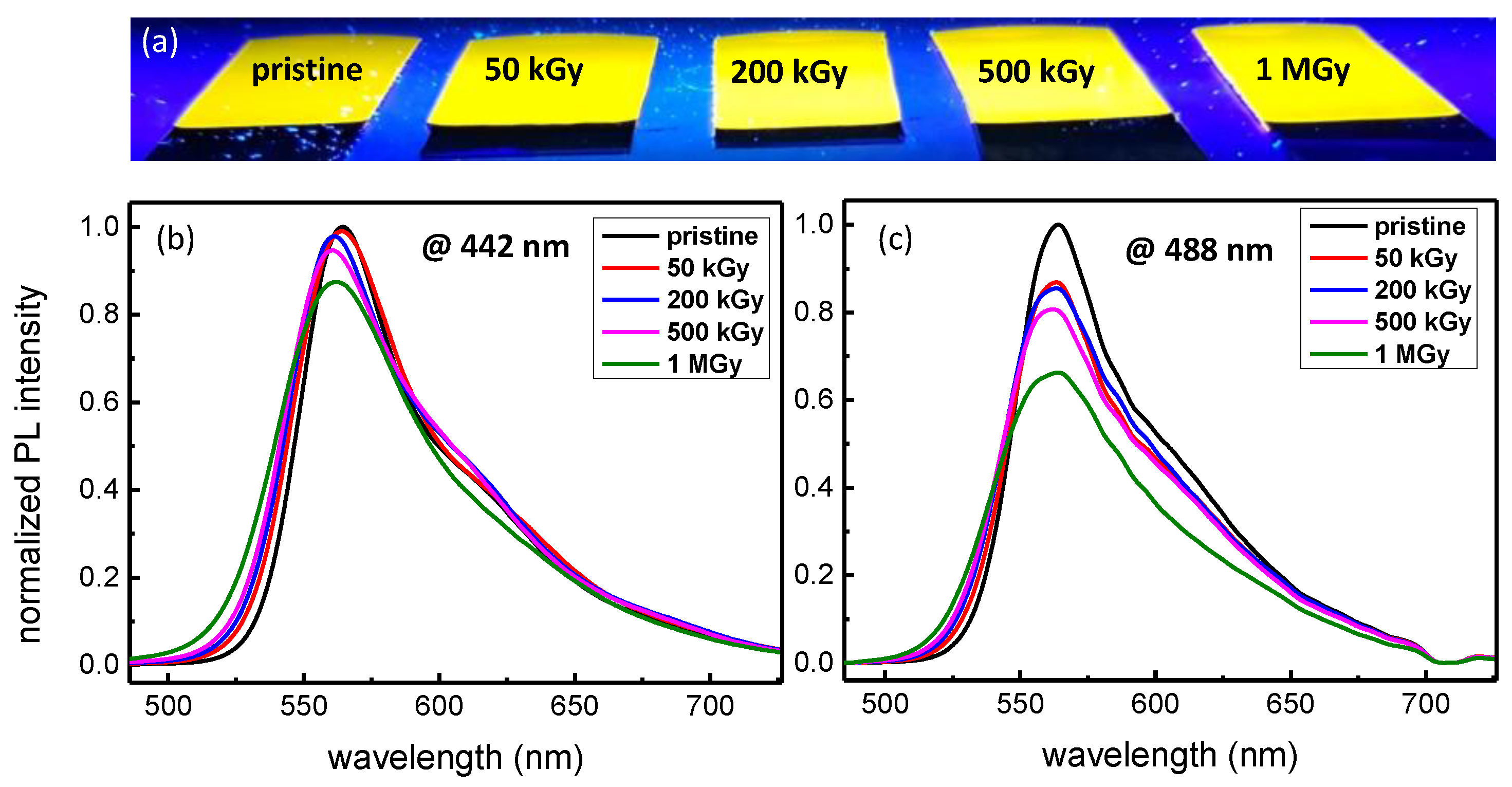
3.3. PL Properties after γ-ray Exposure
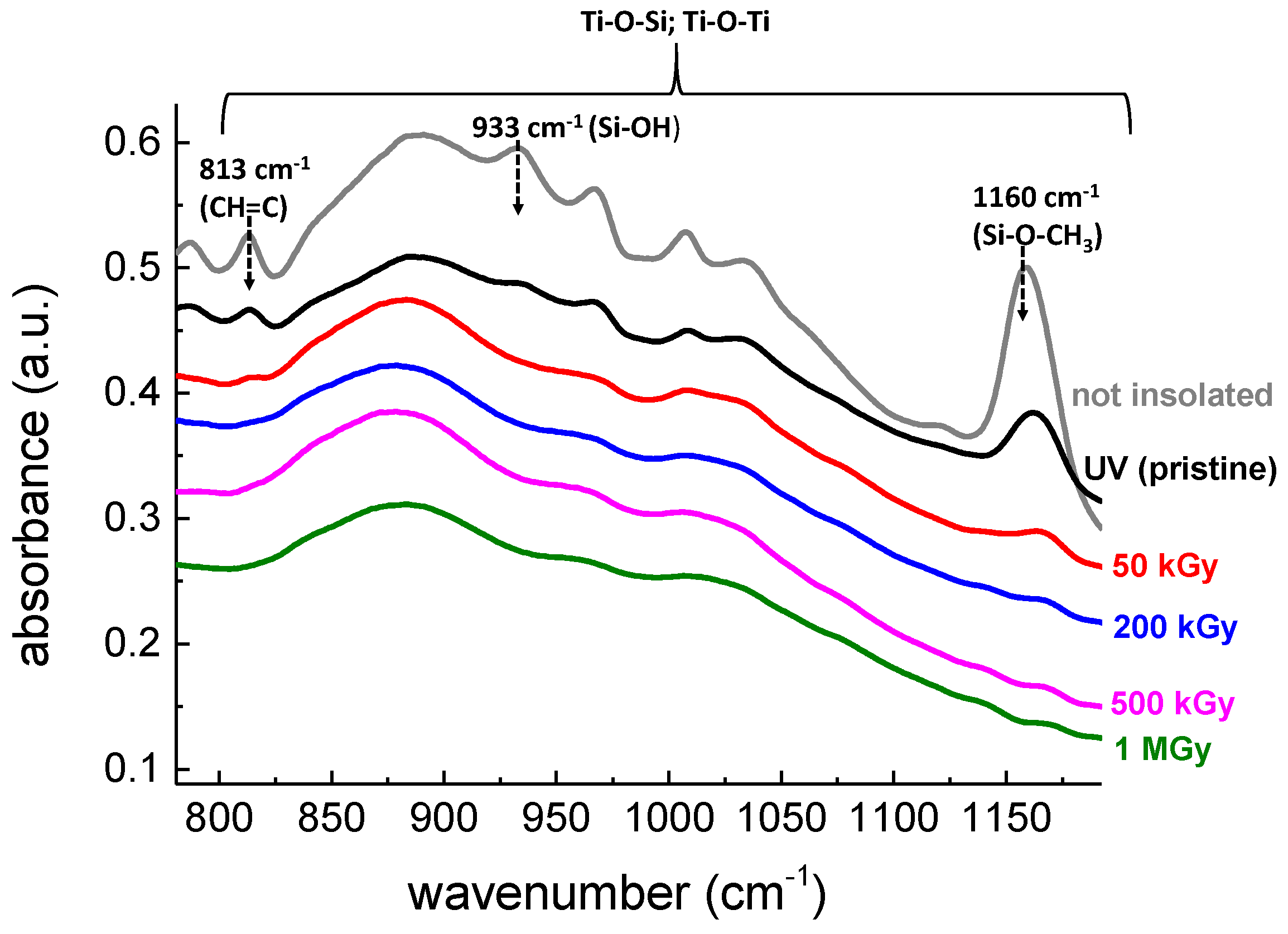
3.4. FTIR Spectroscopy
3.5. Radiation Induced Refractive Index Change
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Agustín-Sáenz, C.; Santa Coloma, P.; Fernández-Carretero, F.J.; Brusciotti, F.; Brizuela, M. Design of Corrosion Protective and Antistatic Hybrid Sol-Gel Coatings on 6XXX AlMgSi Alloys for Aerospace Application. Coatings 2020, 10, 441. [Google Scholar] [CrossRef]
- Wang, D.; Bierwagen, P. Sol-gel coatings on metals for corrosion protection. Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar] [CrossRef]
- Malucelli, G. Sol-gel and layer-by-layer coatings for flame-retardant cotton fabrics: Recent advances. Coatings 2020, 10, 333. [Google Scholar] [CrossRef] [Green Version]
- Nivens, D.A.; Schiza, M.V.; Michael Angel, S. Multilayer sol-gel membranes for optical sensing applications: Single pH and dual layer CO2 and NH3 sensors. Talanta 2002, 58, 543–550. [Google Scholar] [CrossRef]
- Jerónimo, P.C.A.; Araújo, A.N.; Montenegro, M.C.B.S.M. Optical sensors and biosensors based on sol-gel films. Talanta 2007, 72, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Coudray, P.; Etienne, P.; Moreau, Y.; Porque, J.; Najafi, S. Sol-gel channel waveguide on silicon: Fast direct imprinting and low cost fabrication. Opt. Commun. 1997, 143, 199–202. [Google Scholar] [CrossRef]
- Oubaha, M.; Kribich, R.K.; Copperwhite, R.; Etienne, P.; O’Dwyer, K.; MacCraith, B.D.; Moreau, Y. New inorganic sol-gel material with high transparency at 1.55 µm. Opt. Commun. 2005, 253, 346–351. [Google Scholar] [CrossRef]
- Royon, M.; Jamon, D.; Blanchet, T.; Royer, F.; Vocanson, F.; Marin, E.; Morana, A.; Boukenter, A.; Ouerdane, Y.; Jourlin, Y.; et al. Sol–Gel Waveguide-Based Sensor for Structural Health Monitoring on Large Surfaces in Aerospace Domain. Aerospace 2021, 8, 109. [Google Scholar] [CrossRef]
- Gâté, V.; Jourlin, Y.; Vocanson, F.; Dellea, O.; Vercasson, G.; Reynaud, S.; Riassetto, D.; Langlet, M. Sub-micrometric patterns written using a DIL method coupled to a TiO2 photo-resist. Opt. Mater. 2013, 35, 1706–1713. [Google Scholar] [CrossRef]
- Gâté, V.; Bernaud, G.; Veillas, C.; Cazier, A.; Vocanson, F.; Jourlin, Y.; Langlet, M. Fast dynamic interferometric lithography for large submicrometric period diffraction gratings production. Opt. Eng. 2013, 52, 091712. [Google Scholar] [CrossRef]
- Gombert, A.; Rose, K.; Heinzel, A.; Horbelt, W.; Zanke, C.; Bläsi, B.; Wittwer, V. Antireflective submicrometer surface-relief gratings for solar applications. Sol. Energy Mater. Sol. Cells 1998, 54, 333–342. [Google Scholar] [CrossRef]
- Sanchez, C.; Julián, B.; Belleville, P.; Popall, M.; Julián-López, B. Applications of hybrid organic–inorganic nanocomposites. J. Mater. Chem. 2005, 15, 3559–3592. [Google Scholar] [CrossRef]
- Kaczmarek, H.; Galka, P. Effect of irgacure 651 initiator on poly(methyl methacryltate) photostability studied by UV-Vis spectroscopy. Open Process Chem. J. 2008, 1, 8–11. [Google Scholar] [CrossRef]
- Äyräs, P.; Rantala, J.T.; Honkanen, S.; Mendes, S.B.; Peyghambarian, N. Diffraction gratings in sol-gel films by direct contact printing using a UV-mercury lamp. Opt. Commun. 1999, 162, 215–218. [Google Scholar] [CrossRef]
- Bidaud, C.; Berling, D.; Jamon, D.; Gamet, E.; Neveu, S.; Royer, F.; Soppera, O. Photocrosslinking and photopatterning of magneto-optical nanocomposite sol-gel thin film under deep-UV irradiation. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Reisfeld, R. Prospects of sol-gel technology towards luminescent materials. Opt. Mater. 2001, 16, 1–7. [Google Scholar] [CrossRef]
- Tripathi, S.K.; Monga, A.; Saini, G.S.S. Characterization of thermally evaporated thin films of Rhodamine 6G. Smart Mater. Struct. 2009, 18, 125012. [Google Scholar] [CrossRef]
- Zheng, Y.M.; Farida Yunus, R.; Nadeeshani Nanayakkara, K.G.; Paul Chen, J. Electrochemical decoloration of synthetic wastewater containing Rhodamine 6G: Behaviors and mechanism. Ind. Eng. Chem. 2012, 51, 5953–5960. [Google Scholar] [CrossRef]
- Wu, C.; Chen, E.; Wei, J. Surface enhanced Raman spectroscopy of Rhodamine 6G on agglomerates of different-sized silver truncated nanotriangles. Colloids Surf. A Physicochem. Eng. Asp. 2016, 506, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Parejo, P.G.; Alvarez-Herrero, A.; Zayat, M. Thermo-optic properties of hybrid sol-gel thin films doped with Rhodamine 6G at high vacuum conditions. J. Mater. Sci. 2015, 50, 6677–6687. [Google Scholar] [CrossRef]
- Purcar, V.; Donescu, D.; Petcu, C.; Vasilescu, M. Nanostructured hybrid systems with Rhodamine 6G. J. Disper. Sci. Technol. 2008, 29, 1233–1239. [Google Scholar] [CrossRef]
- Cao, J.; Lu, H.; Yang, M.; Wang, Q.; Wei, Z.; Li, J. Luminescent Rhodamine 6G/silica hybrid nanofibers with potential temperature sensing ability. J. Non-Cryst. Solids 2018, 482, 40–45. [Google Scholar] [CrossRef]
- Ou, J.; Hu, Y.; Huang, L.; Zhang, R.; Xu, T.; Zhao, J. pH-sensitive nanocarriers for Ganoderma applanatum polysaccharide release via host-guest interactions. J. Mater. Sci. 2018, 53, 7963–7975. [Google Scholar] [CrossRef]
- Lasio, B.; Malfatti, L.; Innocenzi, P. Photodegradation of rhodamine 6G dimers in silica sol-gel films. J. Photochem. Photobiol. A 2013, 271, 93–98. [Google Scholar] [CrossRef]
- Royon, M.; Vocanson, F.; Jamon, D.; Marin, E.; Morana, A.; Boukenter, A.; Girard, S.; Ouerdane, Y.; Royer, F.; Jourlin, Y. Comparison between the UV and X-ray Photosensitivities of Hybrid TiO2-SiO2 Thin Layers. Materials 2020, 13, 3730. [Google Scholar] [CrossRef] [PubMed]
- Zehentbauer, F.M.; Moretto, C.; Stephen, R.; Thevar, T.; Gilchrist, J.R.; Pokrajac, D.; Richard, K.L.; Kiefer, J. Fluorescence spectroscopy of Rhodamine 6G: Concentration and solvent effects. Spectroc. Acta A 2014, 121, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.; Torge, R. Measurement of thin film parameters with a prism coupler. Appl. Opt. 1973, 12, 2901–2908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franc, J.; Blanc, D.; Zerroukhi, A.; Chalamet, Y.; Last, A.; Destouches, N. Organo-silica–titania nanocomposite elaborated by sol–gel processing with tunable optical properties. Mater. Sci. Eng. B 2006, 129, 180–185. [Google Scholar] [CrossRef]
- Murashkevich, A.N.; Lavitskaya, A.S.; Barannikova, T.I.; Zharskii, I.M. Infrared absorption spectra and structure of TiO2-SiO2 composites. J. Appl. Spectrosc. 2008, 75, 730–734. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Royon, M.; Vocanson, F.; Jamon, D.; Royer, F.; Marin, E.; Morana, A.; Campanella, C.; Boukenter, A.; Ouerdane, Y.; Jourlin, Y.; et al. Impact of γ-rays Irradiation on Hybrid TiO2-SiO2 Sol-Gel Films Doped with RHODAMINE 6G. Materials 2021, 14, 5754. https://doi.org/10.3390/ma14195754
Royon M, Vocanson F, Jamon D, Royer F, Marin E, Morana A, Campanella C, Boukenter A, Ouerdane Y, Jourlin Y, et al. Impact of γ-rays Irradiation on Hybrid TiO2-SiO2 Sol-Gel Films Doped with RHODAMINE 6G. Materials. 2021; 14(19):5754. https://doi.org/10.3390/ma14195754
Chicago/Turabian StyleRoyon, Maxime, Francis Vocanson, Damien Jamon, François Royer, Emmanuel Marin, Adriana Morana, Cosimo Campanella, Aziz Boukenter, Youcef Ouerdane, Yves Jourlin, and et al. 2021. "Impact of γ-rays Irradiation on Hybrid TiO2-SiO2 Sol-Gel Films Doped with RHODAMINE 6G" Materials 14, no. 19: 5754. https://doi.org/10.3390/ma14195754
APA StyleRoyon, M., Vocanson, F., Jamon, D., Royer, F., Marin, E., Morana, A., Campanella, C., Boukenter, A., Ouerdane, Y., Jourlin, Y., & Girard, S. (2021). Impact of γ-rays Irradiation on Hybrid TiO2-SiO2 Sol-Gel Films Doped with RHODAMINE 6G. Materials, 14(19), 5754. https://doi.org/10.3390/ma14195754







