Effect of Sulphur-Containing Tailings Content and Curing Temperature on the Properties of M32.5 Cement Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix Proportions
2.3. Test Methods
- Ex = the expansion at x age, %;
- Lx = the comparator reading of specimen at x age, mm; and
- Li = the initial comparator reading of restraining rod, mm.
3. Results and Discussion
3.1. Effect of STs Content on The Properties of M32.5 Cement Mortar
3.1.1. Fluidity
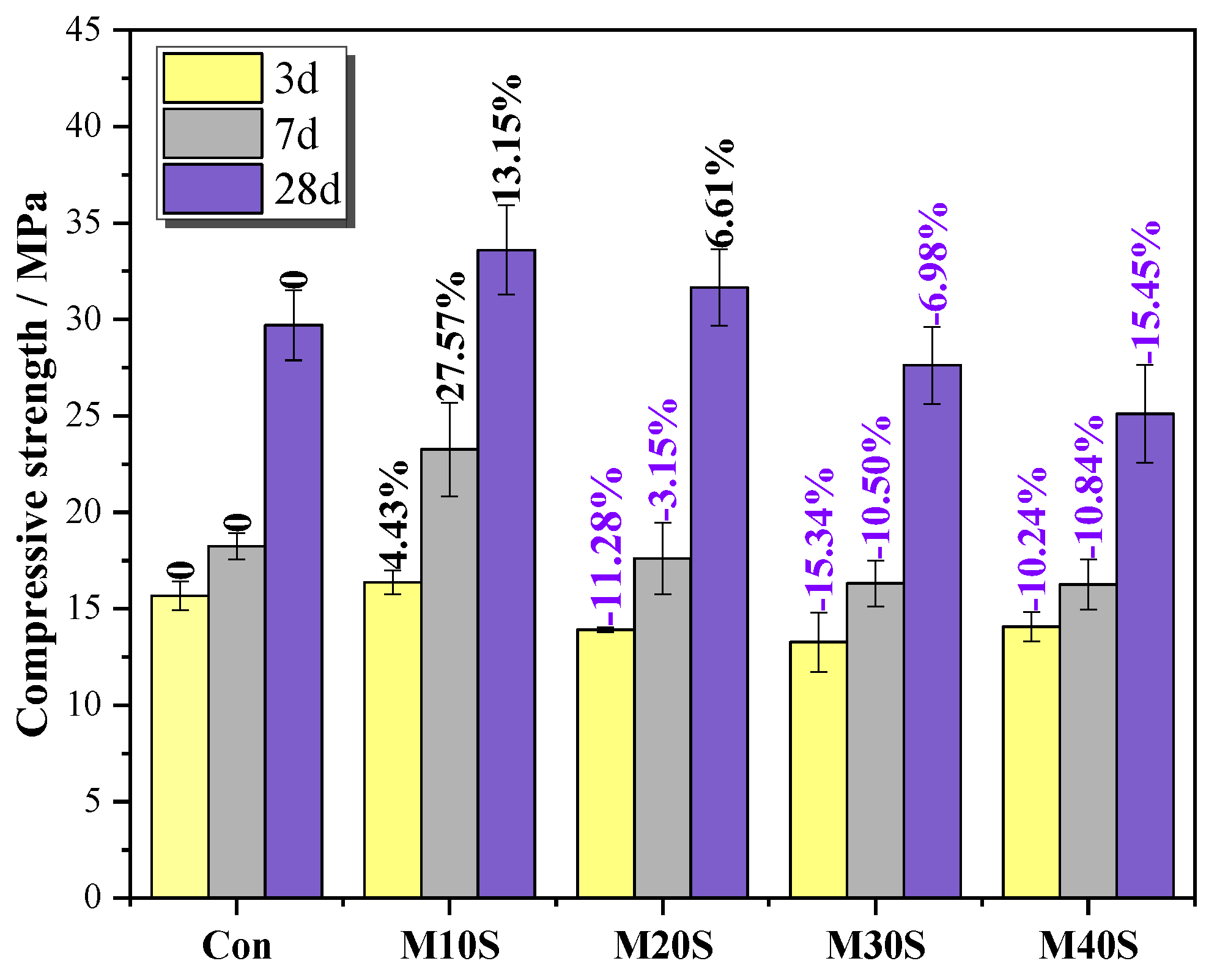
3.1.2. Compressive Strength
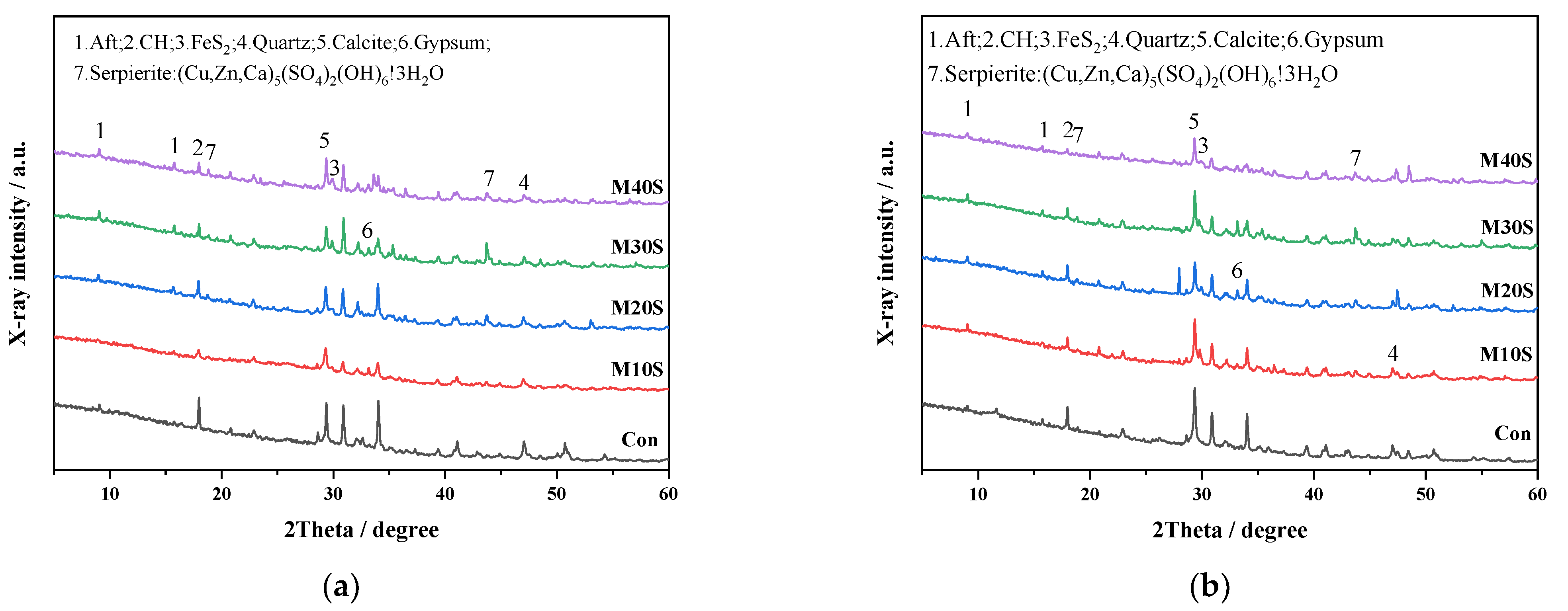
3.1.3. Phase Analysis
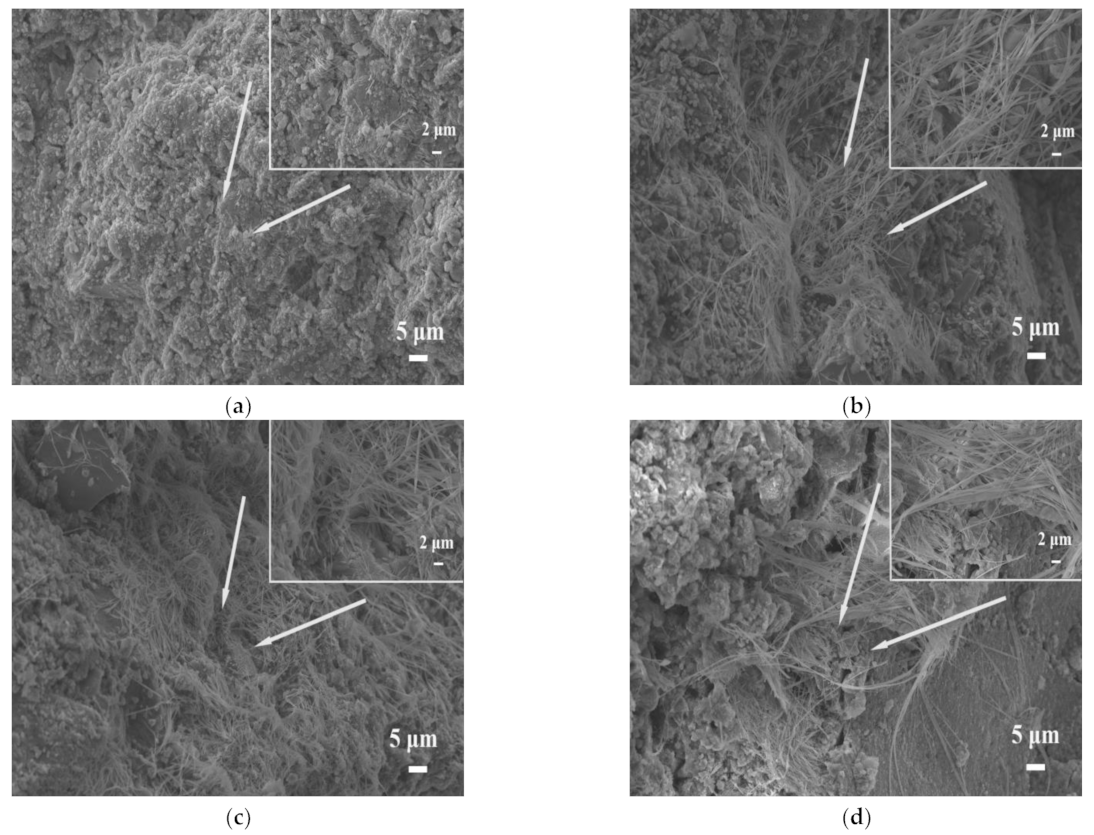
3.1.4. Microstructure Analysis
3.1.5. Expansion
3.2. Effect of Curing Temperature on The Properties of M32.5 Cement Mortar with STs
3.2.1. Compressive Strength
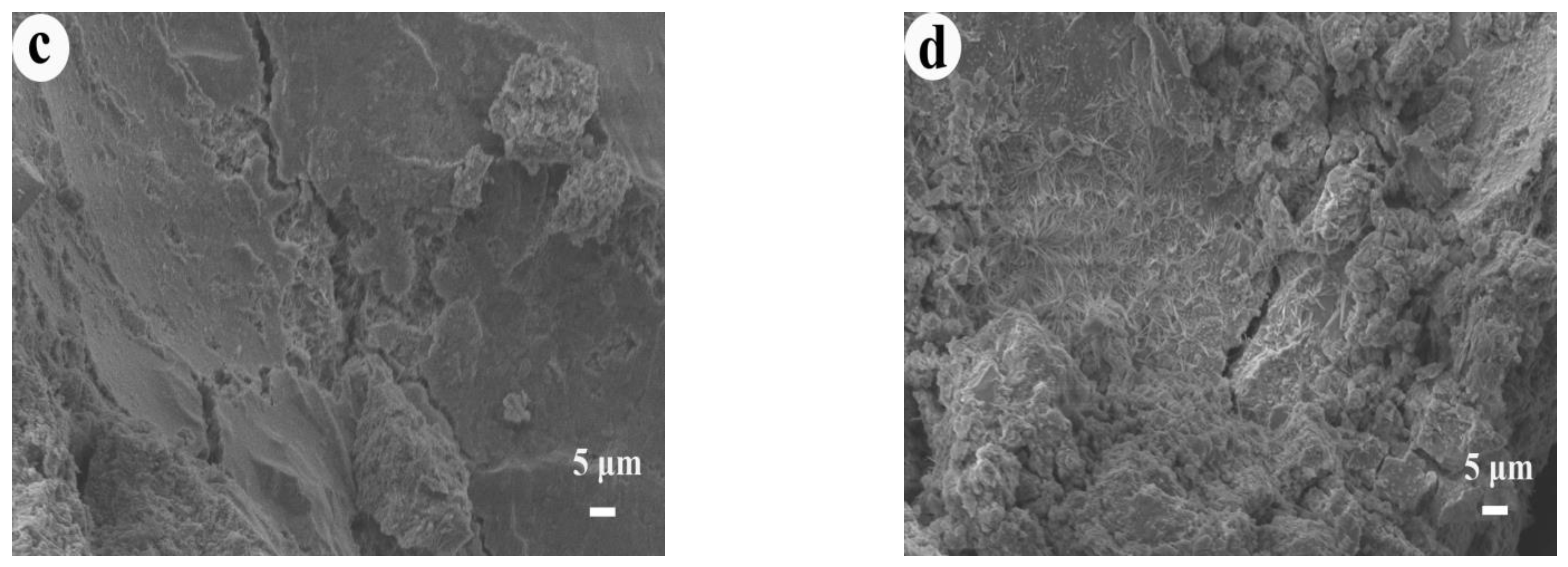
3.2.2. Microstructure Analysis
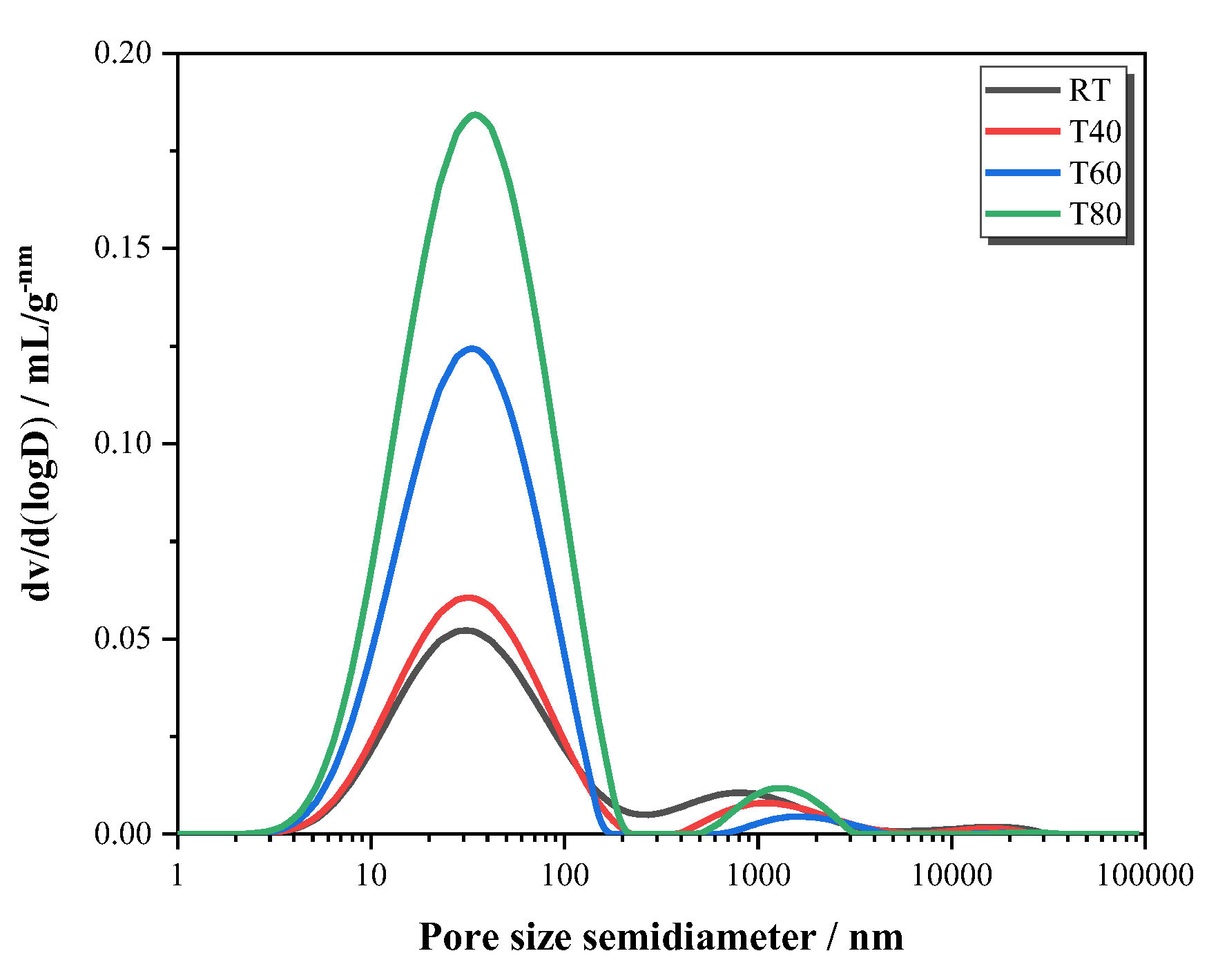
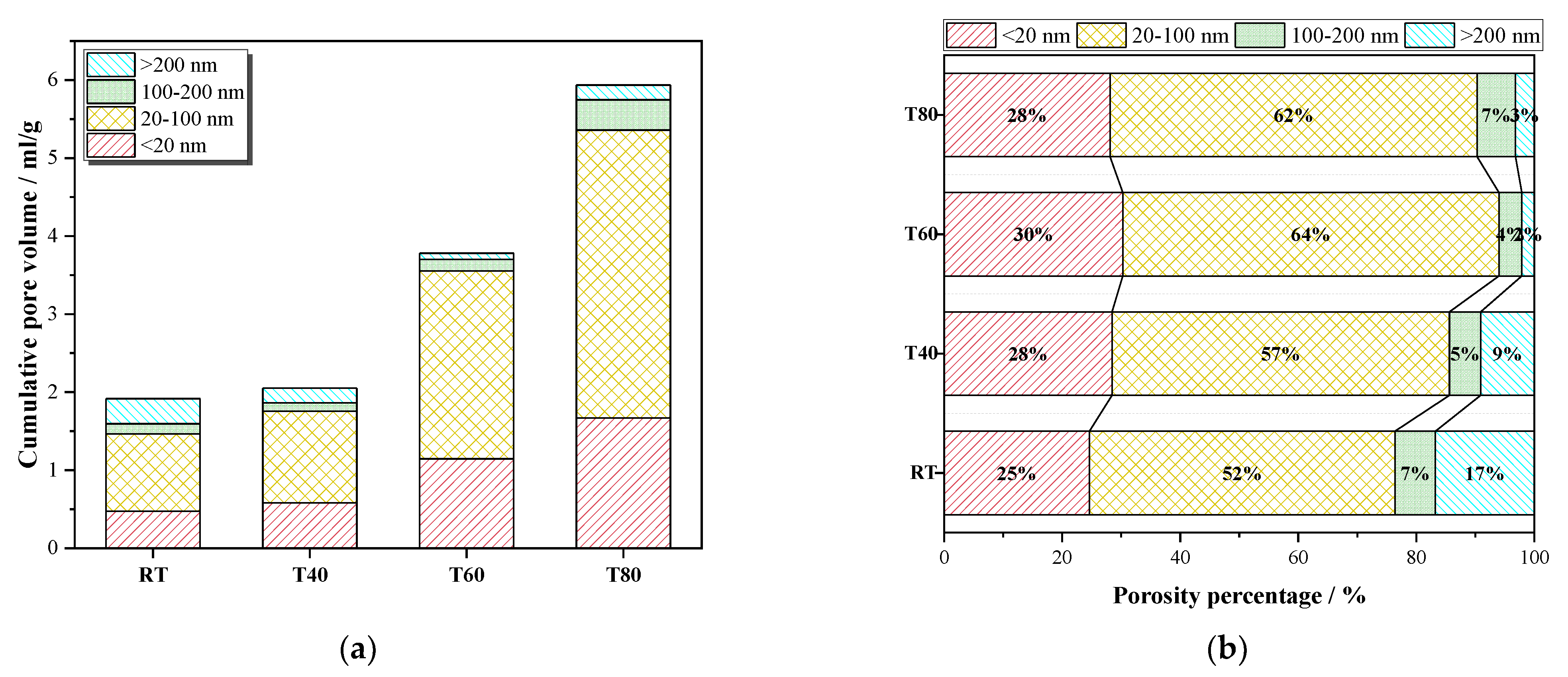
3.2.3. NMR Analysis
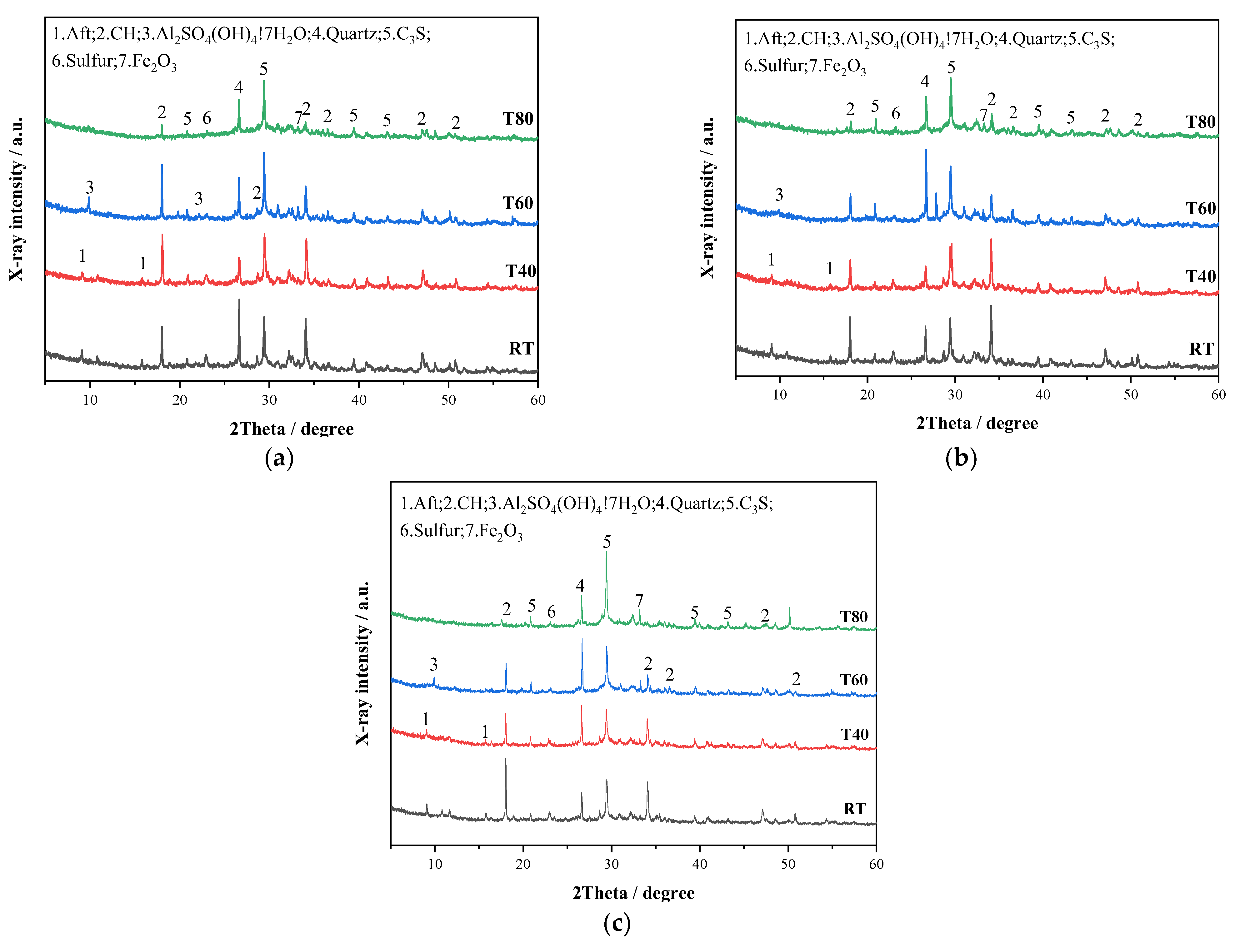
3.2.4. XRD Analysis
4. Conclusions
- The compressive strength of mortars with STs increased at a lower substitution rate (0~20%) but decreased at a higher substitution rate (>20%) with the increase in STs content; adding STs reduced the fluidity of mortar, and the fluidity decreased with the increase of the ST dosage.
- The addition of STs induced the formation of sulfate ions, promoted the cement hydration reaction, and produced a large amount of swelling hydration products, such as ettringite. At a lower dosage (0~20%), it can fill the gap of big pores and increase the strength. However, at a higher dosage (>20%), expansion will occur, destroying the structure of slurry, and reducing the strength.
- The compressive strength of mortars with STs increased at first and then decreased with the increase of the curing temperature. High curing temperatures can accelerate the speed of the hydration reaction, but too high a temperature will cause the formation of more harmful pores and cracks.
- Under high curing temperatures, the early strength development of the mortar mainly depended on the speed of the hydration reaction, and the late strength change is mainly affected by hydration products and the pore size distribution.
- Proper dosage of STs will not cause deterioration of cement mortar in the M32.5 cement system, and these products can be used in the practical application of plaster mortar, road water stabilization, and other aspects. The main practical significance of STs partially replacing river sand is that it can consume a mass of accumulated waste tailings, save a lot of maintenance costs, and have economic and environmental benefits. In the work, the optimal STs replacement ratio of river sand was 10% and the optimal curing temperature was 40 °C.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, C.; Yan, B.; Chen, T.; Quan, S.-X.; Xiao, X.-M. Comprehensive utilization of lead–zinc tailings, part 1: Pollution characteristics and resource recovery of sulfur. J. Environ. Chem. Eng. 2015, 3, 862–869. [Google Scholar] [CrossRef]
- Tao, L.; Wang, X.; Ning, P.; Wang, L.; Fan, W. Removing sulfur dioxide from smelting flue and increasing resource utilization of copper tailing through the liquid catalytic oxidation. Fuel Process. Technol. 2019, 192, 36–44. [Google Scholar] [CrossRef]
- Wei, Z.; Zhao, J.; Wang, W.; Yang, Y.; Zhuang, S.; Lu, T.; Hou, Z. Utilizing gold mine tailings to produce sintered bricks. Constr. Build. Mater. 2021, 282, 122655. [Google Scholar] [CrossRef]
- Zhang, N.; Tang, B.; Liu, X. Cementitious activity of iron ore tailing and its utilization in cementitious materials, bricks and concrete. Constr. Build. Mater. 2021, 288, 123022. [Google Scholar] [CrossRef]
- Lazorenko, G.; Kasprzhitskii, A.; Shaikh, F.; Krishn, R.S.; Mishra, J. Utilization potential of mine tailings in geopolymers: Physicochemical and environmental aspects—ScienceDirect. Process Saf. Environ. Prot. 2021, 147, 559–577. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Z.; Yin, S.; Yu, L. Utilization of Iron Tailings Sand as an Environmentally Friendly Alternative to Natural River Sand in High-Strength Concrete: Shrinkage Characterization and Mitigation Strategies. Materials 2020, 13, 5614. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Gao, W.; Lv, Y.; Tan, H.; Li, X.; Li, B.; Huang, W. Potential utilization of copper tailings in the preparation of low heat cement clinker. Constr. Build. Mater. 2020, 252, 119130. [Google Scholar] [CrossRef]
- Rossetti, A.; Ikumi, T.; Segura, I.; Irassar, E.F. Sulfate performance of blended cements (limestone and illite calcined clay) exposed to aggressive environment after casting. Cem. Concr. Res. 2021, 147, 106495. [Google Scholar] [CrossRef]
- Neto, J.D.S.A.; De la Torre, A.G.; Kirchheim, A.P. Effects of sulfates on the hydration of Portland cement—A review. Constr. Build. Mater. 2021, 279, 122428. [Google Scholar] [CrossRef]
- Hirsch, T.; Lu, Z.; Stephan, D. Effect of different sulphate carriers on Portland cement hydration in the presence of triethanolamine. Constr. Build. Mater. 2021, 294, 123528. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, C.; Hou, D.; Yang, J.; Sun, D.; Zhang, X. Effect of environmental pH values on phase composition and microstructure of Portland cement paste under sulfate attack. Compos. Part B Eng. 2021, 216, 108862. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Y.-T.; Jin, A.-B.; Wang, X. Dynamic characteristics of superfine tailings–blast furnace slag backfill featuring filling surface. Constr. Build. Mater. 2020, 242, 118173. [Google Scholar] [CrossRef]
- Wang, S.; Song, X.; Wei, M.; Liu, W.; Wang, X.; Ke, Y.; Tao, T. Strength characteristics and micro-structure evolution of cemented tailings backfill with rice straw ash as an alternative binder. Constr. Build. Mater. 2021, 297, 123780. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, A.; Li, H. High Sulfur Tailings Backfill Properties and Effect of Chemical Additives. Met. Mine 2017, 496, 171–175. (In Chinese) [Google Scholar]
- Liu, Y.; Hou, D. Strength test of sulfur bearing tailings backfill. Mod. Min. 2013, 29, 195–196. (In Chinese) [Google Scholar]
- Liu, L.; Zhu, C.; Chen, G.; Xin, J.; Fang, Z. Erosion mechanism of sulfur-bearing tailings in micro-scale. J. Xi’an Univ. Sci. Technol. 2018, 38, 43–51. (In Chinese) [Google Scholar]
- Herrera-Mesen, C.; Salvador, R.; Ikumi, T.; Cavalaro, S.; Aguado, A. External sulphate attack of sprayed mortars with sulphate-resisting cement: Influence of accelerator and age of exposition. Cem. Concr. Compos. 2020, 114, 103614. [Google Scholar] [CrossRef]
- Liu, C.; Gao, J.; Chen, F.; Zhao, Y.; Chen, X.; He, Z. Coupled effect of relative humidity and temperature on the degradation of cement mortars partially exposed to sulfate attack. Constr. Build. Mater. 2019, 216, 93–100. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Cui, L.; Si, Z.; Wang, H. Effect of external sulfate attack on the mechanical behavior of cemented paste backfill. Constr. Build. Mater. 2020, 263, 120968. [Google Scholar] [CrossRef]
- Xu, T.; White, S.P.; Pruess, K.; Brimhall, G.H. Modeling of Pyrite Oxidation in Saturated and Unsaturated Subsurface Flow Systems. Transp. Porous Media 2000, 39, 25–56. [Google Scholar] [CrossRef]
- Chandra, A.; Gerson, A. The mechanisms of pyrite oxidation and leaching: A fundamental perspective. Surf. Sci. Rep. 2010, 65, 293–315. [Google Scholar] [CrossRef]
- Zhou, Q.; Glasser, F. Thermal stability and decomposition mechanisms of ettringite at <120 °C. Cem. Concr. Res. 2001, 31, 1333–1339. [Google Scholar] [CrossRef]
- Wei, X.; Li, D.; Ming, F.; Yang, C.; Chen, L.; Liu, Y. Influence of low-temperature curing on the mechanical strength, hydration process, and microstructure of alkali-activated fly ash and ground granulated blast furnace slag mortar. Constr. Build. Mater. 2021, 269, 121811. [Google Scholar] [CrossRef]
- Ogawa, Y.; Uji, K.; Ueno, A.; Kawai, K. Contribution of fly ash to the strength development of mortars cured at different temperatures. Constr. Build. Mater. 2021, 276, 122191. [Google Scholar] [CrossRef]
- Tian, X.; Xu, W.; Song, S.; Rao, F.; Xia, L. Effects of curing temperature on the compressive strength and microstructure of copper tailing-based geopolymers. Chemosphere 2020, 253, 126754. [Google Scholar] [CrossRef]
- ASTM. C1437-20, Standard Test Method for Flow of Hydraulic Cement Mortar; ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM. C109/C109M–20b, Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50 mm] Cube Specimens); ASTM International: West Conshohocken, PA, USA, 2020. [Google Scholar]
- ASTM. C806-18, Standard Test Method for Restrained Expansion of Expansive Cement Mortar; ASTM International: West Con-shohocken, PA, USA, 2018. [Google Scholar]
- Hu, J.; Wen, B. Influence law and effect of machine-made sand gradation on properties of cement mortar. China Concr. 2015, 11, 88–91. (In Chinese) [Google Scholar]
- Liu, L.; Xin, J.; Qi, C.; Jia, H.; Song, K. Experimental investigation of mechanical, hydration, micro-structure and electrical properties of cemented paste backfill. Constr. Build. Mater. 2020, 263, 120137. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Yang, K.; Zhu, X.; Gevaudan, J.P.; Yang, C.; Basheer, M. The long-term failure mechanisms of alkali-activated slag mortar exposed to wet-dry cycles of sodium sulphate. Cem. Concr. Compos. 2021, 116, 103893. [Google Scholar] [CrossRef]
- Jebli, M.; Jamin, F.; Pelissou, C.; Lhopital, E.; El Youssoufi, M. Characterization of the expansion due to the delayed ettringite formation at the cement paste-aggregate interface. Constr. Build. Mater. 2021, 289, 122979. [Google Scholar] [CrossRef]
- Thiebaut, Y.; Multon, S.; Sellier, A.; Lacarrière, L.; Boutillon, L.; Belili, D.; Linger, L.; Cussigh, F.; Hadji, S. Effects of stress on concrete expansion due to delayed ettringite formation. Constr. Build. Mater. 2018, 183, 626–641. [Google Scholar] [CrossRef]
- Wang, P.; Li, N.; Xu, L.; Zhang, G. Hydration Characteristics and Strength Development of Sulphoalu-minate Cement Cured at Low Temperature. J. Chin. Ceram. Soc. 2017, 2, 242–248. (In Chinese) [Google Scholar]
- Xue, S.; Meng, F.; Zhang, P.; Bao, J.; Wang, J.; Zhao, K. Influence of water re-curing on micro-structure of heat-damaged cement mortar characterized by low-field NMR and MIP. Constr. Build. Mater. 2020, 262, 120532. [Google Scholar] [CrossRef]
- Chaohua, J.; Chen, J.; Yizhi, W.; Sheng, Y.; Da, C. Effect of heat curing treatment on the drying shrinkage behavior and microstructure characteristics of mortar incorporating different content ground granulated blast-furnace slag. Constr. Build. Mater. 2018, 186, 379–387. [Google Scholar]
- Moghadam, M.A.; Izadifard, R.A. Effects of zeolite and silica fume substitution on the microstructure and mechanical properties of mortar at high temperatures. Constr. Build. Mater. 2020, 253, 119206. [Google Scholar] [CrossRef]
- O’Farrell, M.; Wild, S.; Sabir, B.B. Pore size distribution and compressive strength of waste clay brick mortar. Cem. Concr. Compos. 2001, 23, 81–91. [Google Scholar] [CrossRef]
- Moretti, J.P.; Sales, A.; Quarcioni, V.A.; Silva, D.C.; Oliveira, M.C.; Pinto, N.S.; Ramos, L.W. Pore size distribution of mortars produced with agroindustrial waste. J. Clean. Prod. 2018, 187, 473–484. [Google Scholar] [CrossRef]
- Ramli, M.; Tabassi, A.A. Effects of polymer modification on the permeability of cement mortars under different curing conditions: A correlational study that includes pore distributions, water absorption and compressive strength. Constr. Build. Mater. 2012, 28, 561–570. [Google Scholar] [CrossRef]
- Francioso, V.; Moro, C.; Martinez-Lage, I.; Velay-Lizancos, M. Curing temperature: A key factor that changes the effect of TiO2 nanoparticles on mechanical properties, calcium hydroxide formation and pore structure of cement mortars. Cem. Concr. Compos. 2019, 104, 103374. [Google Scholar] [CrossRef]
- Xu, L.; Yang, X.; Wang, P.; Wu, K. Influences of Curing Temperature on the Microstructure Evolution of Calcium Sulfoaluminate Cement Based Ternary Blends. J. Build. Mater. 2016, 6, 983–992. (In Chinese) [Google Scholar]
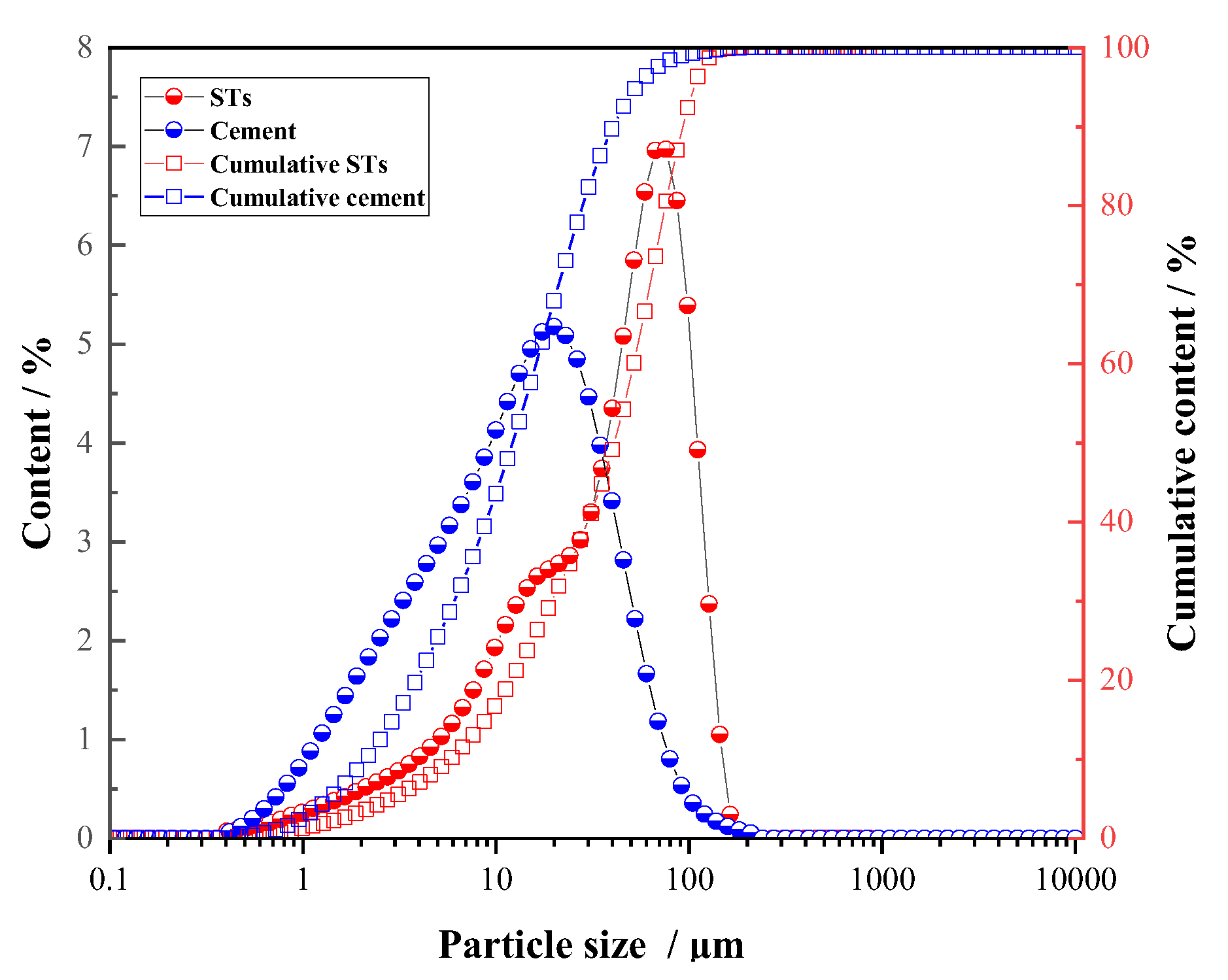
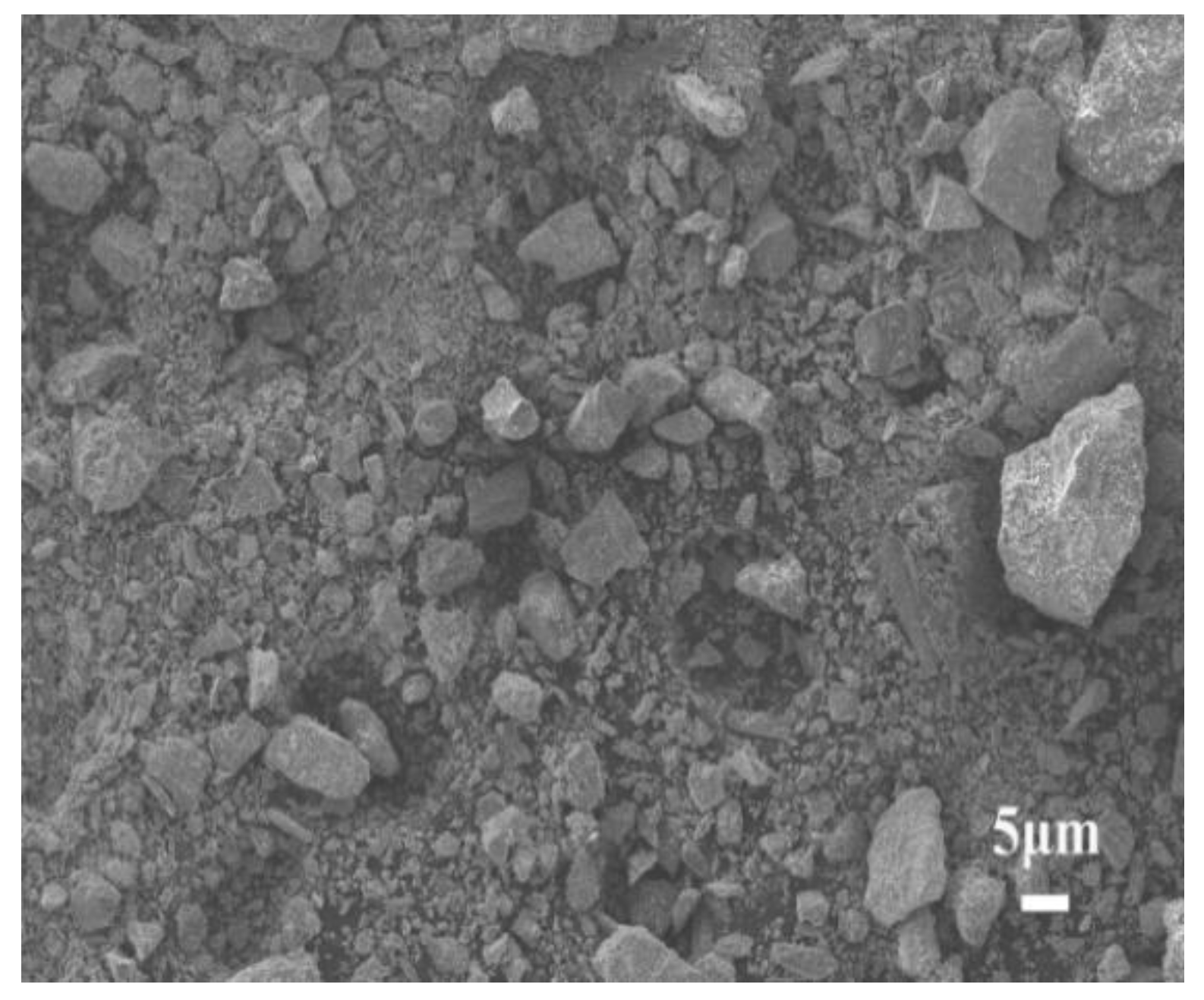
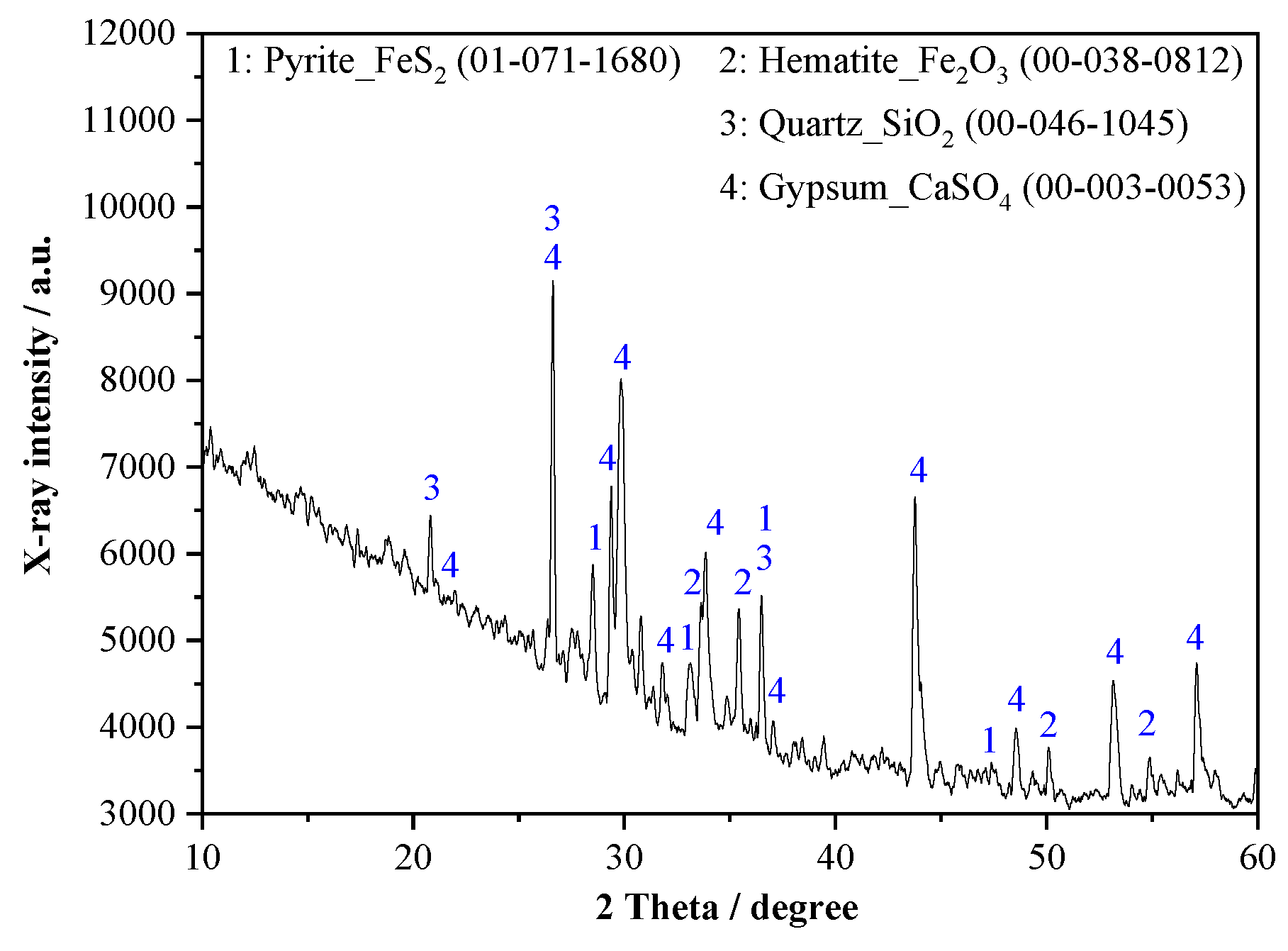
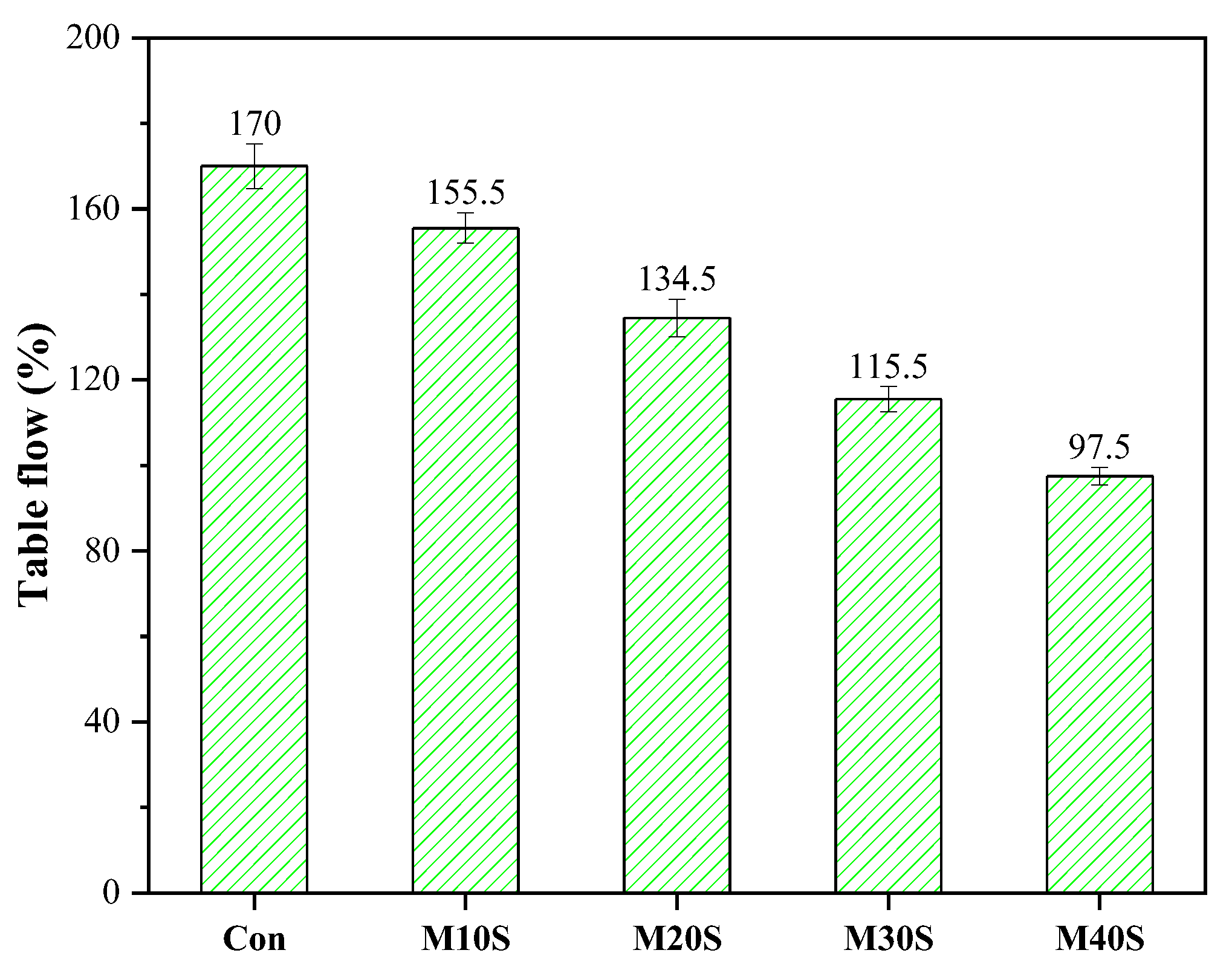
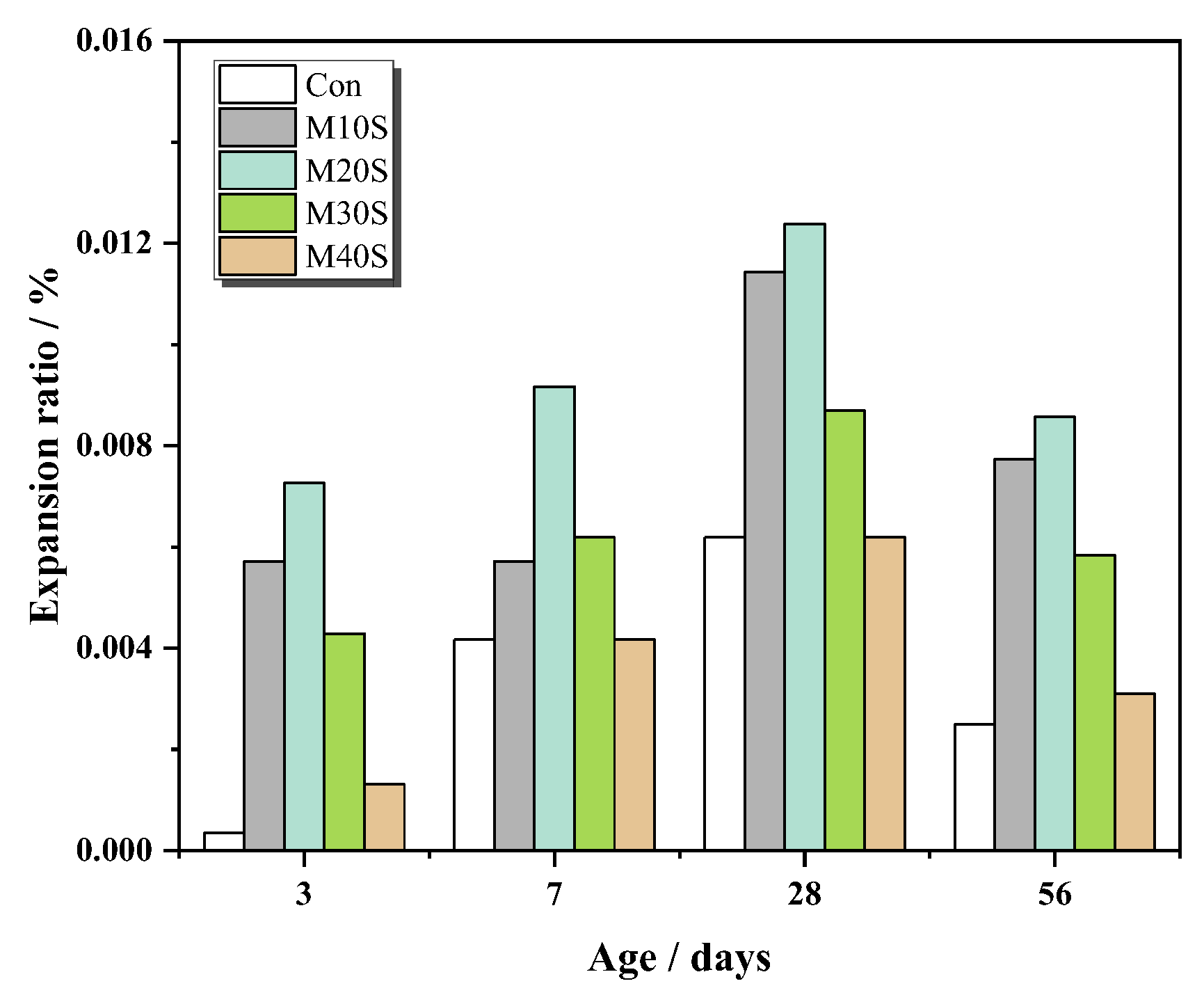
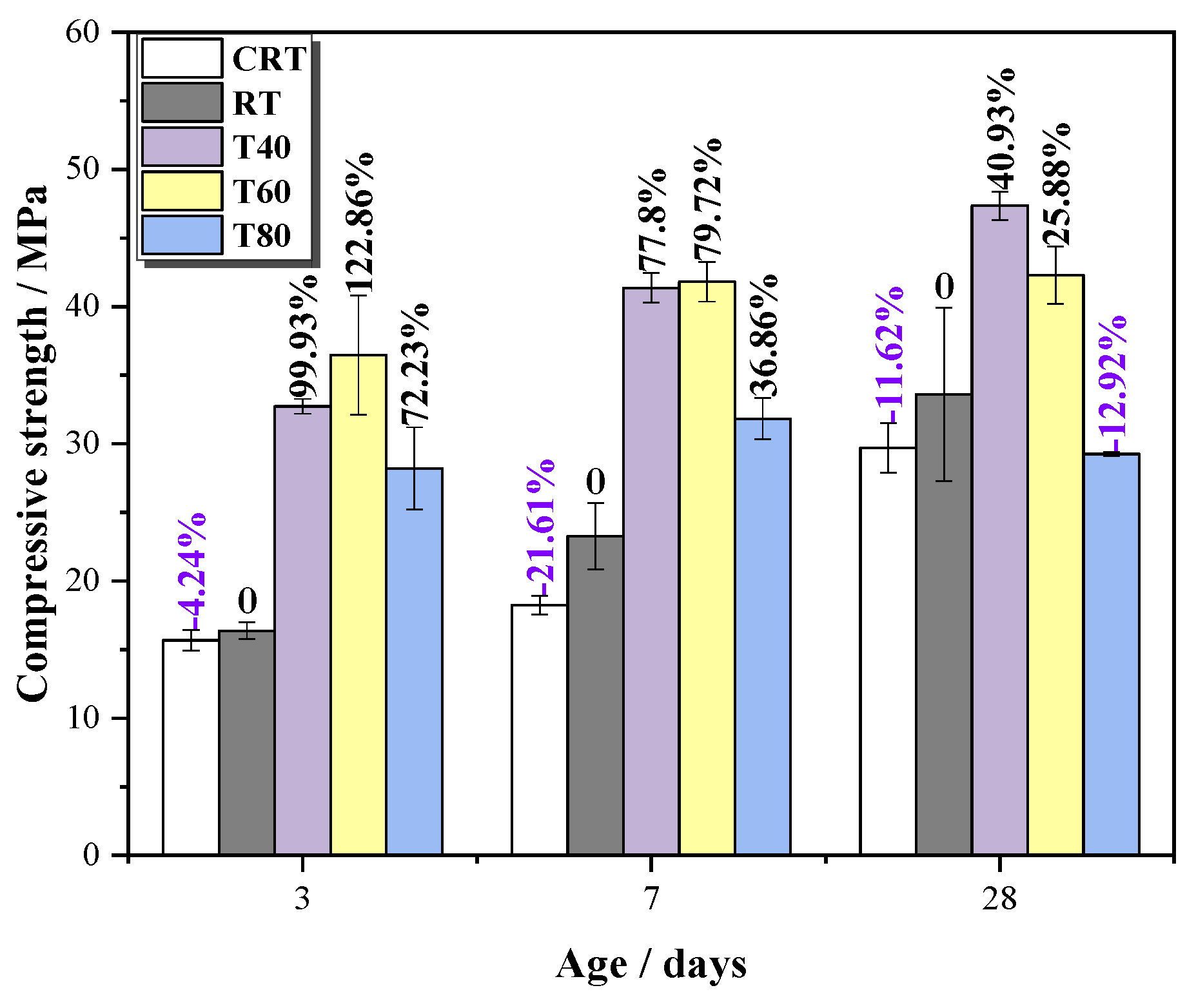

| Material | Al2O3 | SO3 | SiO2 | Fe2O3 | CaO | TiO2 | MgO | Na2O | K2O | CO2 | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| M32.5 Cement | 11.5 | – | 22.1 | 3.1 | 42.0 | 0.5 | 0.7 | 0.2 | 1.1 | 16.4 | 2.4 |
| STs | 5.2 | 21.1 | 22.1 | 30.5 | 10.3 | 0.2 | 5.0 | 0.6 | 0.8 | – | 4.2 |
| Number | M32.5 Cement | STs | Sand | Water |
|---|---|---|---|---|
| Con | 34.5 | 0.0 | 50.0 | 15.5 |
| M10S | 34.5 | 5.0 | 45.0 | 15.5 |
| M20S | 34.5 | 10.0 | 40.0 | 15.5 |
| M30S | 34.5 | 15.0 | 35.0 | 15.5 |
| M40S | 34.5 | 20.0 | 30.0 | 15.5 |
| Number | M32.5 Cement | STs | Sand | Water | Curing Temperature/°C |
|---|---|---|---|---|---|
| CRT | 34.5 | 0.0 | 50.0 | 15.5 | 23 ± 1 |
| RT | 34.5 | 5.0 | 45.0 | 15.5 | 23 ± 1 |
| T40 | 40 | ||||
| T60 | 60 | ||||
| T80 | 80 |
| Number | CRT | RT | T40 | T60 | T80 | |
|---|---|---|---|---|---|---|
| Age | ||||||
| 3 d | 15.68 | 16.37 | 32.73 | 36.49 | 28.20 | |
| 7 d | 18.24 | 23.26 | 41.36 | 41.81 | 31.84 | |
| 28 d | 29.70 | 33.60 | 47.35 | 42.30 | 29.26 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Chen, H.; Wang, P.; Chen, X.; Chen, J. Effect of Sulphur-Containing Tailings Content and Curing Temperature on the Properties of M32.5 Cement Mortar. Materials 2021, 14, 5751. https://doi.org/10.3390/ma14195751
Chen Q, Chen H, Wang P, Chen X, Chen J. Effect of Sulphur-Containing Tailings Content and Curing Temperature on the Properties of M32.5 Cement Mortar. Materials. 2021; 14(19):5751. https://doi.org/10.3390/ma14195751
Chicago/Turabian StyleChen, Qian, Haiming Chen, Pengju Wang, Xiang Chen, and Jie Chen. 2021. "Effect of Sulphur-Containing Tailings Content and Curing Temperature on the Properties of M32.5 Cement Mortar" Materials 14, no. 19: 5751. https://doi.org/10.3390/ma14195751
APA StyleChen, Q., Chen, H., Wang, P., Chen, X., & Chen, J. (2021). Effect of Sulphur-Containing Tailings Content and Curing Temperature on the Properties of M32.5 Cement Mortar. Materials, 14(19), 5751. https://doi.org/10.3390/ma14195751





