Study on the Physical Properties of a SiNW Biosensor to the Sensitivity of DNA Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Fabrication of a SiNW Biosensor Device
2.3. Functionalization of the SiNW Surface
2.4. Characterization of a SiNW Biosensor Device for DNA Detection
3. Results
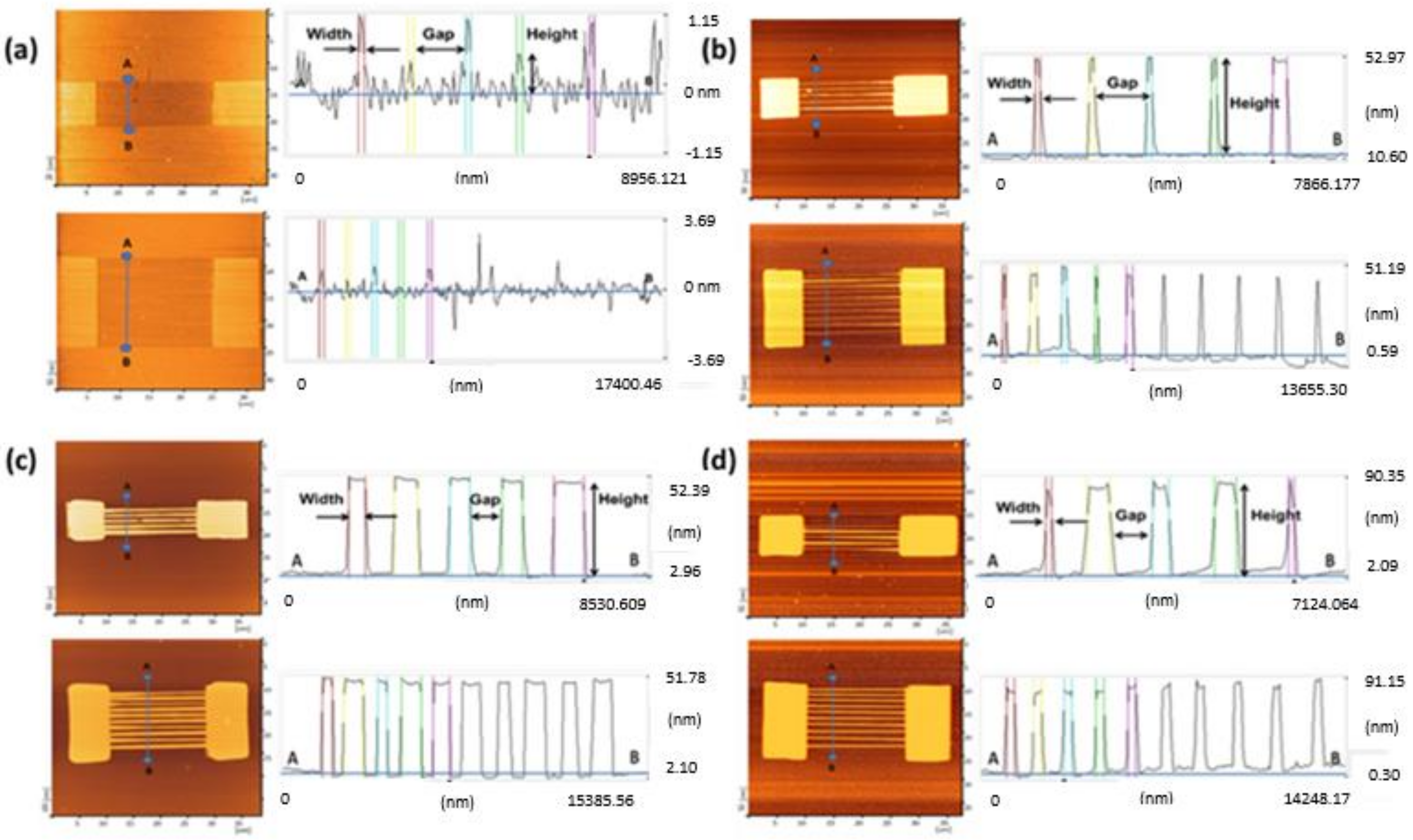
3.1. Fabrication of the SiNW Biosensor Device
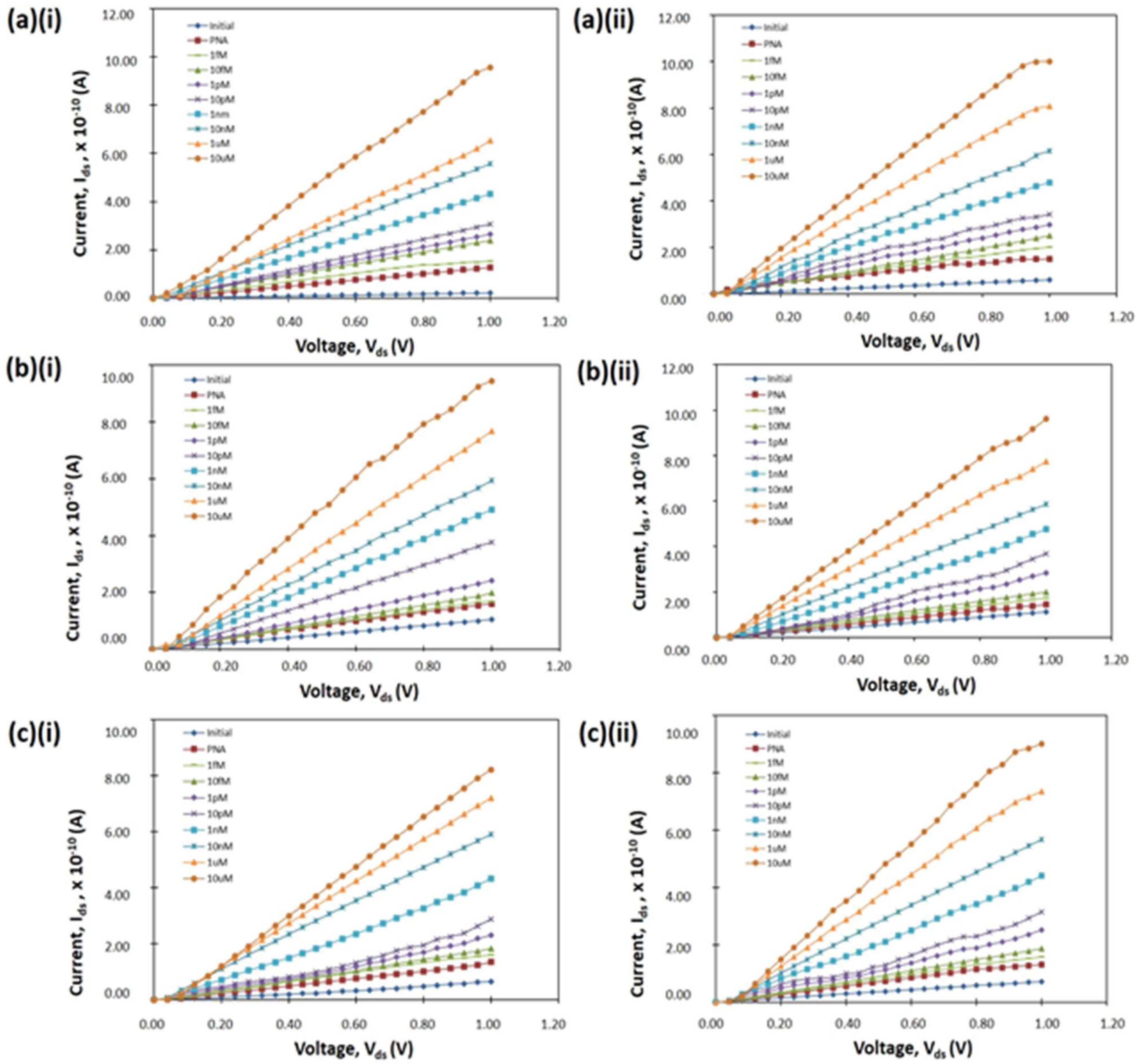
3.2. Electrical Characteristic of a SiNW Biosensor Device
3.3. Factors Affecting Sensitivity a SiNW Biosensor
3.3.1. Number of Wires
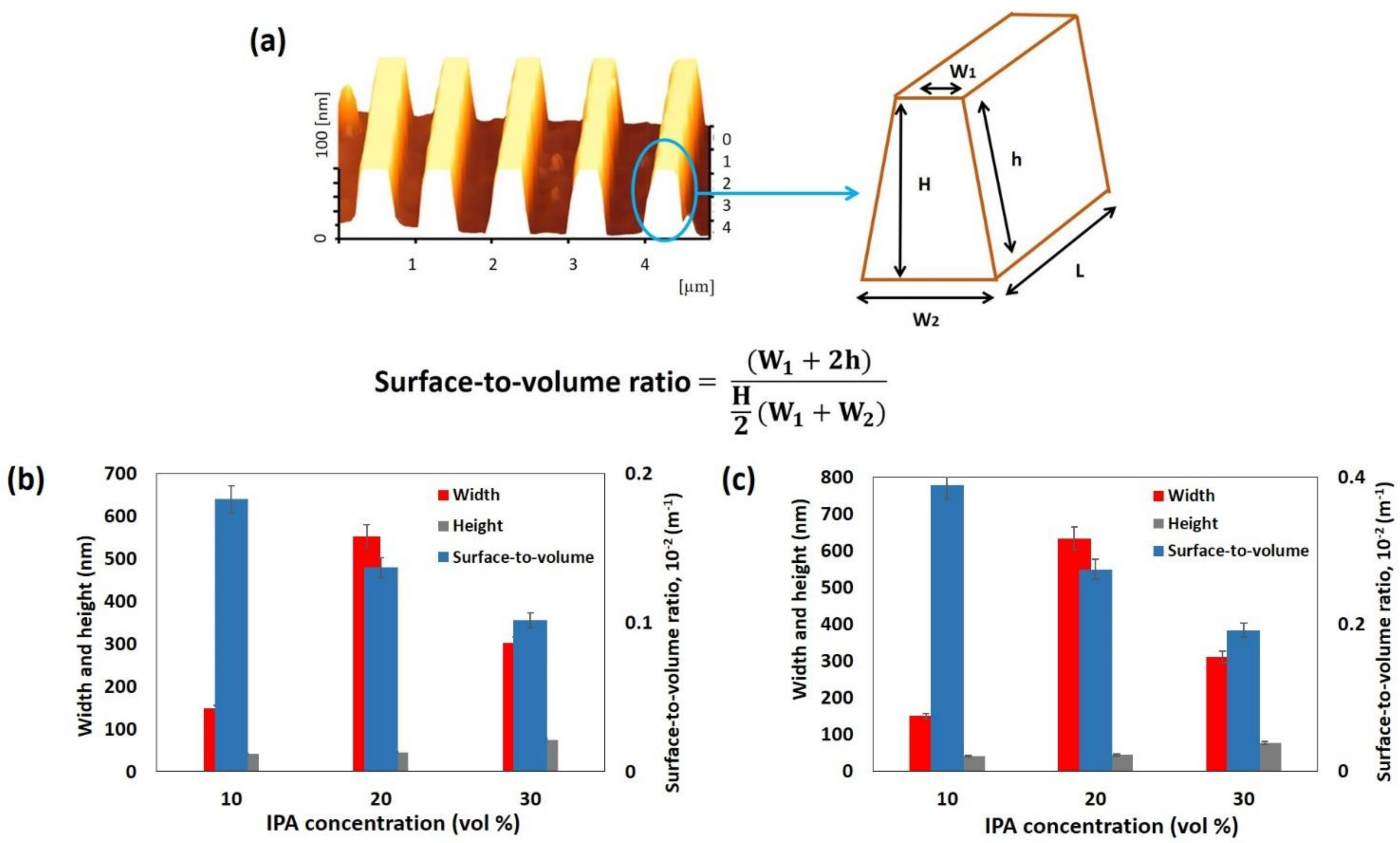
3.3.2. Dimension of Wires
3.3.3. Surface Roughness
3.4. Specificity of a SiNW Biosensor
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coffer, J.L. Semiconducting Silicon Nanowires for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2014; p. 277. [Google Scholar]
- Dhahi TH, S.; Hashim, U.; Ali, M.E.; Nazwa, T. Fabrication of amorphous silicon microgap structure for energy saving devices. Sains Malays. 2012, 41, 755–759. [Google Scholar]
- Yaacob, K.A.; Yusoh, S.N.; Alias, N.N.; Abdullah, A.M. Formation of SiO2 Nanowires by Local Anodic Oxidation Process via AFM Lithography for the Fabrication of Silicon Nanowires. In 1-Dimensional Metal Oxide Nanostructures; Lockman, Z., Ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Noor, M.O.; Krull, U.J. Silicon nanowires as field-effect transducers for biosensor development: A review. Anal. Chim. Acta 2014, 825, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Alias, N.N.; Yaacob, K.A.; Yusoh, S.N.; Abdullah, A.M. Comparison of KOH and TMAH Etching on Sinw Arrays Fabricated via AFM Lithography. J. Phys. Conf. Ser. 2018, 1082, 012051. [Google Scholar] [CrossRef] [Green Version]
- Doucey, M.-A.; Carrara, S. Nanowire Sensors in Cancer. Trends Biotechnol. 2019, 37, 86–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, D.-S.; Chen, P.-Y.; Chiu, H.-C.; Han, C.-C.; Yen, T.-J.; Chen, H.-M. Disease antigens detection by silicon nanowires with the efficiency optimization of their antibodies on a chip. Biosens. Bioelectron. 2019, 141, 111209. [Google Scholar] [CrossRef] [PubMed]
- Yusoh, S.N.; Yaacob, K.A.; Alias, N.N. Trapezoidal SiNWs array fabricated by AFM-LAO for Dengue virus DNA oligomer detection. Mater. Res. Express 2019, 6, 115005. [Google Scholar] [CrossRef]
- Zhang, G.-J.; Zhang, L.; Huang, M.J.; Luo, Z.H.H.; Tay, G.K.I.; Lim, E.-J.A.; Kang, T.G.; Chen, Y. Silicon nanowire biosensor for highly sensitive and rapid detection of Dengue virus. Sens. Actuators B Chem. 2010, 146, 138–144. [Google Scholar] [CrossRef]
- Nikonov, A.M.; Naumova, O.V.; Generalov, V.M.; Safatov, A.S.; Fomin, B.I. Surface Preparation as a Step in the Fabrication of Biosensors Based on Silicon Nanowire Field-Effect Transistors: Review. J. Surf. Investig. X-ray Synchrotron Neutron Technol. 2020, 14, 337–346. [Google Scholar] [CrossRef]
- Gopinath, S.C.; Lakshmipriya, T.; Arshad, M.M.; Uda, N.A.; Al-Douri, Y. Nanoelectronics in Biosensing Applications. In Nanobiosensors for Biomolecular Targeting; Gopinath, S.C.B., Lakshmipriya, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 211–224. [Google Scholar]
- Namdari, P.; Daraee, H.; Eatemadi, A. Recent Advances in Silicon Nanowire Biosensors: Synthesis Methods, Properties, and Applications. Nanoscale Res. Lett. 2016, 11, 406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofacial Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suvarnaphaet, P.; Pechprasarn, S. Graphene-Based Materials for Biosensors: A Review. Sensors 2017, 17, 2161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, Y.; To, S.; You, L.; Sun, Y. Effect of Nanowire Number, Diameter, and Doping Density on Nano-FET Biosensor Sensitivity. ACS Nano 2011, 5, 6661–6668. [Google Scholar] [CrossRef]
- Nuzaihan, M.; Hashim, U.; Arshad, M.M.; Kasjoo, S.R.; Rahman, S.F.A.; Ruslinda, A.R. Electrical detection of dengue virus (DENV) DNA oligomer using silicon nanowire biosensor with novel molecular gate control. Biosens. Bioelectron. 2016, 83, 106–114. [Google Scholar] [CrossRef]
- Yusoh, S.N.; Yaacob, K.A. Effect of tetramethylammonium hydroxide/isopropyl alcohol wet etching on geometry and surface roughness of silicon nanowires fabricated by AFM lithography. Beilstein J. Nanotechnol. 2016, 7, 1461–1470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten, S.; Hashim, U.; Gopinath, S.; Liu, W.W.; Foo, K.; Sam, S.; Rahman, S.; Voon, C.; Nordin, A. Highly sensitive Escherichia coli shear horizontal surface acoustic wave biosensor with silicon dioxide nanostructures. Biosens. Bioelectron. 2017, 93, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, A.; Gupta, V. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21. [Google Scholar] [CrossRef]
- Hubaux, A.; Vos, G. Decision and detection limits for calibration curves. Anal. Chem. 1970, 42, 849–855. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Z.; Li, C.; Tang, T.; Liu, X.; Han, S.; Lei, A.B.; Zhou, C. Detection of NO2 down to ppb Levels Using Individual and Multiple In2O3 Nanowire Devices. Nano Lett. 2004, 4, 1919–1924. [Google Scholar] [CrossRef]
- Leber, M.; Shandhi, M.; Hogan, A.; Solzbacher, F.; Bhandari, R.; Negi, S. Different methods to alter surface morphology of high aspect ratio structures. Appl. Surf. Sci. 2016, 365, 180–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]



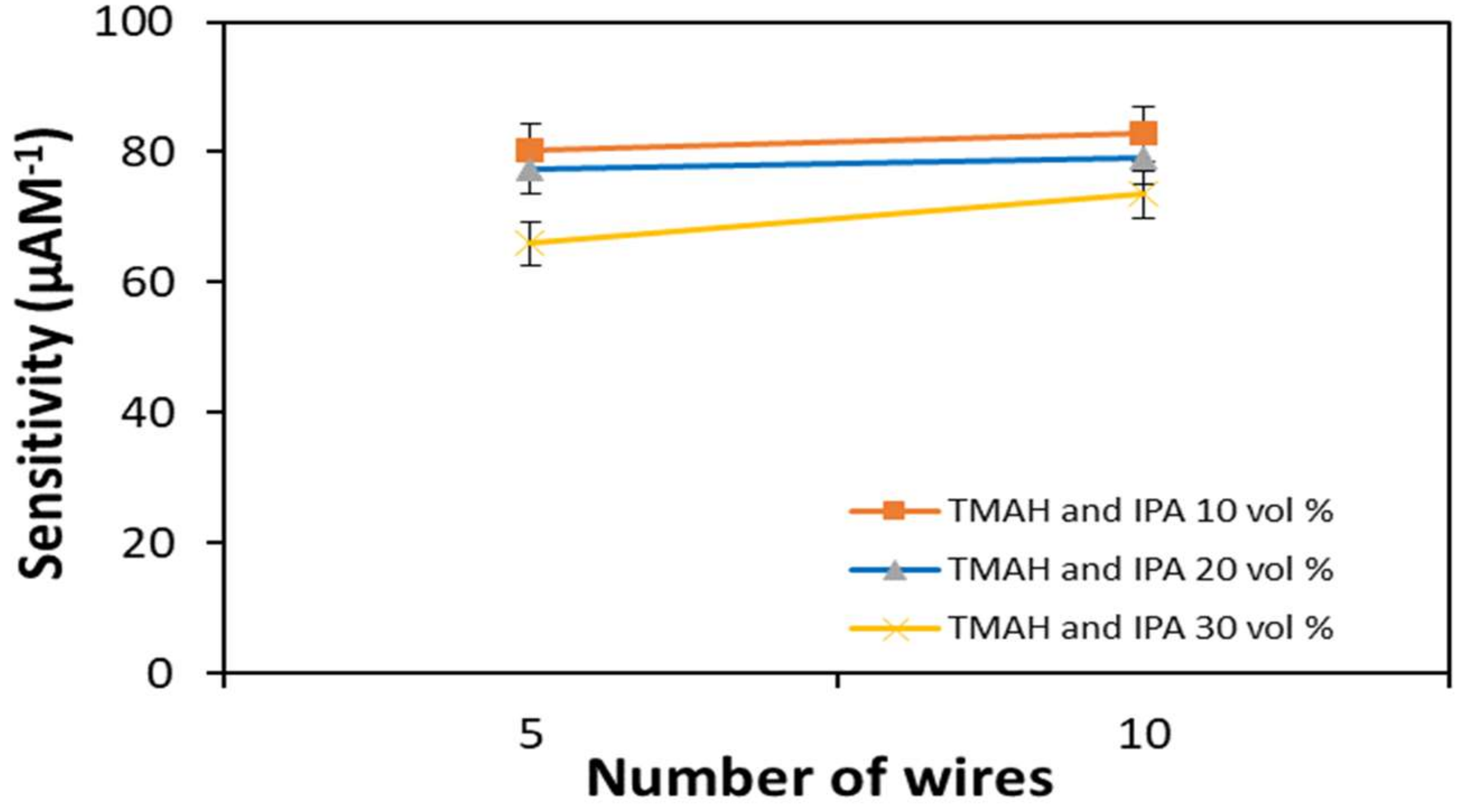



| Etchants | Devices | Sensitivity (µAM−1) | LOD (fM) |
|---|---|---|---|
| TMAH and IPA 10 vol.% | 5-wires | 80.3 | 2.22 |
| 10-wires | 82.8 | 1.93 | |
| TMAH and IPA 20 vol.% | 5-wires | 77.5 | 3.63 |
| 10-wires | 79.0 | 3.20 | |
| TMAH and IPA 30 vol.% | 5-wires | 66.0 | 4.15 |
| 10-wires | 73.5 | 3.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yusoh, S.N.; Yaacob, K.A. Study on the Physical Properties of a SiNW Biosensor to the Sensitivity of DNA Detection. Materials 2021, 14, 5716. https://doi.org/10.3390/ma14195716
Yusoh SN, Yaacob KA. Study on the Physical Properties of a SiNW Biosensor to the Sensitivity of DNA Detection. Materials. 2021; 14(19):5716. https://doi.org/10.3390/ma14195716
Chicago/Turabian StyleYusoh, Siti Noorhaniah, and Khatijah Aisha Yaacob. 2021. "Study on the Physical Properties of a SiNW Biosensor to the Sensitivity of DNA Detection" Materials 14, no. 19: 5716. https://doi.org/10.3390/ma14195716
APA StyleYusoh, S. N., & Yaacob, K. A. (2021). Study on the Physical Properties of a SiNW Biosensor to the Sensitivity of DNA Detection. Materials, 14(19), 5716. https://doi.org/10.3390/ma14195716





