Abstract
The corrosion behavior of L80 casing steel was studied in a simulating annulus environment using the electrochemical measurement method, immersion test, and tensile test under a high-temperature and high-pressure H2S/CO2 environment. The partial pressure of CO2 (), the partial pressure of H2S (), water content, and preloading stress remarkably affected the corrosion behavior of L80 steel. The influence of on stress corrosion cracking (SCC) susceptibility has an inflection point of approximately 1.1 MPa. The SCC susceptibility reaches the maximum when the is about 1.1 MPa. The SCC susceptibility has a positive correlation to and water content. The higher water content of the corrosion medium can increase the electrical conductivity of the corrosion medium and promote the corrosion of L80 steel, which can improve the diffusion of hydrogen into steel and promote the SCC of L80 steel. Preloading stress can promote local corrosion, thereby promoting SCC of steel under stress. The dislocation emergence point caused by preloading stress can accelerate the diffusion of hydrogen into steel and increase SCC susceptibility.
1. Introduction
During oil and gas exploitation, CO2 and H2S, as associated media, cause serious corrosion damage to oil and gas production equipment, thereby restricting the exploitation of oil and gas fields [1,2,3]. CO2 and H2S are important influential parameters for oil and gas equipment corrosion. CO2 primarily causes electrochemical corrosion, which leads to local pitting corrosion and perforation damage of materials [4,5]. Some researchers have also reported that CO2 can cause stress corrosion when dissolved in water [6,7]. On the contrary, H2S is more harmful to equipment. H2S can cause hydrogen-induced cracking and sulfide stress corrosion cracking, which lead to the breakdown of equipment and can cause huge economic losses and heavy casualties [8,9,10,11].
A huge amount of H2S and CO2 is found in the annulus environment because of carbonate, sulphate-reducing bacteria, high temperature, high pressure, and other factors [12,13]. In this environment, serious accidents such as leakage and the fracture of casing steel often occur, which leads to the scrapping of the whole oil well [14,15,16]. Casing is the key structure that supports the structural stability and safety of an oil well, and its integrity determines its lifespan. Controlling stress corrosion is important to maintain the safe and stable production of oil wells [4,17]. In the annulus of an oil well, some oil films and scale layers are found on the surface of casing steel because of the presence of mineralized water and a small amount of infiltrated crude oil, which slows down the uniform corrosion to a certain extent [18,19,20]. Therefore, the corrosion of downhole tubes is due to corrosion perforation and stress corrosion fracture under the scale. This case signifies that the sensitivity of stress corrosion of downhole tubes in the annular environment of a production well may be lower than that without crude oil in the annular environment.
In addition, the water content of the corrosion medium changes with the service life of oil well [18,21]. Thus, the SCC behavioral mechanism of the casing changes because of the change of electrical conductivity of the medium. However, a comprehensive understanding of the effect of these factors on the corrosion behavior of tubing in oil wells is lacking.
Designers of oil field generally use two methods for tubing material selection: (1) Select the material according to the mechanical strength of the tubing and completely ignore the stress corrosion cracking (SCC) of the oil well [22]; (2) Select materials according to the working condition of the oil well annulus and NACE TM0177 standard (a test standard for SCC resistance of pressure vessel materials in acidic sulfide environment without considering the inhibition effect of oil film) [23]. However, these methods cannot meet the demand of the oil field [24,25,26]. Therefore, a comprehensive investigation into the corrosion behavior of tubing in the annulus environment of oil fields, as well as the analysis of the mechanism of SCC and the gaining of control of corrosion are necessary, all of which can provide a reference for material selection and prevention.
In this study, the SCC behavior of L80 casing steel was studied by simulating the annulus environment of production wells using the electrochemical measurement method, immersion test, and tensile test under high temperature and high pressure. The effects of partial pressure of CO2 (), partial pressure of H2S (), water content, and different preloading stresses were studied to fully understand the SCC behavior of L80 steel.
2. Experimental
2.1. Material and Medium
Specimens were obtained from L80 casing steel. The chemical composition of L80 casing steel is listed in Table 1. The microstructure of casing steels is shown in Figure 1, and the mechanical performance is listed in Table 2. The metallographic sample of L80 steel (Length × Width × Height: 10 mm × 10 mm × 2 mm) was ground using sandpaper of up to #2000 grit. Then, the sample was polished with 0.5 μm diamond paste. Finally, the sample was etched by 4% (volume) nital and observed under metallurgical microscope (VHX-2000, Keyence, Japan). L80 steel was made of tempered sorbite.

Table 1.
Chemical composition of casing steels (unit: wt%).
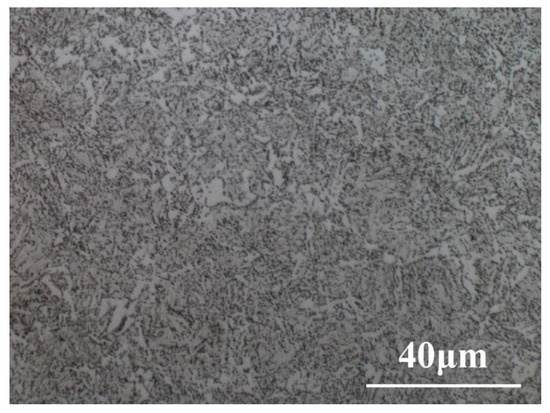
Figure 1.
Microstructure of L80 steel.

Table 2.
Mechanical performance of L80 steel used in this study.
According to the analysis of an oil field, the test medium was an oil–water mixture, which was composed of mineralized water and crude oil, the composition of mineralized water was as follows: 236.5 g·L−1 of NaCl, 1.01 g·L−1 of NaHCO3, 0.64 g·L−1 of Na2SO4, 26.64 g·L−1 of CaCl2, 12.68 g·L−1 of MgCl2·6H2O. The pH of mineralized water was 6.
The water content of the produced medium of oil field was related to the service time of oil well [18]. The proportion of mineralized water (water content) in the test medium was set as 5 wt%, 30 wt%, 50 wt%, 80 wt%, and 100 wt% to simulate the produced medium of an oil field with different service times.
2.2. Potentiodynamic Polarization Measurement
Under the service condition of oil casing, the , , and water content of corrosive medium affected the electrochemical corrosion behavior of oil casing. Therefore, potentiodynamic polarization measurement was used to evaluate the effects of the abovementioned factors on corrosion behavior. The potentiodynamic polarization curves were measured under different conditions (as shown in Table 3) by using a three-electrode system in the autoclave. The L80 steel was used as the research electrode, Ag/AgCl electrode as the reference electrode, and platinum plate electrode as the auxiliary electrode. The size of the electrochemical test sample was 10 mm × 10 mm × 4 mm. After welding with copper wire, the L80 steel was sealed with epoxy resin, and the exposed area was 1 cm2. The exposed surface of specimens was ground using sandpaper of up to #2000 grit. Then, the specimens were rinsed with ethanol, degreased with acetone, and dried with compressed air. The test temperature was set at 80 °C. The scanning rate of the potentiodynamic polarization test was 0.5 mV s−1, and the scanning potential ranged from −0.4 V to 0.7 V (vs. OCP). Before the test, the experimental medium was deoxidized by high-purity nitrogen for 2 h, and the research electrode was polished using 2000-grit sandpapers. After degreasing using acetone, the research electrode was dried for standby. During data processing, all the measured potential were converted into another potential, which refers to the saturated calomel electrode.

Table 3.
Test conditions of polarization measurement.
2.3. Immersion Test
Immersion test was conducted under different conditions (as shown in Table 4). Before the test, specimens were cleaned, dried, and weighed. Then, specimens and test medium were added into the autoclave. Next, the test medium was deoxygenated by purging nitrogen for 2 h. Finally, different experimental conditions were applied, and the immersion test began. All immersion tests lasted for 720 h, and the test temperature was set at 80 °C.

Table 4.
Test conditions of immersion test.
After immersion, all specimens were cleaned, dried, and weighed again. The corrosion rate of specimens was calculated by Equation (1): [27,28]
where vd is the corrosion rate, mm y−1; w0 is the weight of the specimen before immersion, g; w1 is the weight of the specimen after immersion, g; ρ is the density of the steel, which is 7.86 g cm−3; S is the superficial area of the specimen before immersion, mm2; t is the time spent by the immersion test, h. All tests were repeated three times for reliable results.
2.4. Slow Strain Rate Test (SSRT) after Immersion
Tensile tests were conducted on other parallel immersed specimens according to ASTM E8M-09 [29]. The size of the tensile specimens is shown in Figure 2. Before the tensile test, the tensile specimens were immersed for 720 h under different conditions (as shown in Table 4). All tensile tests were conducted with a CORTEST slow strain rate test system (CORTEST, Willoughby, OH, USA). The test system can control temperature and gas pressure during tensile test. The tensile rate was 10−6 s−1. Then, elongations and reductions in the area were obtained from the stress–strain curves. Furthermore, the SCC susceptibility of the L80 steel was calculated by Equations (2)–(4): [30,31,32]
where Iδ, Iψ, and IRm are the susceptibilities of SCC calculated by different parameters; δ, ψ, and Rm are the elongations, reduction of area, and tensile strength, respectively; subscript s and subscript 0 represent the parameters of steel in corrosion medium and air, respectively. All tests were repeated three times for reliable results.
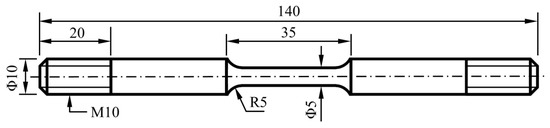
Figure 2.
Size of the tensile specimen (unit: mm).
3. Results
3.1. Corrosion Behavior of L80 Steel without Preloading Stress
3.1.1. Effect of Environmental Factors on the Electrochemical Behavior of L80 Steel without Preloading Stress
Firstly, the effect of temperature on electrochemical corrosion behavior is studied using polarization curves, as shown in Figure 3. It can be found that the corrosion potential of L80 steel shifts negatively as the temperature increases. However, the corrosion current density of L80 steel reaches a maximum at 80 °C. Therefore, 80 °C was chosen to research the effect of other factors on the corrosion behavior of L80 steel.
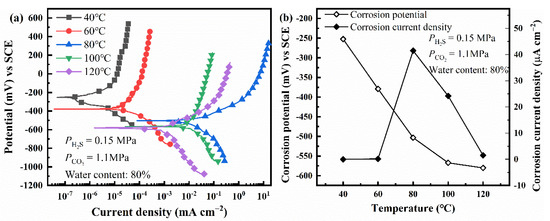
Figure 3.
Polarization curves (a) and fitting results (b) of L80 steels under different temperature.
The polarization curves of L80 steel under different experimental conditions are shown in Figure 4a,c,e. Figure 4b,d,f shows the variation of corrosion potential and corrosion current density with different factors, which is obtained by fitting polarization curves. The environmental factors (, , and water content) can affect the polarization behavior of L80 steel. However, when a single factor changes, all the curves have the same shape, which indicates that the variation of a single factor does not affect the corrosion mechanism of L80 steels and the rate-controlling step of corrosion (electrochemical reaction step).
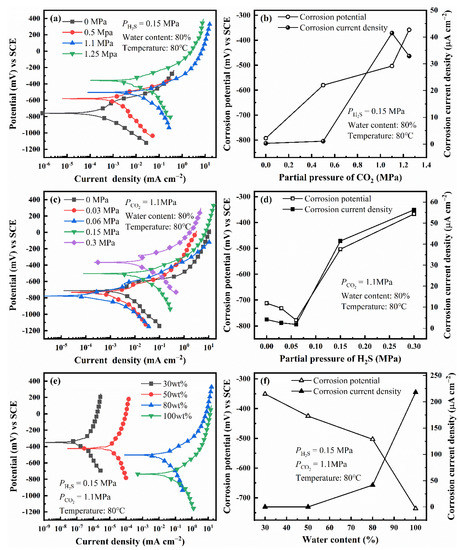
Figure 4.
Polarization curves and fitting results of L80 steels under different partial pressure of CO2 (a,b), different partial pressure of H2S (c,d) and different water content (e,f).
As increases, the corrosion potential of L80 steel shifts positively; meanwhile, the corrosion current density increases initially and then decreases. The partial pressure related to the largest corrosion current density is called critical pressure, which is 1.1 MPa. The effect of on corrosion potential and corrosion current density is partially attributed to the change of pH caused by the solution of CO2 in the corrosion medium [1,4].
As the partial pressure of H2S increases, the corrosion potential of L80 steel shifts negatively first and then shifts positively. In addition, the corrosion current density decreases initially and then increases. Therefore, the increase of restrains the corrosion of L80 steel in the test medium when the is less than the critical pressure (0.06 MPa), which may be related to the competitive adsorption of CO2 and H2S on the surface of L80 steel [28,33].
When the water content changes, the corrosion potential of L80 steel shifts negatively, and the corrosion current density increases with water content. Comparing the four polarization curves under different water content, water content can promote the anodic and cathodic processes of corrosion simultaneously.
In verifying the results from the polarization curves, the L80 steel without preloading stress was immersed under different experimental conditions. The corrosion rate of L80 steel was calculated by Equation (1) (Figure 5).
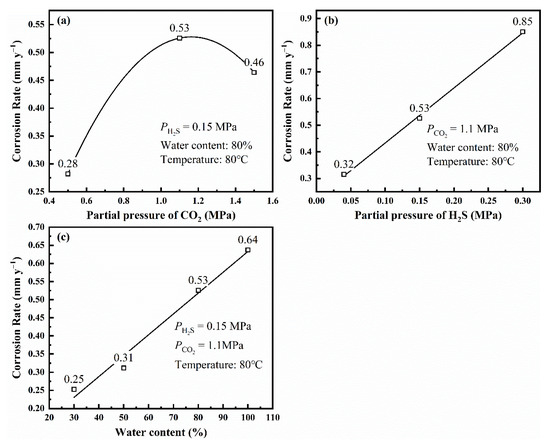
Figure 5.
Corrosion rate of L80 steel without preloading stress obtained from immersion test under different partial pressure of CO2 (a), different partial pressure of H2S (b), and different water content (c).
The results from the immersion test are consistent with those from the polarization curves. When is 1.1 MPa, the L80 steel has the biggest corrosion rate. As (>0.15 MPa) and water content increase, the corrosion rate of L80 steel under experimental condition increases.
3.1.2. SCC Susceptibility of L80 Steel without Preloading Stress after Immersion
Based on the electrochemical behavior of L80 steel, the temperature was set at 80 °C. The , , and water content were changed to study their effect on the SCC susceptibility of L80 steel. Figure 6a,c,e shows the stress–strain curves of L80 steel under different conditions after the 720 h immersion of tensile samples in the test medium.
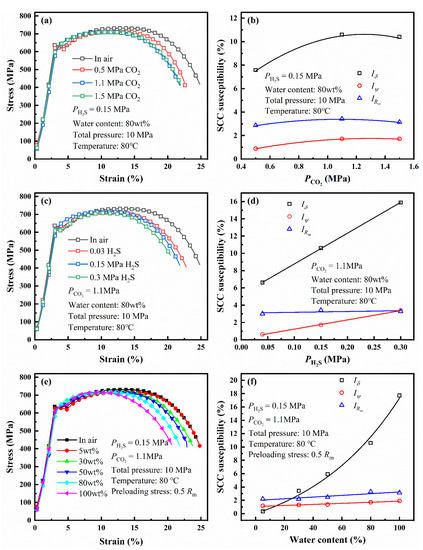
Figure 6.
Stress–strain curves and SCC susceptibility of L80 steel under different partial pressure of CO2 (a,b), different partial pressure of H2S (c,d) and water content (e,f).
Figure 6b,d,f shows the variation of the SCC susceptibility of L80 steel with different , , and water content calculated by Equations (2)–(4). Yield strength (ReL), tensile strength (Rm), and reduction of the area (ψ) change slightly as , , and water content increase. IRm and Iψ are all less than 4%, the change of which is too small to reflect the change of the SCC susceptibility of L80 steel [18]. Moreover, Iδ is fitter for analyzing the change of the SCC susceptibility because of the evident change. The value of Iδ is shown in Table 5. The influence of on Iδ has an inflection point of approximately 1.1 MPa. The Iδ reaches the maximum when the is about 1.1 MPa. Iδ has a positive correlation to and water content.

Table 5.
The SCC susceptibility of L80 steel under different conditions.
When is higher than 1.1 MPa, the stress–strain curves and the SCC susceptibility of L80 steel change slightly, which indicates that slightly affects SCC of steel. However, the variation of the SCC susceptibility of L80 steel with different is larger than that with different . Therefore, the SCC of L80 in the test medium is more sensitive to H2S than CO2. Water content also has a great influence on the SCC susceptibility of L80 steel. The SCC susceptibility of L80 steel increases with the water content of the corrosion medium.
3.2. Corrosion Behavior of L80 Steel with Preloading Stress
After the downhole tubes are adjusted, the tubes must perform under environmental stress, which can affect the SCC behavior of tubes. Therefore, different levels of initial stress (preloading stress) are applied to the tensile specimen to study how preloading stress affects the corrosion behavior of L80 steel.
Figure 7 shows the corrosion rate (calculated by weight–loss method) of L80 steel with different preloading stresses after the 720 h immersion test. The corrosion rate of L80 steel under the experimental conditions increases with preloading stress. Preloading stress can promote the corrosion of L80 steel. The corrosion rate of L80 steel has a positive correlation to preloading stress.
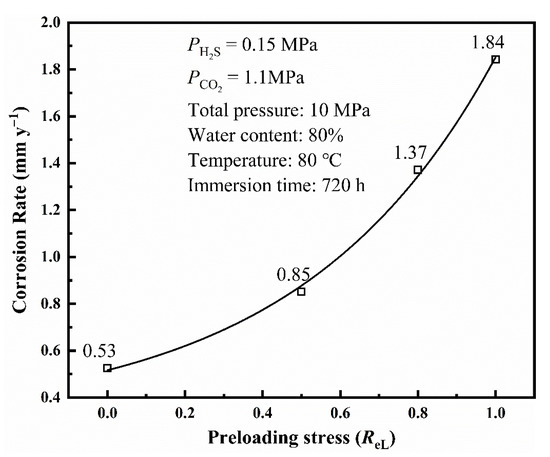
Figure 7.
Corrosion rate of L80 steel with different preloading stresses obtained from immersion test.
Figure 8a shows the stress–strain curves of L80 steel under different preloading stresses after 720 h immersion. The SCC susceptibility of L80 was calculated using Equations (2)–(4) (Figure 8b). The SCC susceptibility increases with preloading stress. Similarly, Iδ is a good parameter to evaluate SCC susceptibility compared with IRm and Iψ. The value of Iδ is shown in Table 6. Iδ also has a positive correlation to preloading stress, which is similar to the relation between corrosion rate and preloading stress.
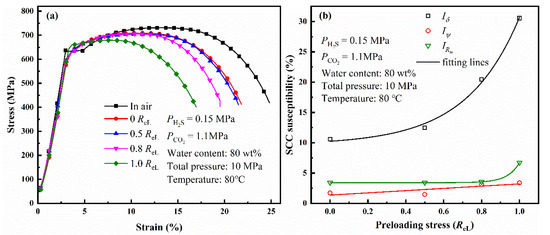
Figure 8.
Stress–strain curves of L80 steel with different preloading stresses (a) and variation of the SCC susceptibility of L80 steel with different preloading stresses (b).

Table 6.
The SCC susceptibility of L80 steel under different preloading stress.
4. Discussion
When downhole tubes service in a CO2/H2S environment, the corrosion behavior of tubes is affected by and , which is complex. After CO2 dissociates into the corrosion medium containing water, the following reactions occur: [34,35]
Carbonic acid (H2CO3) is a weak acid, which can serve as a buffer and keep the pH of the medium at a relatively low value because of the incomplete ionization of H2CO3 [35]. When H2S appears in the solution, reactions similar to Equations (8) and (9) occur, which can also keep the solution acidic [28,36]. The acidic environment can accelerate the corrosion of casing steel. The anodic process is the dissolution of steel (Equation (10)), and the cathodic process is hydrogen evolution reaction (Equation (11)). Hydrogen atoms generated during hydrogen evolution reaction can increase the SCC susceptibility of tubular steels because hydrogen atoms can penetrate the steel substrate [37]. Therefore, every change that can promote hydrogen evolution reaction will increase the SCC susceptibility of tubular steels.
When increases from 0 MPa to 1.5 MPa, the pH of the solution decreases gradually, and the equilibrium potential of hydrogen evolution reaction shifts positively. The mixed potential of the corrosion system, corrosion potential, is the result of the coupling of metal dissolution reaction and hydrogen evolution reaction. Therefore, the corrosion potential of steel in the corrosion medium increases with (Figure 4b) [4]. As changes from 0 MPa to 1.1 MPa, dwindling pH accelerates the corrosion of steel. The further increase of can increase the concentration of CO32−; then, Ca2+, Mg2+, and Fe2+ interact with CO32−, which forms insoluble carbonate precipitation (Equations (12)–(14)) [4,6]. Precipitation can cover the anode surface (where the concentration of Fe2+ is larger than that on the cathodic area), suppress the anodic reaction, and then decrease the corrosion rate of steel. Corrosion is the coupling of anodic and cathodic reactions [38]. Therefore, the hydrogen evolution reaction is also restricted. The less hydrogen formed on the surface of the steel, the lower the SCC susceptibility of steel [39]. Thus, the results of SSRT (Figure 6a,b) show that the L80 steel achieves the highest SCC susceptibility when is 1.1 MPa. Figure 3 shows that the corrosion current density of L80 steel reaches a maximum at 80 °C. This is because the higher temperature can improve the corrosion reaction activity and the precipitation of carbonate (FeCO3 and CaCO3) [40]. When the temperature is lower than 80 °C, the improvement in reaction activity dominates the corrosion process and the corrosion rate increases with temperature. When the temperature is higher than 80 °C, the effect of temperature on the precipitation of FeCO3 and CaCO3 dominates the corrosion process; the corrosion rate decreases with temperature.
Under a CO2/H2S environment, the effect of on the corrosion of steel differs from that of . Some researchers have reported that the CO2/H2S ratio can affect the corrosion behavior of steel [4,6,33,41]. The corrosion product scale can be formed on the surface of steel as corrosion develops in a high-pressure CO2/H2S environment (Equations (12)–(15)) [42,43]. As increases, the corrosion of the L80 steel is under the mixed control of CO2 and H2S. The proportion of FexS in the corrosion product film also increases [9].
In the corrosion medium, H2S can inhibit the hydrolysis of CO2, and the adsorption of H2S competes with CO2 [28]. Moreover, Fe2+ can easily interact with S2− to form precipitation [40]. As the cathode, the precipitation (FeS) can promote the corrosion of L80 steel. When is less than 0.06 MPa, H2S can restrain the corrosion of L80 steel. The corrosion potential shifts negatively, and the corrosion rate decreases. When is higher than 0.06 MPa, H2S also has the abovementioned function (inhibiting the hydrolysis of CO2, the adsorption of H2S competes with CO2, and promoting the precipitation of FeS). However, dissolving enough H2S in the corrosion medium promotes hydrolysis and acidifies the medium, which can make up for the inhibited promoting effect of CO2 on the cathode process [11]. Therefore, an increase in can increase the corrosion rate of steel and positively shift the corrosion potential.
Although the corrosion rate of L80 steel decreases and then increases as increases, the SCC susceptibility of L80 steel always increases as increases because H2S is a poisoning agent, which can increase the hydrogen content in the interior of L80 steel [11]. Hydrogen in the interior of steel can promote stress corrosion.
The water content of the corrosion medium is also an important factor that affects the SCC of L80 steel. As water content increases, the electrical conductivity of the corrosion medium increases. High electrical conductivity can promote the corrosion of L80 steel. In addition, excessive hydrogen can diffuse into the steel and deteriorate the SCC resistance of L80 steel. When the water content is relatively low, the electrical conductivity of the corrosion medium is low, and the corrosion of steel becomes restricted. Moreover, excessive oil can form an oil film on the surface of steel and separate the steel from the corrosion medium, which can also suppress the corrosion of L80 steel.
During the service period, not only the corrosion medium but also the environmental stress affects the corrosion behavior of the L80 steel. In this study, preloading is less than or equal to ReL. The corrosion rate and SCC susceptibility of the L80 steel both have a positive correlation to preloading stress. When the steel is under an elastic stress state, the stress can lead to dislocation emergence, and a small number of dislocations start to produce defects [44]. These defects increase with the stress level. The dislocation emergence point is a high distortion area with high chemical activity, which can promote corrosion reactions. When stress is equal to ReL, the dislocations begin to slip. Then, plastic deformation and many defects occur. These defects can also promote the corrosion of steel. Therefore, as the preloading stress changes from 0 ReL to 1 ReL, the number of defects increases rapidly, and the corrosion rate of the L80 steel increases. A higher corrosion rate can produce more adsorbed hydrogen atoms. Furthermore, the lattice distortion caused by tensile stress can improve the diffusion of hydrogen into steel. According to existing research [45,46,47], interstitial atoms (such as carbon atoms) can interact with dislocations and result in dislocation stacking during plastic deformation. However, hydrogen atoms in steel can promote dislocation emission and motion. Next, hydrogen could cause local stress concentration and initiate hydrogen-induced microcracks [48]. Therefore, more hydrogen can diffuse into the steel and promote the SCC of L80 steel.
, , water content, and preloading stress can affect the SCC behavior of L80 steel. A change in these factors can affect the corrosion of L80 steel and the rate of hydrogen evolution reaction. The faster the rate of hydrogen evolution reaction, the more hydrogen diffused into steel, and the higher the SCC susceptibility.
5. Conclusions
The results of this study suggest that service environmental parameters (, , water content, and preloading stress) can affect the electrochemical corrosion behavior of L80 steel.
- (1)
- The affects the corrosion behavior of steel in two aspects: Keeping the pH of the medium at a relatively low value and promoting form insoluble carbonate precipitation. The influence of on the corrosion rate and SCC susceptibility has an inflection point of approximately 1.1 MPa. The corrosion rate and SCC susceptibility reached the maximum when the is about 1.1 MPa;
- (2)
- The also affects the corrosion behavior of steel from two aspects: Inhibiting the hydrolysis of CO2 and promoting formation of FeS precipitation. The corrosion rate and SCC susceptibility have a positive correlation to ;
- (3)
- Corrosion rate has a linear relation to water content, and SCC susceptibility has a positive correlation to water content. Low water content can decrease the electrical conductivity of the corrosion medium and then restrict the corrosion of L80 steel;
- (4)
- The corrosion rate and SCC susceptibility of L80 steel have a positive correlation to preloading stress. SCC susceptibility of L80 steel can be explained by the local additional potential model. It means that preloading stress can promote the occurrence of defects in the surface of L80 steel. These defects can facilitate local corrosion, accelerate the diffusion of hydrogen into steel, and then increase SCC susceptibility.
Author Contributions
Conceptualization, Z.L. (Zhiyong Liu) and L.L.; methodology, X.S., H.C. and Z.L. (Zhiyong Liu); validation, X.S., H.C., Z.L. (Zhong Li), R.H. and Z.L. (Zhiyong Liu); formal analysis, X.S.; investigation, X.S., H.C., Z.L. (Zhong Li), Z.L. (Zhiyong Liu) and L.L.; resources, Z.L. (Zhiyong Liu); data curation, X.S.; writing—original draft preparation, X.S.; writing—review and editing, X.S., H.C., Z.L. (Zhong Li), Z.L. (Zhiyong Liu) and L.L.; visualization, X.S., H.C., Z.L. (Zhong Li) and R.H.; supervision, Z.L. (Zhiyong Liu) and L.L.; funding acquisition, Z.L. (Zhiyong Liu). All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by Fundamental Research Funds for the Central Universities (No. FRF-NP-20-04).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gao, X.; Zhang, D.; Lu, Y.; Fan, Z.; Du, L.; Yuan, G.; Qiu, C.; Kang, J. CO2 Corrosion Behavior of High-Strength and Toughness V140 Steel for Oil Country Tubular Goods. J. Mater. Eng. Perform. 2020, 29, 8451–8460. [Google Scholar] [CrossRef]
- Sun, C.; Zeng, H.; Luo, J.-L. Unraveling the Effects of CO2 and H2S on the Corrosion Behavior of Electroless Ni-P Coating in CO2/H2S/Cl– Environments at High Temperature and High Pressure. Corros. Sci. 2019, 148, 317–330. [Google Scholar] [CrossRef]
- Abayarathna, D.; Naraghi, A.R.; Wang, S. The Effect of Surface Films on Corrosion of Carbon Steel in a CO2-H2S-H2O System; NACE International: Houston, TX, USA, 2005. [Google Scholar]
- Cui, H.; Mei, P.; Liu, Z.; Lu, L. Effect of CO2 Partial Pressure on the Stress Corrosion Cracking Behavior of N80 Tubing Steel in the Annulus Environment of CO2 Injection Well. Chin. J. Eng. 2020, 42, 1182–1189. [Google Scholar]
- Zhang, C. Study of the Corrosion Inhibition Mechanism and the Synergistic Corrosion Inhibition Effect of Inhibitors CO2/H2S System. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2018. [Google Scholar]
- Liu, Z.Y.; Wang, X.Z.; Liu, R.K.; Du, C.W.; Li, X.G. Electrochemical and Sulfide Stress Corrosion Cracking Behaviors of Tubing Steels in a H2S/CO2 Annular Environment. J. Mater. Eng. Perform. 2014, 23, 1279–1287. [Google Scholar] [CrossRef] [Green Version]
- Parkins, R.N.; Zhou, S. The Stress Corrosion Cracking of C-Mn Steel in CO2-HCO3−-CO32− Solutions. I: Stress Corrosion Data. Corros. Sci. 1997, 39, 159–173. [Google Scholar] [CrossRef]
- Zhou, C.; Zheng, S.; Chen, C.; Lu, G. The Effect of the Partial Pressure of H2S on the Permeation of Hydrogen in Low Carbon Pipeline Steel. Corros. Sci. 2013, 67, 184–192. [Google Scholar] [CrossRef]
- Monnot, M.; Nogueira, R.P.; Roche, V.; Berthomé, G.; Chauveau, E.; Estevez, R.; Mantel, M. Sulfide Stress Corrosion Study of a Super Martensitic Stainless Steel in H2S Sour Environments: Metallic Sulfides Formation and Hydrogen Embrittlement. Appl. Surf. Sci. 2017, 394, 132–141. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Q.; Guo, Q.; Zheng, J.; Chen, X.; Zhu, J.; Zhang, L. Sulphide Stress Cracking Behaviour of the Dissimilar Metal Welded Joint of X60 Pipeline Steel and Inconel 625 Alloy. Corros. Sci. 2016, 110, 242–252. [Google Scholar] [CrossRef]
- Jia, Y. Study on Stress Corrosion Sensitivity of Cold Deformed 316L Austenitic Stainless Steel in H2S Environment; China University of Petroleum: Beijing, China, 2019. [Google Scholar]
- Wang, S. Multiphysical Simulation of CO2 Enhanced Oil Recovery in Unconventional Reservoirs: From Fundamental Physics to Simulator Development; Colorado School of Mines: Golden, CO, USA, 2019. [Google Scholar]
- Liu, Q.; Li, Z.; Liu, Z.Y.; Li, X.G.; Wang, S.Q. Effects of H2S/HS− on Stress Corrosion Cracking Behavior of X100 Pipeline Steel Under Simulated Sulfate-Reducing Bacteria Metabolite Conditions. J. Mater. Eng. Perform. 2017, 26, 2763–2775. [Google Scholar] [CrossRef]
- Vyboishchik, M.A.; Ioffe, A.V.; Zyryanov, A.O. Failure of Pump–Compressor Pipes in Highly Aggressive Oil-Field Media. Russ. Metall. 2019, 2019, 1067–1073. [Google Scholar] [CrossRef]
- Khalaf, A.M.; Seibi, A.C. Failure Analysis of Lube Oil Feed Tube of a Gas Turbine Operating in Oil Fields. Eng. Fail. Anal. 2011, 18, 1341–1350. [Google Scholar] [CrossRef]
- De Assis Severiano, J.; Silva, A.S.; Sussushi, E.M.; da Silva Sant’Anna, M.V.; da Cunha, M.A.; Bergmann, C.P.; Griza, S. Corrosion Damages of Flow Regulation Valves for Water Injection in Oil Fields. Eng. Fail. Anal. 2019, 96, 362–373. [Google Scholar] [CrossRef]
- Liu, R.; Li, J.; Liu, Z.; Du, C.; Dong, C.; Li, X. Effect of PH and H2S Concentration on Sulfide Stress Corrosion Cracking (SSCC) of API 2205 Duplex Stainless Steel. IJMR 2015, 106, 608–613. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Z.; Du, C.; Hu, J.; Li, X. A Modelling Study for Predicting Life of Downhole Tubes Considering Service Environmental Parameters and Stress. Materials 2016, 9, 741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yucheng, L.; Yinlong, Z.; Jianmei, Y.; Mengjing, Y.; Junzhong, X. Research on Corrosion Perforation on Pipeline by Media of High Salinity Acidic Oil–Water Mixture. Eng. Fail. Anal. 2013, 34, 35–40. [Google Scholar] [CrossRef]
- Bouazaze, H.; Huet, F.; Nogueira, R.P. A New Approach for Monitoring Corrosion and Flow Characteristics in Oil/Brine Mixtures. Electrochim. Acta 2005, 50, 2081–2090. [Google Scholar] [CrossRef]
- Liu, R. Stress Corrosion Cracking Behaviour and Prevention of High Strength Tubing Steels in Typical H2S/CO2 Annulus Environments. Ph.D. Thesis, University of Science and Technology Beijing, Beijing, China, 2015. [Google Scholar]
- Hong, C.; Estefen, S.F.; Wang, Y.; Lourenço, M.I. Mixed-Integer Nonlinear Programming Model for Layout Design of Subsea Satellite Well System in Deep Water Oil Field. Autom. Constr. 2021, 123, 103524. [Google Scholar] [CrossRef]
- NACE International Standard: TM0177-2005 Laboratory Testing of Metals for Resistance to Sulfide Stress Cracking and Stress Corrosion Cracking in H2S Environments; NACE International: Houston, TX, USA, 2005.
- Li, N.; Ding, N.; Liu, L.; Hu, S.; Sun, S.; Qu, S.; Wu, C.-M.L. Stress Corrosion Cracking of an Electrohydraulic Oil Pipe. J. Fail. Anal. Prev. 2019, 19, 29–35. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Shu, Z.; Zhao, Q.; Han, L. Failure Analysis of Casing Dropping in Shale Oil Well During Large Scale Volume Fracturing. Eng. Fail. Anal. 2020, 118, 104849. [Google Scholar] [CrossRef]
- Tian, J.; Wang, L.; Sun, W.; Yang, Y.; Liu, Z.; Wang, G.; Zhao, L.; Zhou, Y.; Liu, G. Failure Analysis of Steam Jet Pump at Top of Crude Oil Vacuum Distillation Tower. Eng. Fail. Anal. 2019, 103, 9–19. [Google Scholar] [CrossRef]
- Liu, H.; Gu, T.; Zhang, G.; Wang, W.; Dong, S.; Cheng, Y.; Liu, H. Corrosion Inhibition of Carbon Steel in CO2-Containing Oilfield Produced Water in the Presence of Iron-Oxidizing Bacteria and Inhibitors. Corros. Sci. 2016, 105, 149–160. [Google Scholar] [CrossRef]
- Zhang, G.A.; Zeng, Y.; Guo, X.P.; Jiang, F.; Shi, D.Y.; Chen, Z.Y. Electrochemical Corrosion Behavior of Carbon Steel under Dynamic High Pressure H2S/CO2 Environment. Corros. Sci. 2012, 65, 37–47. [Google Scholar] [CrossRef]
- American Society for Testing and Materials. Standard Test Method for Tension Testing of Metallic Materials. In Annual Book of ASTM Standards; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- Wu, W.; Hao, W.; Liu, Z.; Li, X.; Du, C. Comparative Study of The Stress Corrosion Behavior of a Multiuse Bainite Steel in the Simulated Tropical Marine Atmosphere and Seawater Environments. Constr. Build. Mater. 2020, 239, 117903. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Hao, W.K.; Wu, W.; Luo, H.; Li, X.G. Fundamental Investigation of Stress Corrosion Cracking of E690 Steel in Simulated Marine Thin Electrolyte Layer. Corros. Sci. 2019, 148, 388–396. [Google Scholar] [CrossRef]
- Wu, W.; Cheng, X.; Zhao, J.; Li, X. Benefit of The Corrosion Product Film Formed on a New Weathering Steel Containing 3% Nickel under Marine Atmosphere in Maldives. Corros. Sci. 2020, 165, 108416. [Google Scholar] [CrossRef]
- Zheng, Y.; Ning, J.; Brown, B.; Nešić, S. Electrochemical Model of Mild Steel Corrosion in a Mixed H2S/CO2 Aqueous Environment in the Absence of Protective Corrosion Product Layers. Corrosion 2014, 71, 316–325. [Google Scholar] [CrossRef]
- Barker, R.; Burkle, D.; Charpentier, T.; Thompson, H.; Neville, A. A Review of Iron Carbonate (FeCO3) Formation in the Oil and Gas Industry. Corros. Sci. 2018, 142, 312–341. [Google Scholar] [CrossRef]
- Cui, G.; Yang, Z.; Liu, J.; Li, Z. A Comprehensive Review of Metal Corrosion in a Supercritical CO2 Environment. Int. J. Greenh. Gas Control 2019, 90, 102814. [Google Scholar] [CrossRef]
- Li, Z.; Xu, X.; Li, Y.; Liu, Z. Effect of Imidazoline Inhibitor on Stress Corrosion Cracking of P110 Steel in Simulated Annulus Environments of CO2 Injection Well. J. Electroanal. Chem. 2021, 886, 115105. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Z.; Ke, L.; Huang, L.; Du, C.; Li, X. Stress Corrosion Cracking Behavior of TP95S Tube Steel in an Acidic Gas Field Environment. Int. J. Electrochem. Sci. 2016, 5021–5034. [Google Scholar] [CrossRef]
- Cao, C. Principles of Electrochemistry of Corrosion, 3rd ed.; Chemical Industry Press: Beijing, China, 2008. [Google Scholar]
- Chen, Z.; Li, L.; Zhang, G.; Qiu, Y.; Guo, X. Inhibition Effect of Propargyl Alcohol on the Stress Corrosion Cracking of Super 13Cr Steel in a Completion Fluid. Corros. Sci. 2013, 69, 205–210. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics, 94th ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Liu, R.; Yin, G.; Wei, C.; Wang, X.; Ma, F.; Liu, Z.; Du, C.; Li, X. Electrochemical Corrosion Behaviour of P110 Tubing Steel in Annular Environment. Corros. Sci. Prot. Technol. 2013, 25, 450–454. [Google Scholar]
- Zhao, X.; Huang, W.; Li, G.; Feng, Y.; Zhang, J. Effect of CO2/H2S and Applied Stress on Corrosion Behavior of 15Cr Tubing in Oil Field Environment. Metals 2020, 10, 409. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Wu, W.; Li, Q. Under-Deposit Corrosion of Tubing Served for Injection and Production Wells of CO2 Flooding. Eng. Fail. Anal. 2021, 127, 105540. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Li, X.G.; Du, C.W.; Cheng, Y.F. Local Additional Potential Model for Effect of Strain Rate on SCC of Pipeline Steel in an Acidic Soil Solution. Corros. Sci. 2009, 51, 2863–2871. [Google Scholar] [CrossRef]
- Brechtl, J.; Chen, B.; Xie, X.; Ren, Y.; Venable, J.D.; Liaw, P.K.; Zinkle, S.J. Entropy Modeling on Serrated Flows in Carburized Steels. Mater. Sci. Eng. A 2019, 753, 135–145. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, L.; Yu, L.; Sun, B.; Cao, Y.; Zhao, Y.; Zhang, Y. The Mechanism for the Serrated Flow Induced by Suzuki Segregation in a Ni Alloy. Mater. Sci. Eng. A 2021, 820, 141575. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, J.P.; Chen, S.Y.; Xie, X.; Liaw, P.K.; Dahmen, K.A.; Qiao, J.W.; Wang, Y.L. Serration and Noise Behaviors in Materials. Prog. Mater. Sci. 2017, 90, 358–460. [Google Scholar] [CrossRef]
- Song, L.; Liu, Z.; Li, X.; Du, C. Characteristics of Hydrogen Embrittlement in High-PH Stress Corrosion Cracking of X100 Pipeline Steel in Carbonate/Bicarbonate Solution. Constr. Build. Mater. 2020, 263, 120124. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).