High-Performance Washable PM2.5 Filter Fabricated with Laser-Induced Graphene
Abstract
:1. Introduction
2. Materials and Methods
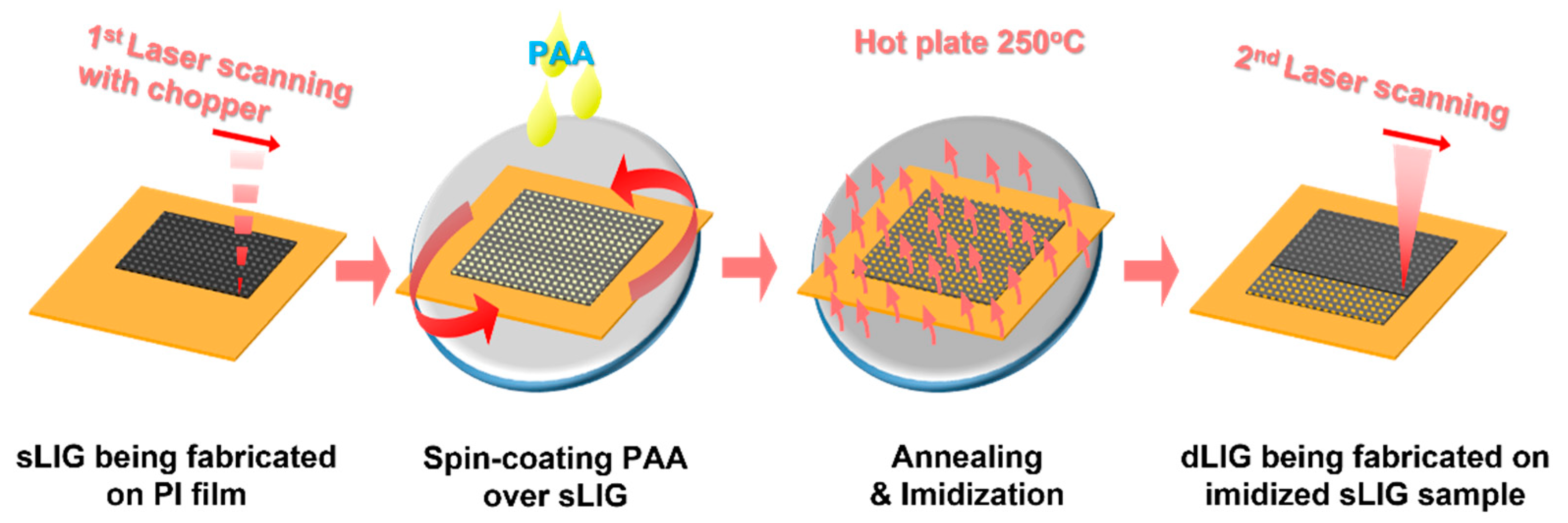
2.1. Materials and Laser Irradiation
2.2. Characterization
2.3. PM Generation and Filtration
3. Results and Discussion
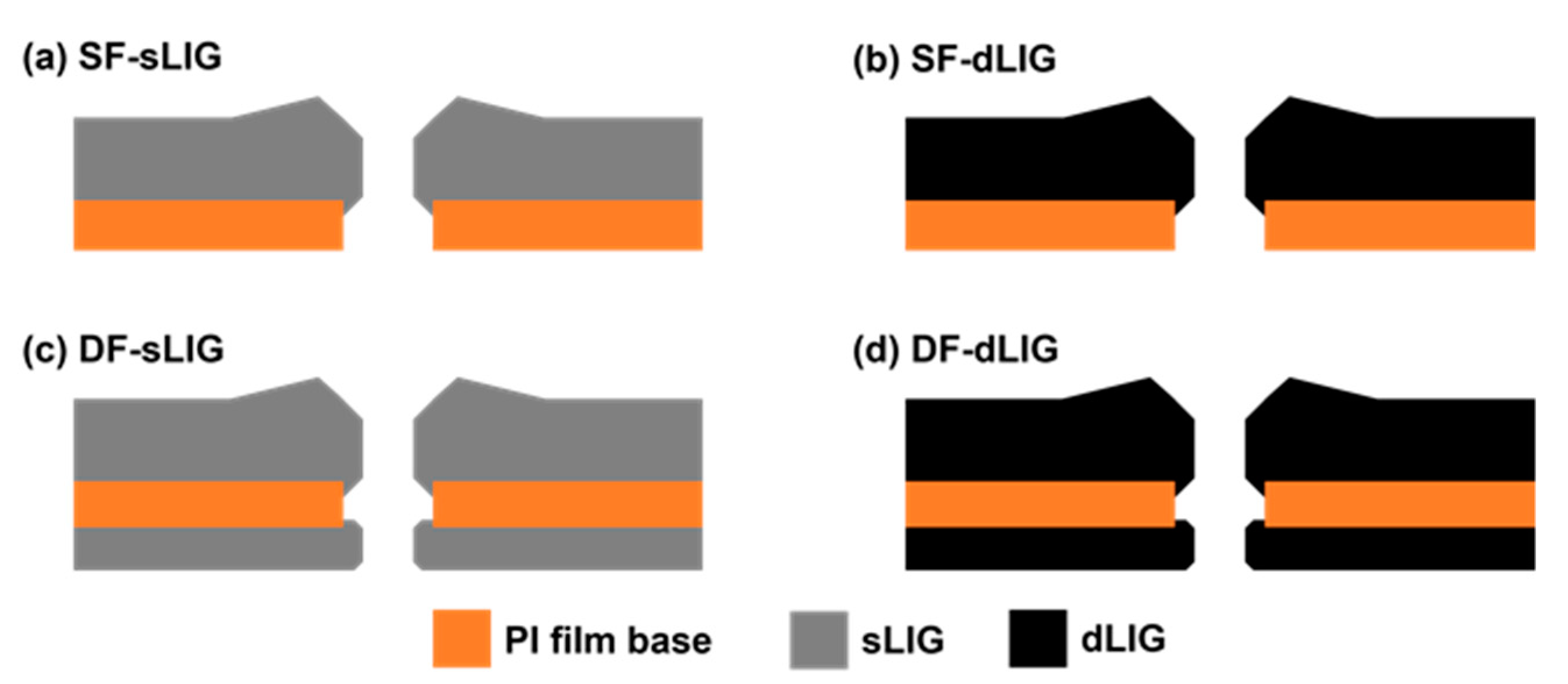
3.1. Fabrication of LIG Filters
3.2. Morphology of LIG Filters
3.3. Filtration Performance
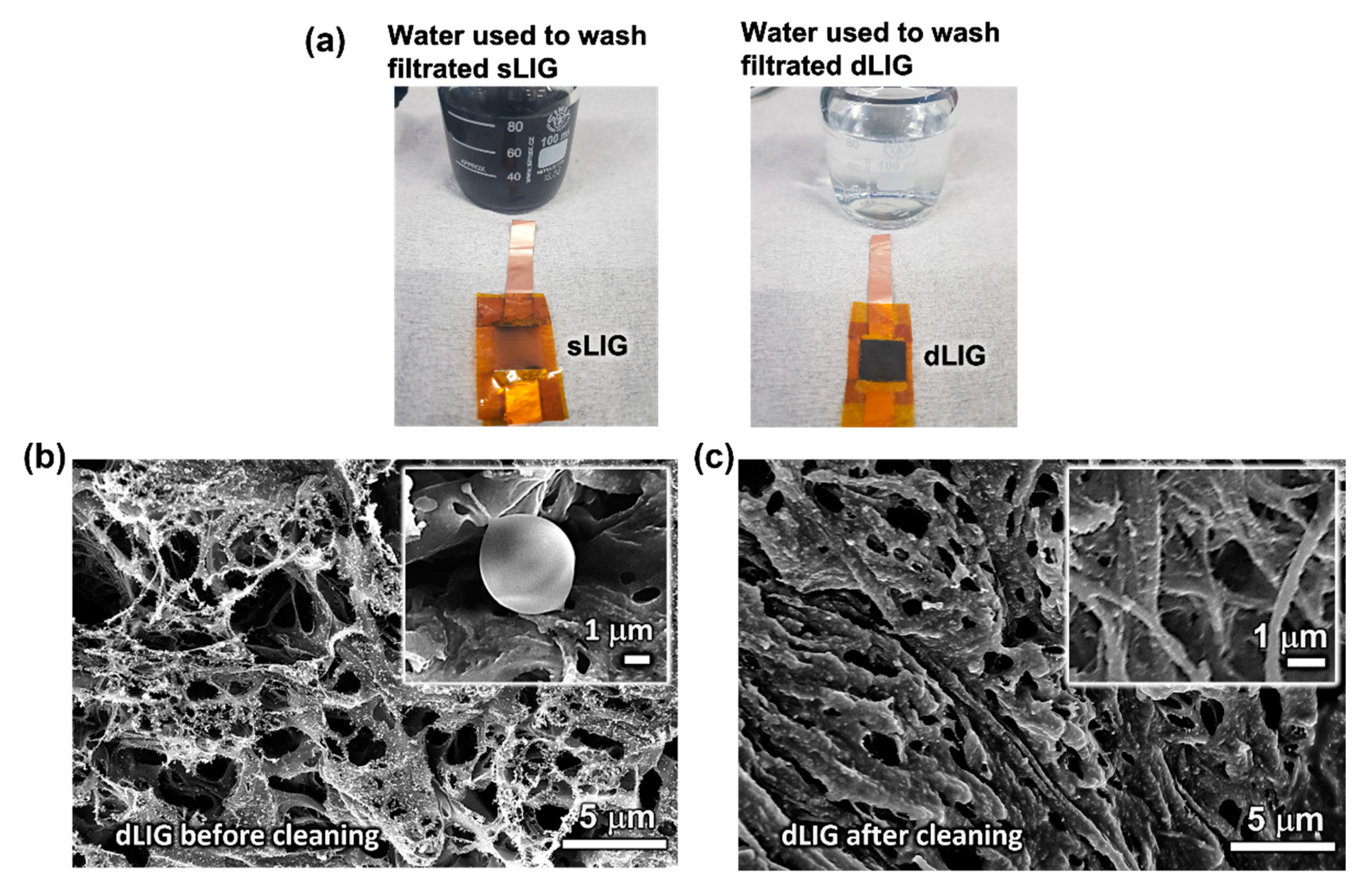
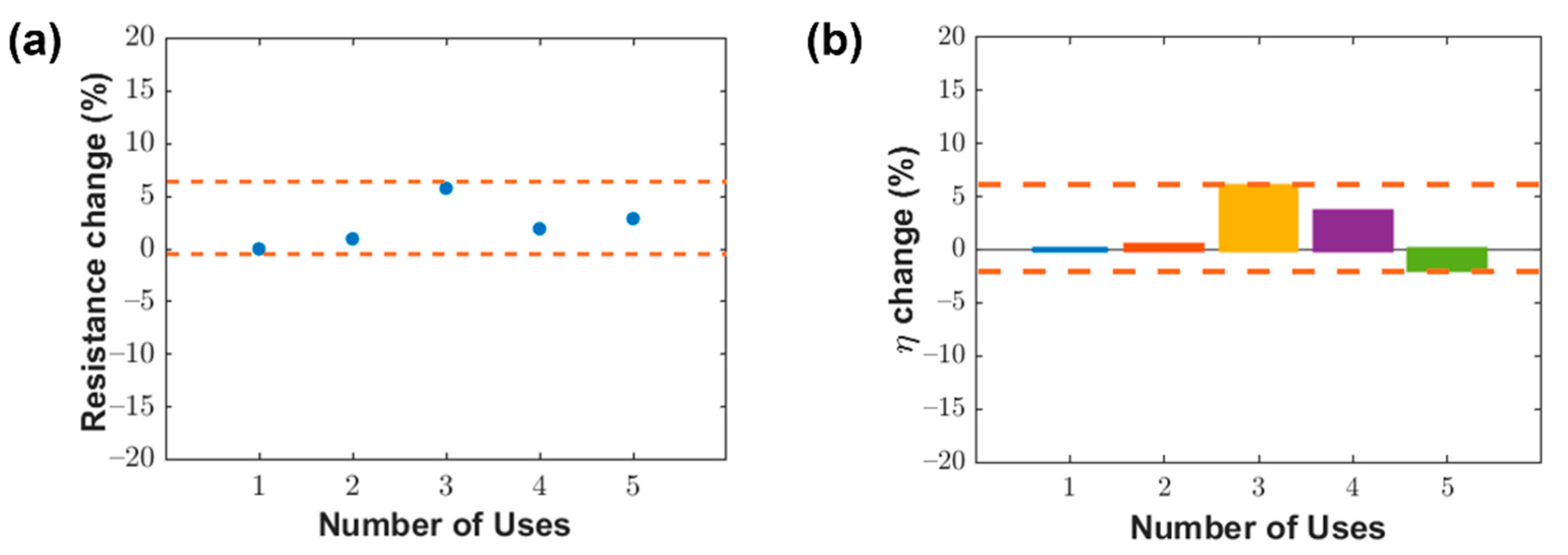
3.4. Washability of LIG Filters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brunekreef, B.; Holgate, S.T. Air pollution and health. Lancet 2002, 360, 1233–1242. [Google Scholar] [CrossRef]
- Rockström, J.; Steffen, W.; Noone, K.; Persson, Å.; Chapin, F.S.; Lambin, E.F.; Lenton, T.M.; Scheffer, M.; Folke, C.; Schellnhuber, H.J.; et al. A safe operating space for humanity. Nature 2009, 461, 472–475. [Google Scholar] [CrossRef]
- Sharma, P.; Yadav, P.; Ghosh, C.; Singh, B. Heavy metal capture from the suspended particulate matter by Morus alba and evidence of foliar uptake and translocation of PM associated zinc using radiotracer (65Zn). Chemosphere 2020, 254, 126863. [Google Scholar] [CrossRef]
- Gilmour, P.S.; Brown, D.M.; Lindsay, T.G.; Beswick, P.H.; MacNee, W.; Donaldson, K. Adverse health effects of PM10 particles: Involvement of iron in generation of hydroxyl radical. Occup. Environ. Med. 1996, 53, 817–822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, C.A., 3rd; Burnett, R.T.; Thun, M.J.; Calle, E.E.; Krewski, D.; Ito, K.; Thurston, G.D. Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. JAMA 2002, 287, 1132–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Nethery, R.C.; Sabath, M.B.; Braun, D.; Dominici, F. Air pollution and COVID-19 mortality in the United States: Strengths and limitations of an ecological regression analysis. Science Advances 2020, 6, eabd4049. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.; Das, D.; Das, A. Electret Air Filters. Sep. Purif. Rev. 2013, 42, 87–129. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X.; Yin, X.; Yu, J.; Ding, B. Electret Polyvinylidene Fluoride Nanofibers Hybridized by Polytetrafluoroethylene Nanoparticles for High-Efficiency Air Filtration. ACS Appl. Mater. Interfaces 2016, 8, 23985–23994. [Google Scholar] [CrossRef] [PubMed]
- Khalid, B.; Bai, X.; Wei, H.; Huang, Y.; Wu, H.; Cui, Y. Direct Blow-Spinning of Nanofibers on a Window Screen for Highly Efficient PM2.5 Removal. Nano Lett. 2017, 17, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hsu, P.-C.; Lee, H.-W.; Ye, M.; Zheng, G.; Liu, N.; Li, W.; Cui, Y. Transparent air filter for high-efficiency PM2.5 capture. Nat. Commun. 2015, 6, 6205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Wang, C.; Zhang, Y.; Wei, F. Air Filtration in the Free Molecular Flow Regime: A Review of High-Efficiency Particulate Air Filters Based on Carbon Nanotubes. Small 2014, 10, 4543–4561. [Google Scholar] [CrossRef]
- Li, P.; Zong, Y.; Zhang, Y.; Yang, M.; Zhang, R.; Li, S.; Wei, F. In situ fabrication of depth-type hierarchical CNT/quartz fiber filters for high efficiency filtration of sub-micron aerosols and high water repellency. Nanoscale 2013, 5, 3367–3372. [Google Scholar] [CrossRef] [PubMed]
- Joannou, C.J. Ionizing and Polarizing Electronic Air Filter (US5573577A). USPTO: 1996. Available online: https://patents.google.com/patent/US5573577 (accessed on 10 August 2021).
- Rammig, J.; Schmierer, U.; Hornfeck, U.; Straub, R.; Multi-layer Filter Structure and Use of a Multi-Layer Filter Structure (US6966939B2). USPTO: 2005. Available online: https://patents.google.com/patent/US6966939B2 (accessed on 10 August 2021).
- Fjeld, R.; Owens, T. The effect of particle charge on penetration in an electret filter. IEEE Trans. Ind. Appl. 1988, 24, 725–731. [Google Scholar] [CrossRef]
- Chen, T.-M.; Tsai, C.-J.; Yan, S.-Y.; Li, S.-N. An efficient wet electrostatic precipitator for removing nanoparticles, submicron and micron-sized particles. Sep. Purif. Technol. 2014, 136, 27–35. [Google Scholar] [CrossRef]
- Wen, T.-Y.; Wang, H.-C.; Krichtafovitch, I.; Mamishev, A.V. Novel electrodes of an electrostatic precipitator for air filtration. J. Electrost. 2015, 73, 117–124. [Google Scholar] [CrossRef]
- Bin In, J.; Hsia, B.; Yoo, J.-H.; Hyun, S.; Carraro, C.; Maboudian, R.; Grigoropoulos, C.P. Facile fabrication of flexible all solid-state micro-supercapacitor by direct laser writing of porous carbon in polyimide. Carbon 2015, 83, 144–151. [Google Scholar] [CrossRef]
- Lin, J.; Peng, Z.; Liu, Y.; Ruiz-Zepeda, F.; Ye, R.; Samuel, E.L.G.; Yacaman, M.J.; Yakobson, B.I.; Tour, J.M. Laser-induced porous graphene films from commercial polymers. Nat. Commun. 2014, 5, 5714. [Google Scholar] [CrossRef]
- El-Kady, M.F.; Strong, V.; Dubin, S.; Kaner, R.B. Laser Scribing of High-Performance and Flexible Graphene-Based Electrochemical Capacitors. Science 2012, 335, 1326–1330. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Hoang, P.T.; Liu, T. Direct laser writing for creating porous graphitic structures and their use for flexible and highly sensitive sensor and sensor arrays. Carbon 2016, 96, 522–531. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved Synthesis of Graphene Oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Wang, Q.; Bai, Y.; Xie, J.; Jiang, Q.; Qiu, Y. Synthesis and filtration properties of polyimide nanofiber membrane/carbon woven fabric sandwiched hot gas filters for removal of PM2.5 particles. Powder Technol. 2016, 292, 54–63. [Google Scholar] [CrossRef]
- Konios, D.; Stylianakis, M.M.; Stratakis, E.; Kymakis, E. Dispersion behaviour of graphene oxide and reduced graphene oxide. J. Colloid Interface Sci. 2014, 430, 108–112. [Google Scholar] [CrossRef]
- Jeong, S.; Cho, H.; Han, S.; Won, P.; Lee, H.; Hong, S.; Yeo, J.; Kwon, J.; Ko, S.H. High Efficiency, Transparent, Reusable, and Active PM2.5 Filters by Hierarchical Ag Nanowire Percolation Network. Nano Lett. 2017, 17, 4339–4346. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.Y.; Choi, H.; Van Tran, C.; Bin In, J. Simultaneous densification and nitrogen doping of laser-induced graphene by duplicated pyrolysis for supercapacitor applications. J. Power Sources 2019, 441, 227199. [Google Scholar] [CrossRef]
- Khandelwal, M.; Tran, C.V.; Lee, J.; In, J.B. Nitrogen and boron co-doped densified laser-induced graphene for supercapacitor applications. Chem. Eng. J. 2022, 428, 131119. [Google Scholar] [CrossRef]
- Luo, J.; Yao, Y.; Duan, X.; Liu, T. Force and humidity dual sensors fabricated by laser writing on polyimide/paper bilayer structure for pulse and respiration monitoring. J. Mater. Chem. C 2018, 6, 4727–4736. [Google Scholar] [CrossRef]
- Duy, L.X.; Peng, Z.; Li, Y.; Zhang, J.; Ji, Y.; Tour, J.M. Laser-induced graphene fibers. Carbon 2018, 126, 472–479. [Google Scholar] [CrossRef]
- Lee, J.B.; Jang, J.; Zhou, H.; Lee, Y.; Bin In, J. Densified Laser-Induced Graphene for Flexible Microsupercapacitors. Energies 2020, 13, 6567. [Google Scholar] [CrossRef]
- Lee, H.; Jeon, S. Polyacrylonitrile Nanofiber Membranes Modified with Ni-Based Conductive Metal Organic Frameworks for Air Filtration and Respiration Monitoring. ACS Appl. Nano Mater. 2020, 3, 8192–8198. [Google Scholar] [CrossRef]
- Seoul Metropolitan Government. Seoul Tap Water Arisu. Available online: https://www.metropolis.org/sites/default/files/seoul_tap_water_arisu_english.pdf (accessed on 19 August 2021).
- Lee, S.; Cho, A.R.; Park, D.; Kim, J.K.; Han, K.S.; Yoon, I.-J.; Lee, M.H.; Nah, J. Reusable Polybenzimidazole Nanofiber Membrane Filter for Highly Breathable PM2.5 Dust Proof Mask. ACS Appl. Mater. Interfaces 2019, 11, 2750–2757. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-T.; Cen, X.; Ren, H.-T.; Wu, L.; Peng, H.-K.; Wang, W.; Gao, B.; Lou, C.-W.; Lin, J.-H. Zeolitic Imidazolate Framework-8/Polypropylene–Polycarbonate Barklike Meltblown Fibrous Membranes by a Facile in Situ Growth Method for Efficient PM2.5 Capture. ACS Appl. Mater. Interfaces 2020, 12, 8730–8739. [Google Scholar] [CrossRef] [PubMed]



| Sample | E (%) | ∆P (Pa) | QF (Pa-1) |
|---|---|---|---|
| SF-sLIG | 96.82 | 278 | 0.0124 |
| DF-sLIG | 98.87 | 157 | 0.0285 |
| SF-dLIG | 98.01 | 366 | 0.0107 |
| DF-dLIG | 99.86 | 255 | 0.00771 |
| Commercial | 97.47 | 3764 | 0.00097 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, A.-P.; Kang, W.-K.; Lee, J.-B.; In, J.-B. High-Performance Washable PM2.5 Filter Fabricated with Laser-Induced Graphene. Materials 2021, 14, 5551. https://doi.org/10.3390/ma14195551
Nguyen A-P, Kang W-K, Lee J-B, In J-B. High-Performance Washable PM2.5 Filter Fabricated with Laser-Induced Graphene. Materials. 2021; 14(19):5551. https://doi.org/10.3390/ma14195551
Chicago/Turabian StyleNguyen, Anh-Phan, Won-Kyu Kang, Jung-Bae Lee, and Jung-Bin In. 2021. "High-Performance Washable PM2.5 Filter Fabricated with Laser-Induced Graphene" Materials 14, no. 19: 5551. https://doi.org/10.3390/ma14195551
APA StyleNguyen, A.-P., Kang, W.-K., Lee, J.-B., & In, J.-B. (2021). High-Performance Washable PM2.5 Filter Fabricated with Laser-Induced Graphene. Materials, 14(19), 5551. https://doi.org/10.3390/ma14195551






