3D-Printed Polymer-Infiltrated Ceramic Network with Biocompatible Adhesive to Potentiate Dental Implant Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of 3D-Printed Cubic Samples, Ceramic Functionalization, and Copolymer Covalent Deposition
2.3. Scaffold Characterization
2.4. Human Cells Adhesion and Proliferation
3. Results and Discussion
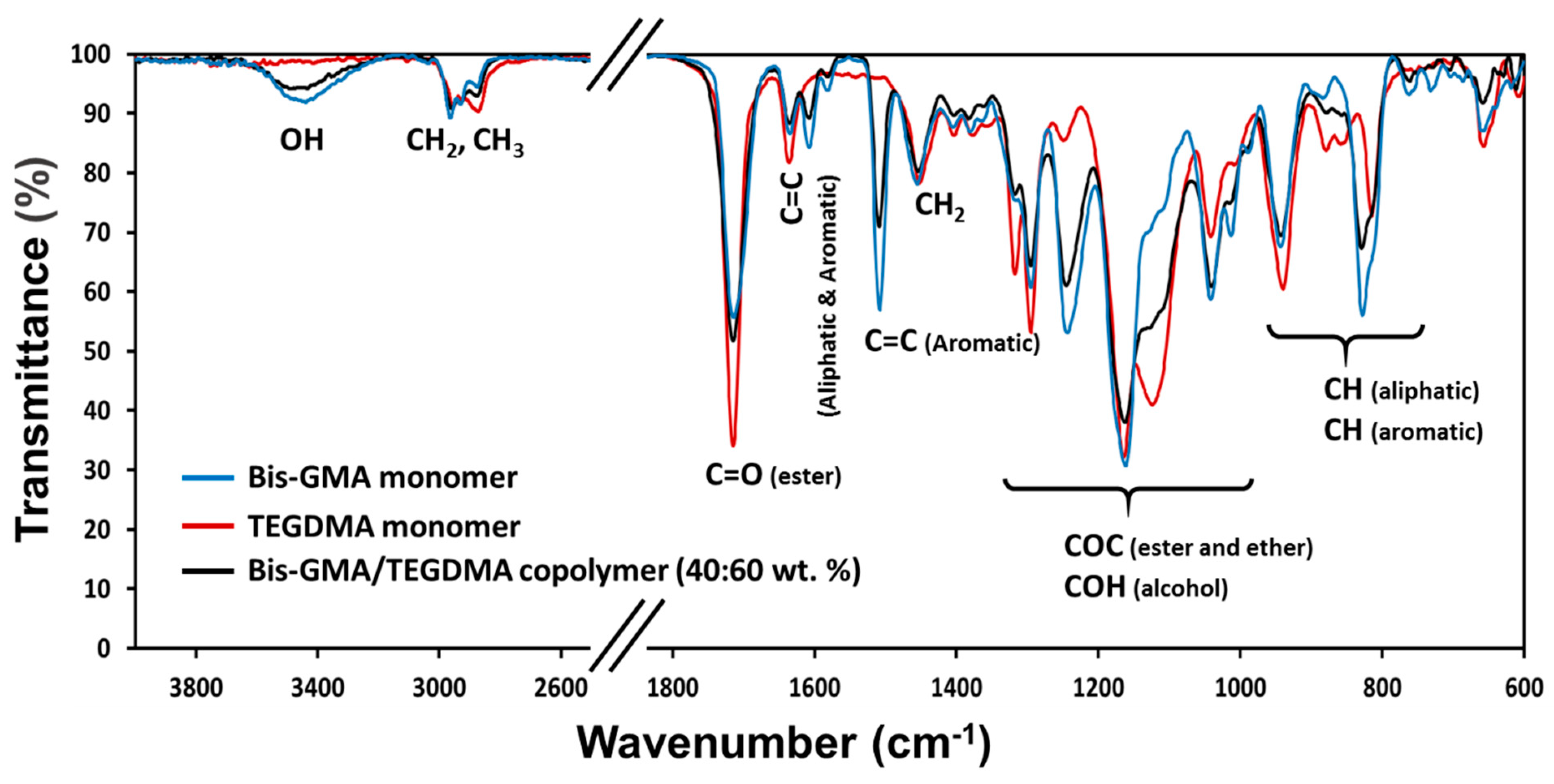
3.1. Hybrid Material Characterization
3.2. Mechanical Tests
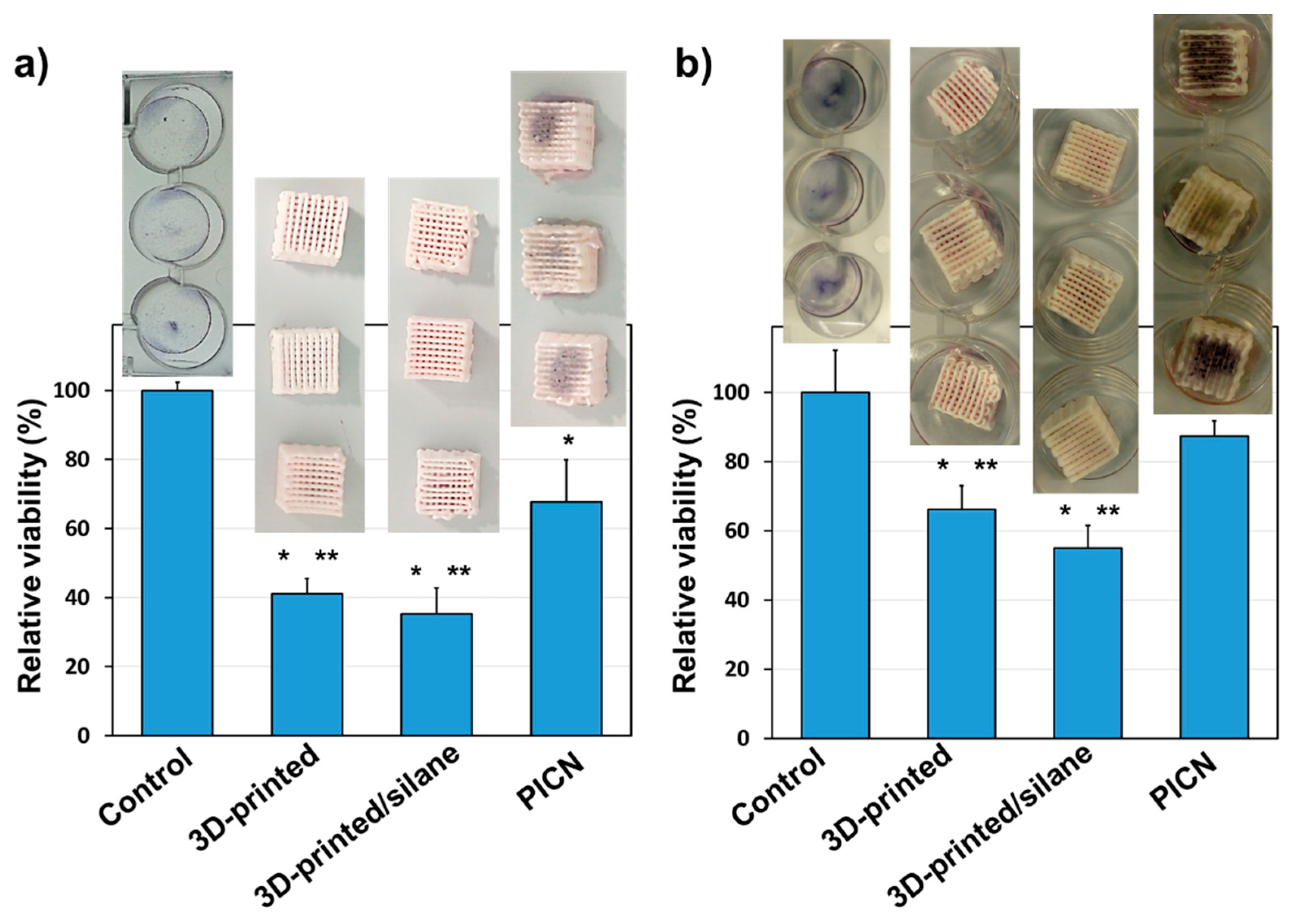
3.3. In Vitro Human Cell Adhesion and Proliferation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abraham, C.M. A Brief Historical Perspective on Dental Implants, Their Surface Coatings and Treatments. Open Dent. J. 2014, 8, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, L.; Röstlund, T.; Albrektsson, B.; Albrektsson, T.; Brånemark, P.-I. Osseointegration of titanium implants. Acta Orthop. Scand. 1986, 57, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Stefanic, M.; Krnel, K.; Pribosic, I.; Kosmac, T. Rapid biomimetic deposition of octacalcium phosphate coatings on zirconia ceramics (Y-TZP) for dental implant applications. Appl. Surf. Sci. 2012, 258, 4649–4656. [Google Scholar] [CrossRef]
- Jonhson, A.; Sinthuprasirt, P.; Fathi, H.; Pollington, S. Current Glass-Ceramic Systems Used in Dentistry. In Current Trends on Glass and Ceramic Materials; Nandyala, S.H., Dos Santos, J., Eds.; Bentham Science Publishers: Sharjak, United Arab Emirates, 2013; pp. 49–72. [Google Scholar]
- Zafar, M.S.; Fareed, M.A.; Riaz, S.; Latif, M.; Habib, S.R.; Khurshid, Z. Customized Therapeutic Surface Coatings for Dental Implants. Coatings 2020, 10, 568. [Google Scholar] [CrossRef]
- Trincă, L.C.; Mareci, D.; Souto, R.M.; Lozano-Gorrín, A.D.; Izquierdo, J.; Burtan, L.; Motrescu, I.; Vulpe, V.; Pavel, G.; Strungaru, S.; et al. Osseointegration evaluation of ZrTi alloys with hydroxyapatite-zirconia-silver layer in pig’s tibiae. Appl. Surf. Sci. 2019, 487, 127–137. [Google Scholar] [CrossRef]
- Cranin, A.N.; Schnitman, P.A.; Rabkin, M.; Dennison, T.; Onesto, E.J. Alumina and zirconia coated vitallium oral endosteal implants in beagles. J. Biomed. Mater. Res. 1975, 9, 257–262. [Google Scholar] [CrossRef]
- Brum, R.S.; Monich, P.R.; Fredel, M.C.; Contri, G.; Ramoa, S.D.A.S.; Magini, R.S.; Benfatti, C.A.M. Polymer coatings based on sulfonated-poly-ether-ether-ketone films for implant dentistry applications. J. Mater. Sci. Mater. Med. 2018, 29, 132. [Google Scholar] [CrossRef]
- Natale, L.C.; Rodrigues, M.C.; Alania, Y.; Chiari, M.; Vilela, H.S.; Vieira, D.N.; Arana-Chavez, V.; Meier, M.M.; Vichi, F.M.; Braga, R.R. Development of calcium phosphate/ethylene glycol dimethacrylate particles for dental applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 107, 708–715. [Google Scholar] [CrossRef]
- Gautam, R.; Singh, R.D.; Sharma, V.P.; Siddhartha, R.; Chand, P.; Kumar, R. Biocompatibility of polymethylmethacrylate resins used in dentistry. J. Biomed. Mater. Res. Part B Appl. Biomater. 2012, 100, 1444–1450. [Google Scholar] [CrossRef]
- Rokaya, D.; Srimaneepong, V.; Sapkota, J.; Qin, J.; Siraleartmukul, K.; Siriwongrungson, V. Polymeric materials and films in dentistry: An overview. J. Adv. Res. 2018, 14, 25–34. [Google Scholar] [CrossRef]
- Hisbergues, M.; Vendeville, S.; Vendeville, P. Zirconia: Established facts and perspectives for a biomaterial in dental implantology. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 88, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Özkurt, Z.; Kazazoglu, E. Zirconia Dental Implants: A Literature Review. J. Oral Implant. 2011, 37, 367–376. [Google Scholar] [CrossRef]
- Sivaraman, K.; Chopra, A.; Narayan, A.I.; Balakrishnan, D. Is zirconia a viable alternative to titanium for oral implant? A critical review. J. Prosthodont. Res. 2018, 62, 121–133. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C 2019, 98, 1294–1305. [Google Scholar] [CrossRef]
- Al-Amleh, B.; Lyons, K.; Swain, M. Clinical trials in zirconia: A systematic review. J. Oral Rehabil. 2010, 37, 641–652. [Google Scholar] [CrossRef]
- Kolgeci, L.; Mericske, E.; Worni, A.; Walker, P.; Katsoulis, J.; Mericske-Stern, R. Technical complications and failures of zirconia-based prostheses supported by implants followed up to 7 years: A case series. Int. J. Prosthodont. 2014, 27, 544–552. [Google Scholar] [CrossRef]
- Osman, R.B.; Swain, M.V.; Atieh, M.; Ma, S.; Duncan, W. Ceramic implants (Y-TZP): Are they a viable alternative to titanium implants for the support of overdentures? A randomized clinical trial. Clin. Oral Implant. Res. 2013, 25, 1366–1377. [Google Scholar] [CrossRef]
- Nejatidanesh, F.; Abbasi, M.; Savabi, G.; Bonakdarchian, M.; Atash, R.; Savabi, O. Five year clinical outcomes of metal ceramic and zirconia-based implant-supported dental prostheses: A retrospective study. J. Dent. 2020, 100, 103420. [Google Scholar] [CrossRef] [PubMed]
- Zarone, F.; Di Mauro, M.I.; Spagnuolo, G.; Gherlone, E.; Sorrentino, R. Fourteen-year evaluation of posterior zirconia-based three-unit fixed dental prostheses. J. Dent. 2020, 101, 103419. [Google Scholar] [CrossRef] [PubMed]
- Chia, H.N.; Wu, B.M. Recent advances in 3D printing of biomaterials. J. Biol. Eng. 2015, 9, 1–14. [Google Scholar] [CrossRef]
- Hwa, L.C.; Rajoo, S.; Noor, A.M.; Ahmad, N.; Uday, M. Recent advances in 3D printing of porous ceramics: A review. Curr. Opin. Solid State Mater. Sci. 2017, 21, 323–347. [Google Scholar] [CrossRef]
- Juneja, M.; Thakur, N.; Kumar, D.; Gupta, A.; Bajwa, B.; Jindal, P. Accuracy in dental surgical guide fabrication using different 3-D printing techniques. Addit. Manuf. 2018, 22, 243–255. [Google Scholar] [CrossRef]
- Zhang, D.; Peng, E.; Borayek, R.; Ding, J. Controllable Ceramic Green-Body Configuration for Complex Ceramic Architectures with Fine Features. Adv. Funct. Mater. 2019, 29, 1–2. [Google Scholar] [CrossRef]
- Branco, A.C.; Silva, R.; Santos, T.; Jorge, H.; Rodrigues, A.; Fernandes, R.; Bandarra, S.; Barahona, I.; Matos, A.; Lorenz, K.; et al. Suitability of 3D printed pieces of nanocrystalline zirconia for dental applications. Dent. Mater. 2020, 36, 442–455. [Google Scholar] [CrossRef]
- Zhang, D.; Jonhson, W.; Herng, T.S.; Ang, Y.Q.; Yang, L.; Tan, S.C.; Peng, E.; He, H.; Ding, J. A 3D-printing method of fabrication for metals, ceramics, and multi-materials using a universal self-curable technique for robocasting. Mater. Horizons 2019, 7, 1083–1090. [Google Scholar] [CrossRef]
- Hodásová, L.; Sans, J.; Molina, B.G.; Alemán, C.; Llanes, L.; Fargas, G.; Armelin, E. Polymer infiltrated ceramic networks with biocompatible adhesive and 3D-printed highly porous scaffolds. Addit. Manuf. 2021, 39, 101850. [Google Scholar] [CrossRef]
- White, S.; Miklus, V.; McLaren, E.; Lang, L.; Caputo, A. Flexural strength of a layered zirconia and porcelain dental all-ceramic system. J. Prosthet. Dent. 2005, 94, 125–131. [Google Scholar] [CrossRef]
- Ziskind, D.; Hasday, M.; Cohen, S.; Wagner, H.D. Young’s modulus of peritubular and intertubular human dentin by nano-indentation tests. J. Struct. Biol. 2011, 174, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, X.-H.; Cui, B.-C.; Lin, Y.-H.; Deng, X.-L.; Li, M.; Nan, C.-W. Mechanical performance of polymer-infiltrated zirconia ceramics. J. Dent. 2017, 58, 60–66. [Google Scholar] [CrossRef]
- Wille, S.; Sieper, K.; Kern, M. Wear resistance of crowns made from different CAM/CAD materials. Dent. Mater. 2021, 37, e407–e413. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Zinelis, S.; Thomas, A.; Syres, K.; Silikas, N.; Eliades, G. Surface characterization of zirconia dental implants. Dent. Mater. 2010, 26, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Tabares, J.; Jiménez-Piqué, E.; Reyes-Gasga, J.; Anglada, M. Microstructural changes in ground 3Y-TZP and their effect on mechanical properties. Acta Mater. 2011, 59, 6670–6683. [Google Scholar] [CrossRef]
- Maridurai, T.; Balaji, D.; Sagadevan, S. Synthesis and Characterization of Yttrium Stabilized Zirconia Nanoparticles. Mater. Res. 2016, 19, 812–816. [Google Scholar] [CrossRef]
- Karmaker, A.C.; DiBenedetto, A.T.; Goldberg, A.J. Extent of conversion and its effect on the mechanical performance of Bis-GMA/PEGDMA-based resins and their composites with continuous glass fibres. J. Mater. Sci. Mater. Electron. 1997, 8, 369–374. [Google Scholar] [CrossRef]
- Teotia, M.; Chauhan, M.; Choudhary, P.; Soni, R.K. Photocured characteristics of fast photocurable acrylic formulations and investigations by differential photo calorimeter. J. Therm. Anal. Calorim. 2018, 137, 133–141. [Google Scholar] [CrossRef]
- Lin, C.-H.; Lin, Y.-M.; Lai, Y.-L.; Lee, S.-Y. Mechanical properties, accuracy, and cytotoxicity of UV-polymerized 3D printing resins composed of Bis-EMA, UDMA, and TEGDMA. J. Prosthet. Dent. 2019, 123, 349–354. [Google Scholar] [CrossRef]
- Coldea, A.; Swain, M.; Thiel, N. Mechanical properties of polymer-infiltrated-ceramic-network materials. Dent. Mater. 2013, 29, 419–426. [Google Scholar] [CrossRef]
- Josset, Y.; Oum’Hamed, Z.; Zarrinpour, A.; Lorenzato, M.; Adnet, J.J.; Laurent-Maquin, D. In vitro reactions of human osteoblasts in culture with zirconia and alumina ceramics. J. Biomed. Mater. Res. 1999, 47, 481–493. [Google Scholar] [CrossRef]
- Carinci, F.; Pezzetti, F.; Volinia, S.; Francioso, F.; Arcelli, D.; Farina, E.; Piattelli, A. Zirconium oxide: Analysis of MG63 osteoblast-like cell response by means of a microarray technology. Biomaterials 2003, 25, 215–228. [Google Scholar] [CrossRef]
- Tonda-Turo, C.; Gentile, P.; Saracino, S.; Chiono, V.; Nandagiri, V.; Muzio, G.; Canuto, R.; Ciardelli, G. Comparative analysis of gelatin scaffolds crosslinked by genipin and silane coupling agent. Int. J. Biol. Macromol. 2011, 49, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Ren, S.; Xu, X.; Wen, H.; Fang, K.; Song, W.; Wang, L.; Jia, S. Immobilization of chitosan film containing semaphorin 3A onto a microarc oxidized titanium implant surface via silane reaction to improve MG63 osteogenic differentiation. Int. J. Nanomed. 2014, 9, 4649–4657. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hodásová, L.; Quintana, R.; Czuba, U.; del Valle, L.J.; Fargas, G.; Alemán, C.; Armelin, E. Atmospheric pressure plasma liquid assisted deposition of polydopamine/acrylate copolymer on zirconia (Y-TZP) ceramics: A biocompatible and adherent nanofilm. RSC Adv. 2021, 11, 17360–17368. [Google Scholar] [CrossRef]

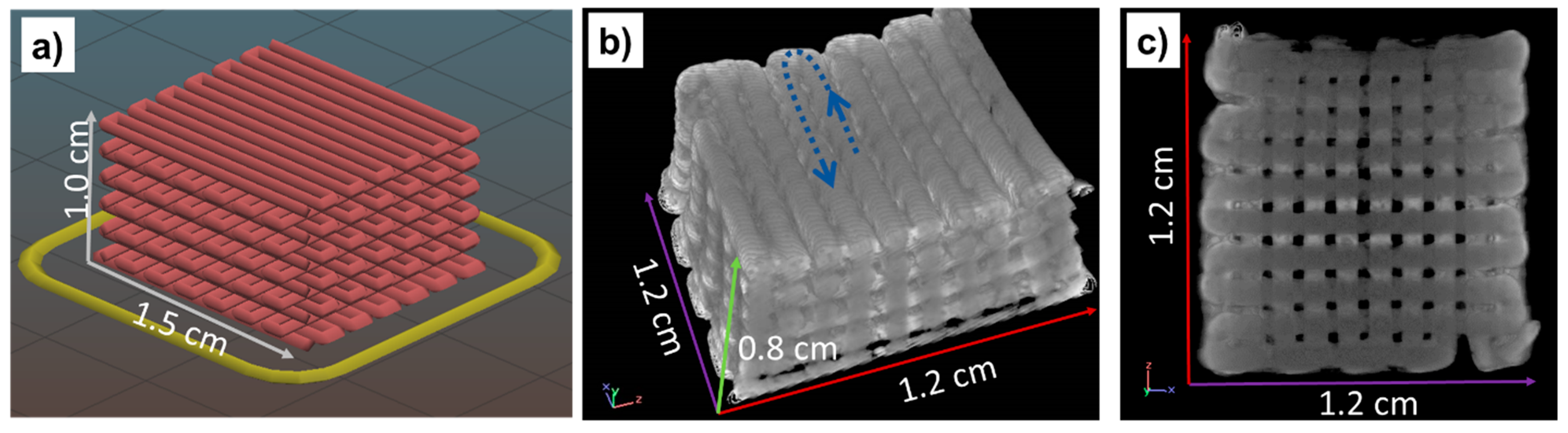


| Sample | Phase | 2θ | Cos (θ) | B (rad) | t (nm) |
|---|---|---|---|---|---|
| 3Y-TZP powder | Monoclinic | 28 | 0.970 | 0.005 | 28 |
| Tetragonal | 30 | 0.966 | 0.004 | 35 | |
| 3D-printed | Monoclinic | - | - | - | - |
| Tetragonal | 30 | 0.966 | 0.003 | 51 |
| Sample | σmax (MPa) | σbreak (MPa) | εcompression 2 (%) |
|---|---|---|---|
| 3D-printed zirconia 50% infill | 86 | 51 | 4.1 |
| 3D-printed zirconia 100% infill | 124 | 124 | 5.2 |
| PICN | 102 | 102 | 5.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hodásová, Ľ.; Alemán, C.; del Valle, L.J.; Llanes, L.; Fargas, G.; Armelin, E. 3D-Printed Polymer-Infiltrated Ceramic Network with Biocompatible Adhesive to Potentiate Dental Implant Applications. Materials 2021, 14, 5513. https://doi.org/10.3390/ma14195513
Hodásová Ľ, Alemán C, del Valle LJ, Llanes L, Fargas G, Armelin E. 3D-Printed Polymer-Infiltrated Ceramic Network with Biocompatible Adhesive to Potentiate Dental Implant Applications. Materials. 2021; 14(19):5513. https://doi.org/10.3390/ma14195513
Chicago/Turabian StyleHodásová, Ľudmila, Carlos Alemán, Luís J. del Valle, Luis Llanes, Gemma Fargas, and Elaine Armelin. 2021. "3D-Printed Polymer-Infiltrated Ceramic Network with Biocompatible Adhesive to Potentiate Dental Implant Applications" Materials 14, no. 19: 5513. https://doi.org/10.3390/ma14195513
APA StyleHodásová, Ľ., Alemán, C., del Valle, L. J., Llanes, L., Fargas, G., & Armelin, E. (2021). 3D-Printed Polymer-Infiltrated Ceramic Network with Biocompatible Adhesive to Potentiate Dental Implant Applications. Materials, 14(19), 5513. https://doi.org/10.3390/ma14195513









