Analysis of the Structural Aspects of Tannin-Based Adhesives by 2D-NMR
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Apparatus and Procedure for Recording NMR Spectra
3. Results
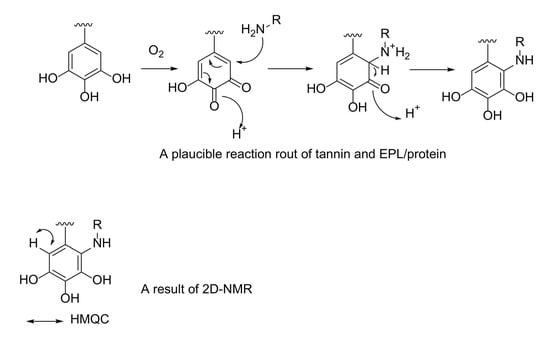
3.1. 2D-NMR Analysis of Simplified Model Compounds
3.2. 2D-NMR, HMQC, Analysis of Gelatin-Tannin C and EPL-Tannin C Complex
3.3. 2D-NMR, HMBC, Analysis of EPL-Tannic Acid Complex
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omura, S.; Kawazoe, Y.; Uemura, D. Development of a novel adhesive composed of all-natural components. Int. J. Adhes. Adhes. 2017, 74, 35–39. [Google Scholar] [CrossRef]
- Shirmohammadli, Y.; Efhamisisi, D.; Pizzi, A. Tannins as a sustainable raw material for green chemistry: A review. Ind. Crop. Prod. 2018, 126, 316–332. [Google Scholar] [CrossRef]
- Zhao, H.; Waite, J.H. Coating proteins: Structure and cross-linking in fp-1 from the green shell mussel Perna canaliculus. Biochemistry 2005, 44, 15915–15923. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Pizzi, A.; Li, J.; Zhang, W. MALDI-TOF MS analysis of the acceleration of the curing of phenol-formaldehyde (PF) resins induced by propylene carbonate. Eur. J. Wood Wood Prod. 2015, 73, 135–138. [Google Scholar] [CrossRef]
- Ping, L.; Pizzi, A.; Guo, Z.D.; Brosse, N. Condensed tannins from grape pomace: Characterization by FTIR and MALDI TOF and production of environment friendly wood adhesive. Ind. Crop. Prod. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- Vázquez, G.; Pizzi, A.; Freire, M.S.; Santos, J.; Antorrena, G.; González-Álvarez, J. MALDI-TOF, HPLC-ESI-TOF and 13C-NMR characterization of chestnut (Castanea sativa) shell tannins for wood adhesives. Wood Sci. Technol. 2012, 47, 523–535. [Google Scholar] [CrossRef]
- Liang, Z.; Zhihua, S. In situ spectroscopic study on the effect of UV radiation on stabilized collagen fiber: Role of plant tannin. J. Soc. Leather Technol. Chem. 2012, 96, 210–215. [Google Scholar]
- Zhao, Y.; Yan, N.; Feng, M. Characterization of phenol-formaldehyde resins derived from liquefied lodgepole pine barks. Int. J. Adhes. Adhes. 2010, 30, 689–695. [Google Scholar] [CrossRef]
- Vázquez, G.; Santos, J.; Freire, M.S.; Antorrena, G.; González-Álvarez, J. DSC and DMA study of chestnut shell tannins for their application as wood adhesives without formaldehyde emission. J. Therm. Anal. Calorim. 2012, 108, 605–611. [Google Scholar] [CrossRef]
- Moubarik, A.; Charrier, B.; Allal, A.; Charrier, F.; Pizzi, A. Development and optimization of a new formaldehyde-free cornstarch and tannin wood adhesive. Eur. J. Wood Wood Prod. 2009, 68, 167–177. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, J.; Yi, Z.; Yang, H.; Zhao, B.; Zhang, W.; Li, J. Preparation and characterization of a novel environmentally friendly phenol-formaldehyde adhesive modified with tannin and urea. Int. J. Adhes. Adhes. 2016, 66, 26–32. [Google Scholar] [CrossRef]
- Silber, M.L.; Davitt, B.B.; Khairutdinov, R.F.; Hurst, J.K. A Mathematical Model Describing Tannin-Protein Association. Anal. Biochem. 1998, 263, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, A. Tannery row—The story of some natural and synthetic wood adhesives. Wood Sci. Technol. 2000, 34, 277–316. [Google Scholar] [CrossRef]
- Li, K.; Geng, X.; Simonsen, J.; Karchesy, J. Novel wood adhesives from condensed tannins and polyethylenimine. Int. J. Adhes. Adhes. 2004, 24, 327–333. [Google Scholar] [CrossRef]
- Zhao, Z.; Umemura, K. Investigation of a new natural particleboard adhesive composed of tannin and sucrose. J. Wood Sci. 2014, 60, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Do, M.D.; Casey, P.; Sulistio, A.; Qiao, G.G.; Lundin, L.; Lillford, P.; Kosaraju, S. Chemical Cross-Linking Gelatin with Natural Phenolic Compounds as Studied by High-Resolution NMR Spectroscopy. Biomacromolecules 2010, 11, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Hoong, Y.B.; Pizzi, A.; Tahir, P.M.; Pasch, H. Characterization of Acacia mangium polyflavonoid tannins by MALDI-TOF mass spectrometry and CP-MAS 13C NMR. Eur. Polym. J. 2010, 46, 1268–1277. [Google Scholar] [CrossRef]
- Pizzi, A. Tannin-Based Biofoams—A Review. J. Renew. Mater. 2019, 7, 474–489. [Google Scholar] [CrossRef] [Green Version]
- Roux, D.G.; Ferreira, D.; Botha, J.J. Structural considerations in predicting the utilization of tannins. J. Agric. Food Chem. 1980, 28, 216–222. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omura, S.; Kawazoe, Y.; Uemura, D. Analysis of the Structural Aspects of Tannin-Based Adhesives by 2D-NMR. Materials 2021, 14, 5479. https://doi.org/10.3390/ma14195479
Omura S, Kawazoe Y, Uemura D. Analysis of the Structural Aspects of Tannin-Based Adhesives by 2D-NMR. Materials. 2021; 14(19):5479. https://doi.org/10.3390/ma14195479
Chicago/Turabian StyleOmura, Sachikazu, Yoshinori Kawazoe, and Daisuke Uemura. 2021. "Analysis of the Structural Aspects of Tannin-Based Adhesives by 2D-NMR" Materials 14, no. 19: 5479. https://doi.org/10.3390/ma14195479
APA StyleOmura, S., Kawazoe, Y., & Uemura, D. (2021). Analysis of the Structural Aspects of Tannin-Based Adhesives by 2D-NMR. Materials, 14(19), 5479. https://doi.org/10.3390/ma14195479