Preparation and Characterization of High-Strength Glass-Ceramics via Ion-Exchange Method
Abstract
:1. Introduction
2. Experiment Procedures
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
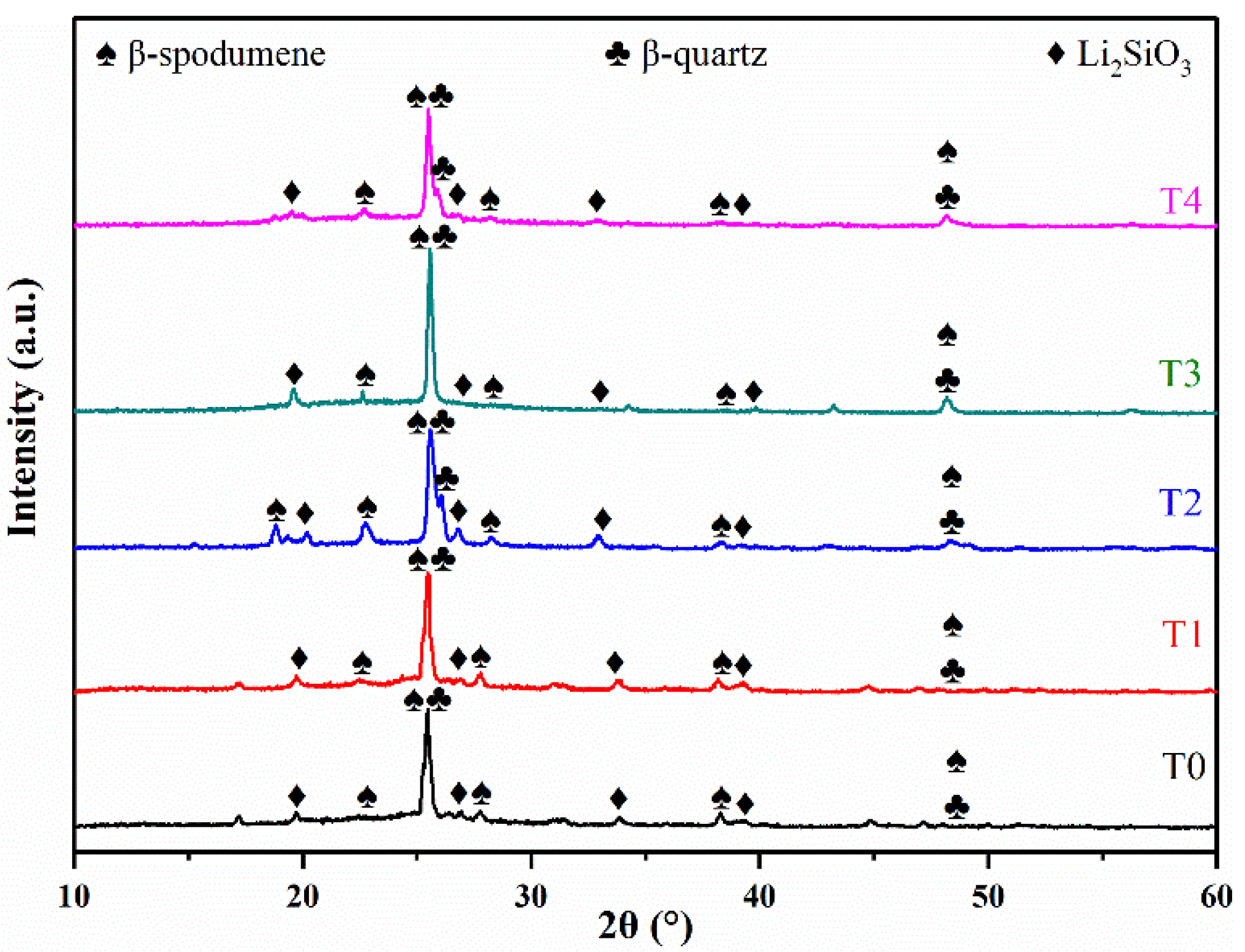
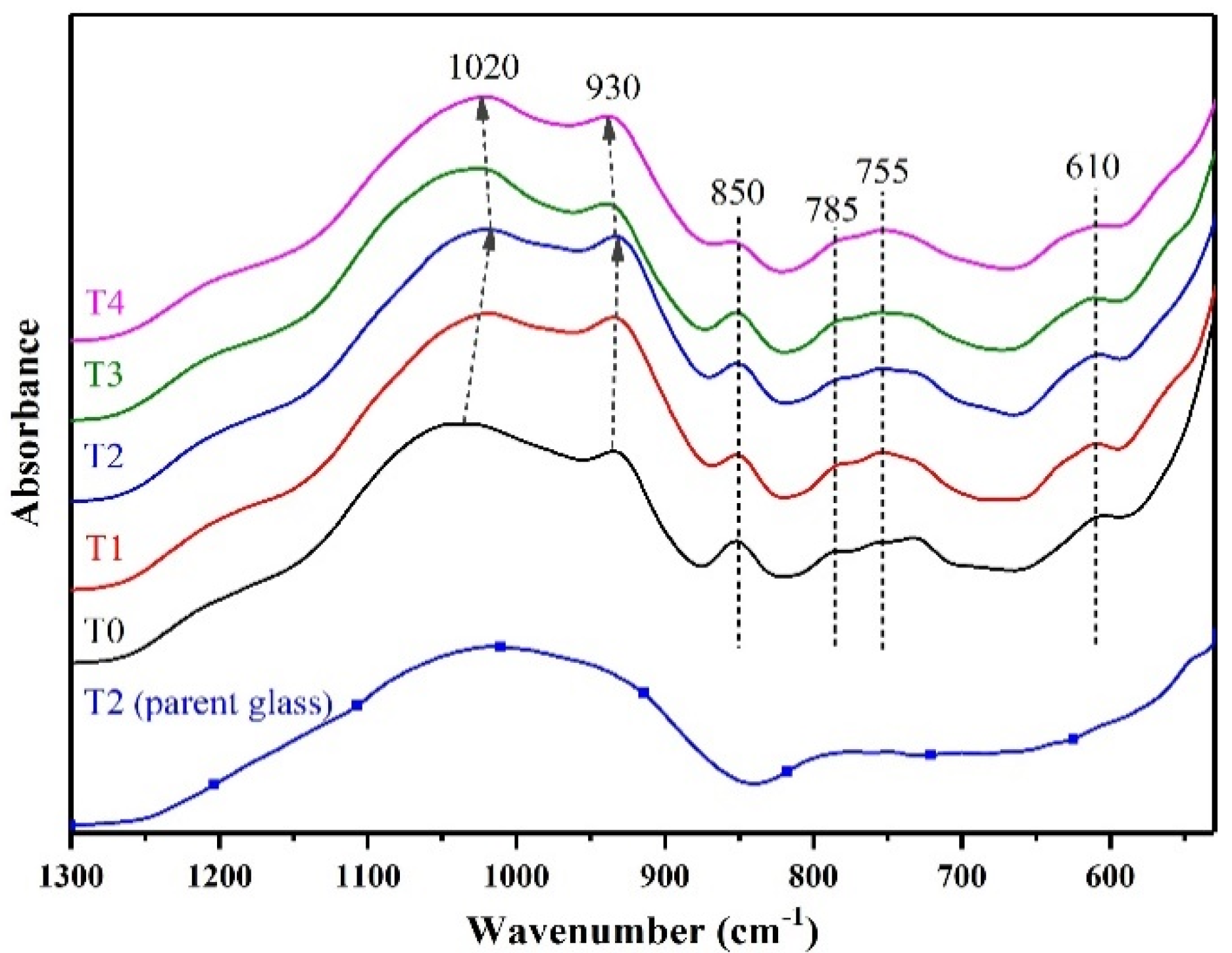
3.1. Effect of TiO2/(TiO2 + ZrO2) Ratio on Properties of LAS GCs
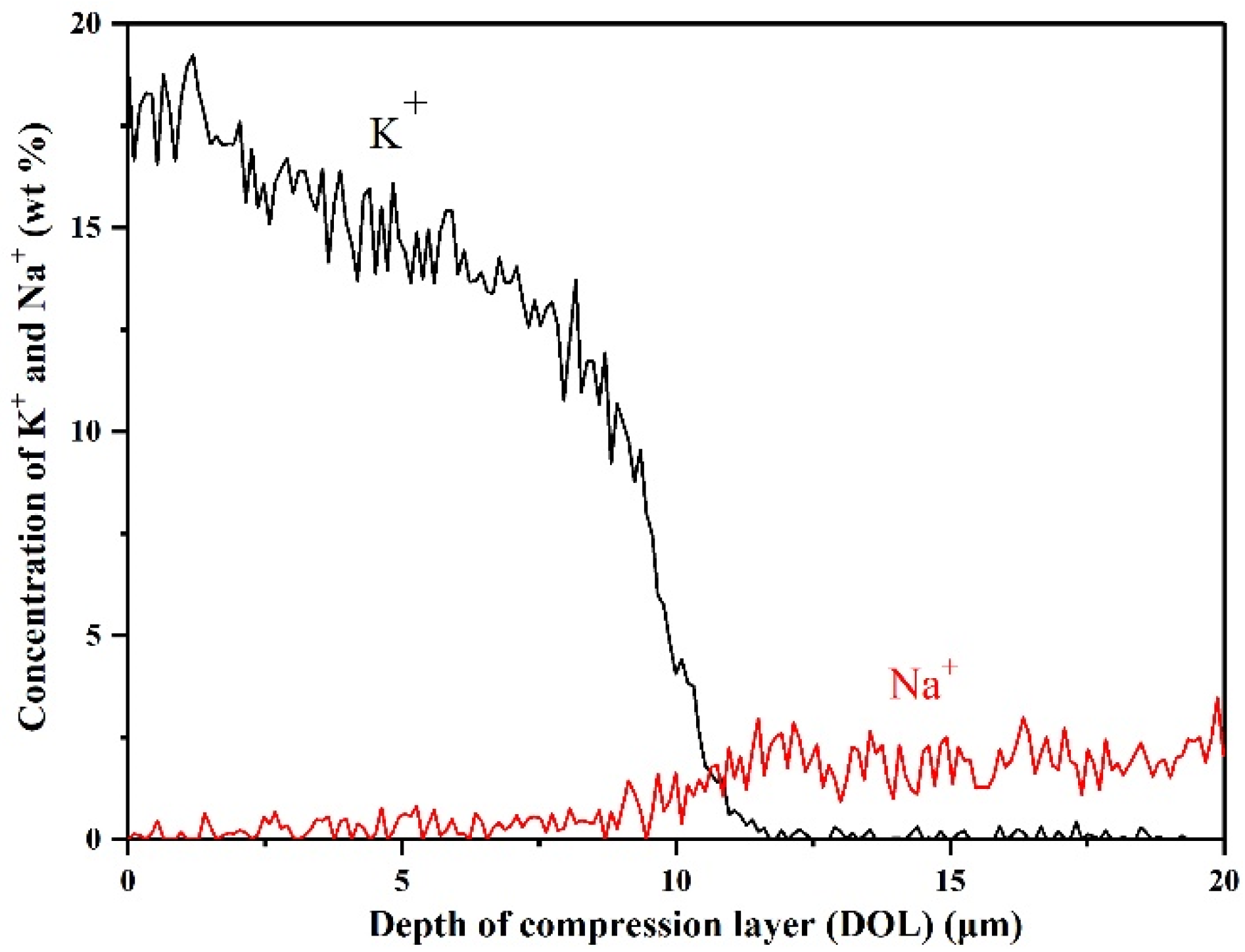
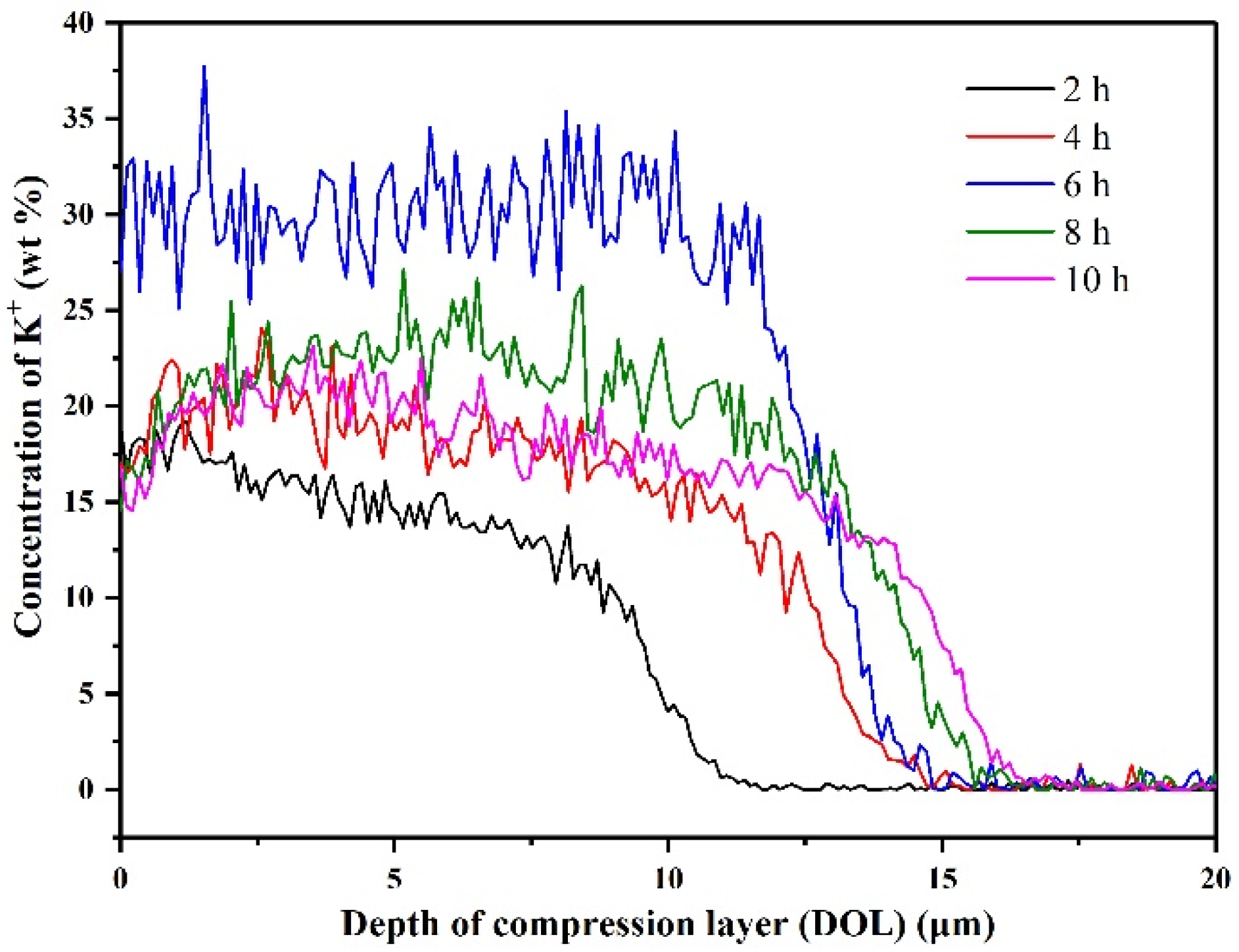
3.2. Ion-Exchange Strengthening Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Soares, V.O.; Serbena, F.C.; Oliveira, G.D.; da Cruz, C.; Muniz, R.F.; Zanotto, E.D. Highly translucent nanostructured glass-ceramic. Ceram. Int. 2021, 47, 4707–4714. [Google Scholar] [CrossRef]
- Deubener, J.; Allix, M.; Davis, M.J.; Duran, A.; Höche, T.; Honma, T.; Komatsu, T.; Krüger, S.; Mitra, I.; Müller, R.; et al. Updated definition of glass-ceramics. J. Non-Cryst. Solids 2018, 501, 3–10. [Google Scholar] [CrossRef]
- Zanotto, E.D. A bright future for glass-ceramics. Am. Ceram. Soc. Bull. 2010, 89, 19–27. [Google Scholar]
- Ritzberger, C.; Apel, E.; Holand, W.; Peschke, A.; Rheinberger, V.M. Properties and Clinical Application of Three Types of Dental Glass-Ceramics and Ceramics for CAD-CAM Technologies. Materials 2010, 3, 3700–3713. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.A.F.E.; Lai, B.C.; Hsu, Y.F.; Lu, C.A. Dielectric properties of CaO-B2O3-SiO2 glass-ceramic systems in the millimeter-wave frequency range of 20–60 GHz. Ceram. Int. 2021, 47, 22627–22635. [Google Scholar] [CrossRef]
- Guo, Y.L.; Liu, C.; Wang, J.; Ruan, J.; Li, X.Y.; Han, J.J.; Xie, J. Effect of ZrO2 crystallization on ion exchange properties in aluminosilicate glass. J. Eur. Ceram. Soc. 2020, 40, 2179–2184. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, J.S.; Tang, L.Y.; Tian, P.J. Effect of nucleating agents and heat treatments on the crystallization of magnesium aluminosilicate transparent glass-ceramics. J. Wuhan Univ. Technol. 2013, 28, 69–72. [Google Scholar] [CrossRef]
- Hu, A.M.; Li, M.; Mao, D.L. Controlled crystallization of glass-ceramics with two nucleating agents. Mater. Charact. 2009, 60, 1529–1533. [Google Scholar] [CrossRef]
- Dressler, M.; Rudinger, B.; Deubener, J. Crystallization kinetics in a lithium alumosilicate glass using SnO2 and ZrO2 additives. J. Non-Cryst. Solids 2014, 389, 60–65. [Google Scholar] [CrossRef]
- Lilensten, L.; Fu, Q.; Wheaton, B.R.; Credle, A.J.; Stewart, R.L.; Kohli, J.T. Kinetic study on lithium-aluminosilicate (LAS) glass-ceramics containing MgO and ZnO. Ceram. Int. 2014, 40, 11657–11661. [Google Scholar] [CrossRef]
- Dressler, M.; Rudinger, B.; Deubener, J. An In Situ High-Temperature X-Ray Diffraction Study of Early-Stage Crystallization in Lithium Alumosilicate Glass-Ceramics. J. Am. Ceram. Soc. 2011, 94, 1421–1426. [Google Scholar] [CrossRef]
- Deng, B.; Luo, J.; Harris, J.T.; Smith, C.M. Critical stress map for ZrO2 tetragonal to monoclinic phase transformation in ZrO2-toughened glass-ceramics. Materialia 2020, 9, 100548. [Google Scholar] [CrossRef]
- Deng, B.; Harris, J.T.; Luo, J. Atomic picture of crack propagation in Li2O-2SiO2 glass-ceramics revealed by molecular dynamics simulations. J. Am. Ceram. Soc. 2020, 103, 4304–4312. [Google Scholar] [CrossRef]
- Maeda, K.; Iwasaki, K.; Urata, S.; Akatsuka, K.; Yasumori, A. 3D microstructure and crack pathways of toughened CaO-Al2O3-SiO2 glass by precipitation of hexagonal CaAl2Si2O8 crystal. J. Am. Ceram. Soc. 2019, 102, 5535–5544. [Google Scholar] [CrossRef]
- Deng, B.; Luo, J.; Harris, J.T.; Smith, C.M.; McKenzie, M.E. Molecular dynamics simulations on fracture toughness of Al2O3-SiO2 glass-ceramics. Scr. Mater. 2019, 162, 277–280. [Google Scholar] [CrossRef]
- Serbena, F.C.; Mathias, I.; Foerster, C.E.; Zanotto, E.D. Crystallization toughening of a model glass-ceramic. Acta Mater. 2015, 86, 216–228. [Google Scholar] [CrossRef]
- Li, D.; Guo, J.W.; Wang, X.S.; Zhang, S.F.; He, L. Effects of crystal size on the mechanical properties of a lithium disilicate glass-ceramic. Mater. Sci. Eng. A 2016, 669, 332–339. [Google Scholar] [CrossRef]
- Beall, G.H.; Comte, M.; Dejneka, M.J.; Marques, P.; Pradeau, P.; Smith, C. Ion-Exchange in Glass-Ceramics. Front. Mater. 2016, 3, 41. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wang, J.; Ruan, J.; Han, J.; Xie, J.; Liu, C. Microstructure and ion-exchange properties of glass-ceramics containing ZnAl2O4 and β-quartz solid solution nanocrystals. J. Eur. Ceram. Soc. 2021, 41, 5331–5340. [Google Scholar] [CrossRef]
- Laczka, K.; Cholewa-Kowalska, K.; Sroda, M.; Rysz, J.; Marzec, M.M.; Laczka, M. Glass-ceramics of LAS (Li2O-Al2O3-SiO2) system enhanced by ion-exchange in KNO3 salt bath. J. Non-Cryst. Solids 2015, 428, 90–97. [Google Scholar] [CrossRef]
- Torres-Castanon, J.J.; Gorokhovskii, A.V.; Zhabrev, V.A.; Fuentes, A.F.; Escalante-Garcia, J.I.; German, E.V. Kinetics and mechanism of the interaction of alkali calcium silicate glasses with salt melts in the KNO3-Pb(NO3)2 system. Glass Phys. Chem. 2004, 30, 167–172. [Google Scholar] [CrossRef]
- Li, X.Y.; Jiang, L.B.; Wang, Y.; Mohagheghian, I.; Dear, J.P.; Li, L.; Yan, Y. Correlation between K+-Na+ diffusion coefficient and flexural strength of chemically tempered aluminosilicate glass. J. Non-Cryst. Solids 2017, 471, 72–81. [Google Scholar] [CrossRef]
- Karapetyan, G.O.; Loboda, V.V.; Tagantsev, D.K. Influence of ion exchange on liquid-liquid phase separation in alkali borosilicate glasses: Effect of ion-exchange-induced metastable glass homogenization. J. Non-Cryst. Solids 2000, 270, 154–162. [Google Scholar] [CrossRef]
- Jiang, L.B.; Guo, X.T.; Li, X.Y.; Li, L.; Zhang, G.L.; Yan, Y. Different K+-Na+ inter-diffusion kinetics between the air side and tin side of an ion-exchanged float aluminosilicate glass. Appl. Surf. Sci. 2013, 265, 889–894. [Google Scholar] [CrossRef]
- Mauro, J.C.; Yue, Y.; Ellison, A.J.; Gupta, P.K.; Allan, D.C. Viscosity of Glass-Forming Liquids. Proc. Natl. Acad. Sci. USA 2009, 106, 19780–19784. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Q.J.; Zhang, Y.F.; Montazerian, M.; Gulbiten, O.; Mauro, J.C.; Zanotto, E.D.; Yue, Y.Z. Understanding Glass through Differential Scanning Calorimetry. Chem. Rev. 2019, 119, 7848–7939. [Google Scholar] [CrossRef]
- Daguano, J.K.M.E.; Strecker, K.; Ziemath, E.C.; Rogero, S.O.; Fernandes, M.H.V.; Santos, C. Effect of partial crystallization on the mechanical properties and cytotoxicity of bioactive glass from the 3CaO center dot P2O5-SiO2-MgO system. J. Mech. Behav. Biomed. 2012, 14, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Kleebusch, E.; Patzig, C.; Höche, T.; Rüssel, C. Effect of the concentrations of nucleating agents ZrO2 and TiO2 on the crystallization of Li2O-Al2O3-SiO2 glass: An X-ray diffraction and TEM investigation. J. Mater. Sci. 2016, 51, 10127–10138. [Google Scholar] [CrossRef]
- Wu, J.Q.; Lin, C.W.; Liu, J.L.; Han, L.; Gui, H.; Li, C.; Liu, T.Y.; Lu, A.X. The effect of complex nucleating agent on the crystallization, phase formation and performances in lithium aluminum silicate (LAS) glasses. J. Non-Cryst. Solids 2019, 521, 119486. [Google Scholar] [CrossRef]
- Arvind, A.; Kumar, R.; Deo, M.N.; Shrikhande, V.K.; Kothiyal, G.P. Preparation, structural and thermo-mechanical properties of lithium aluminum silicate glass-ceramics. Ceram. Int. 2009, 35, 1661–1666. [Google Scholar] [CrossRef]
- Hou, Z.X.; Zhang, Y.M.; Zhang, H.S.; Zhang, H.B.; Shao, J.; Su, C.H. Study on crystallization and microstructure of Li2O-Al2O3-SiO2 glass ceramics. J. Univ. Sci. Technol. B 2006, 13, 564–569. [Google Scholar] [CrossRef]
- Liu, T.; Li, C.; Huang, Q.; Liu, C.; Lin, C.; Zhang, Q.; Luo, Z.; Zhu, L.; Lu, A. Characterization of structure and properties of MgO-Al2O3-SiO2-B2O3-Cr2O3 glass-ceramics. J. Non-Cryst. Solids 2020, 543, 120154. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Q.; Luo, L.; Yan, T.; Liu, J.; Ding, L.; Jiang, W. Effects of high-temperature treatment and iron reduction index on tensile strength of basalt continuous fiber. J. Non-Cryst. Solids 2021, 564, 120836. [Google Scholar] [CrossRef]
- Pisciella, P.; Pelino, M. FTIR spectroscopy investigation of the crystallisation process in an iron rich glass. J. Eur. Ceram. Soc. 2005, 25, 1855–1861. [Google Scholar] [CrossRef]
- Gy, R. Ion exchange for glass strengthening. Mater. Sci. Eng. B-Adv. 2008, 149, 159–165. [Google Scholar] [CrossRef]
- Li, X.C.; Meng, M.; Li, D.; Wei, R.; He, L.; Zhang, S.F. Strengthening and toughening of a multi-component lithium disilicate glass-ceramic by ion-exchange. J. Eur. Ceram. Soc. 2020, 40, 4635–4646. [Google Scholar] [CrossRef]
- Seaman, J.H.; Lezzi, P.J.; Blanchet, T.A.; Tomozawa, M. Degradation of ion-exchange strengthened glasses due to surface stress relaxation. J. Non-Cryst. Solids 2014, 403, 113–123. [Google Scholar] [CrossRef]


| Sample | SiO2 | Li2O | Al2O3 | Na2O | TiO2 | ZrO2 | Others | HV (Kg/mm−2) | Flexural Strength (MPa) | CI% | Transparency |
|---|---|---|---|---|---|---|---|---|---|---|---|
| T0 | 70 | 12.4 | 7.4 | 2.8 | 0 | 4 | 3.4 | 520 ± 21 | 86 ± 15 | 47 | Transparent |
| T1 | 70 | 12.4 | 7.4 | 2.8 | 1 | 3 | 3.4 | 528 ± 18 | 101 ± 11 | 54 | Translucent |
| T2 | 70 | 12.4 | 7.4 | 2.8 | 2 | 2 | 3.4 | 525 ± 17 | 109 ± 17 | 64 | Opaque |
| T3 | 70 | 12.4 | 7.4 | 2.8 | 3 | 1 | 3.4 | 503 ± 18 | 96 ± 16 | 45 | Opaque |
| T4 | 70 | 12.4 | 7.4 | 2.8 | 4 | 0 | 3.4 | 490 ± 25 | 81 ± 14 | 51 | Opaque |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Wang, H.; Zhu, J.; Zheng, Q.; Ding, L.; Jiang, W. Preparation and Characterization of High-Strength Glass-Ceramics via Ion-Exchange Method. Materials 2021, 14, 5477. https://doi.org/10.3390/ma14195477
Lu J, Wang H, Zhu J, Zheng Q, Ding L, Jiang W. Preparation and Characterization of High-Strength Glass-Ceramics via Ion-Exchange Method. Materials. 2021; 14(19):5477. https://doi.org/10.3390/ma14195477
Chicago/Turabian StyleLu, Jianwei, Haifeng Wang, Juanjuan Zhu, Qiuju Zheng, Linfeng Ding, and Weizhong Jiang. 2021. "Preparation and Characterization of High-Strength Glass-Ceramics via Ion-Exchange Method" Materials 14, no. 19: 5477. https://doi.org/10.3390/ma14195477
APA StyleLu, J., Wang, H., Zhu, J., Zheng, Q., Ding, L., & Jiang, W. (2021). Preparation and Characterization of High-Strength Glass-Ceramics via Ion-Exchange Method. Materials, 14(19), 5477. https://doi.org/10.3390/ma14195477






