Chemical and Structural Changes by Gold Addition Using Recharge Method in NiW/Al2O3-CeO2-TiO2 Nanomaterials
Abstract
1. Introduction
2. Materials and Methods
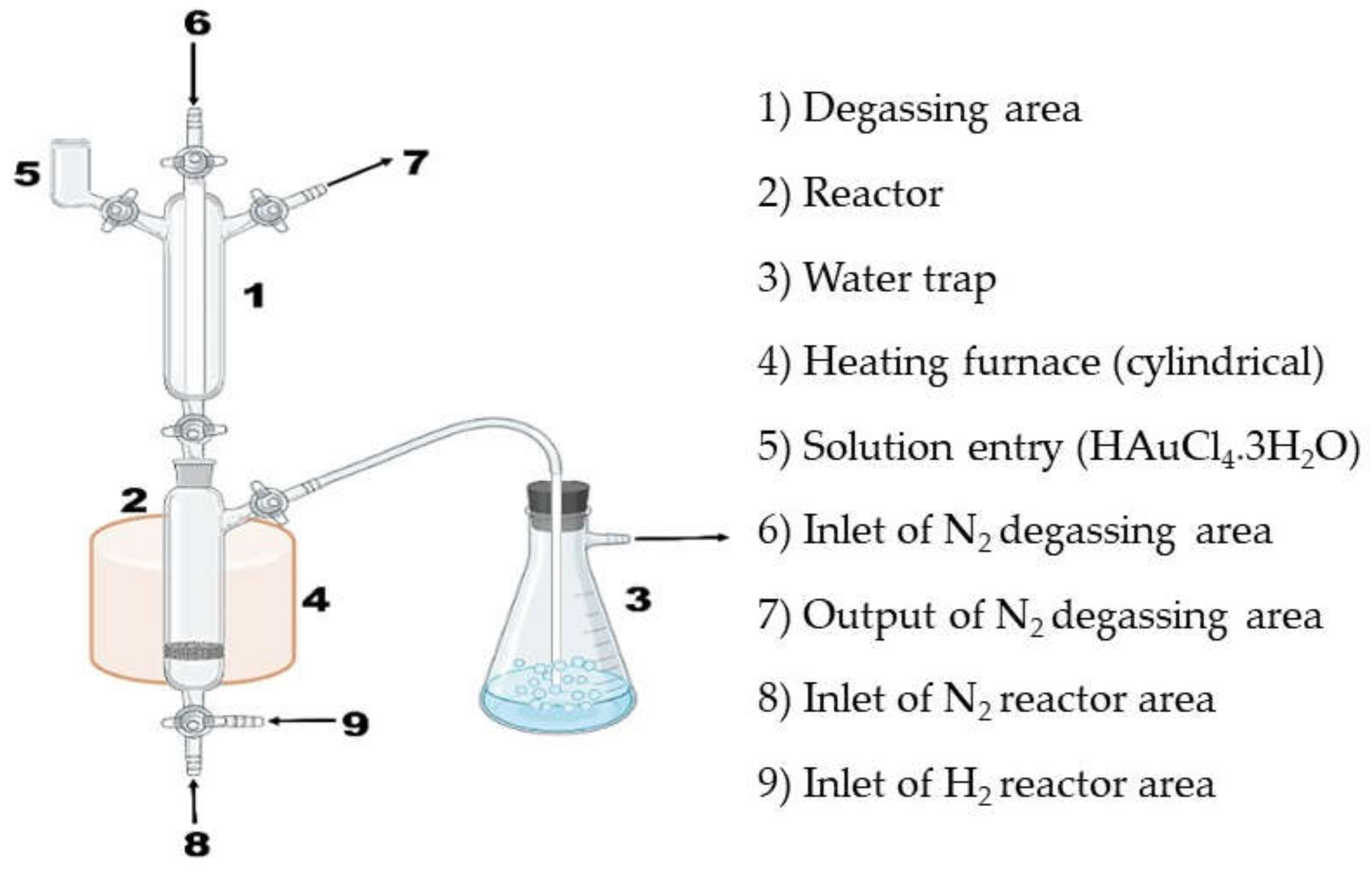
2.1. Materials Preparation
2.2. Materials Characterization
2.2.1. X-ray Diffraction
2.2.2. UV-Vis Diffuse Reflectance Spectroscopy (UV-Vis DRS)
2.2.3. BET Specific Surface Area (SSA)
2.2.4. Scanning Electron Microscopy (SEM)
2.2.5. Transmission Electron Microscopy (TEM)
2.2.6. Temperature-Programmed Desorption of Hydrogen (TPD-H2)
3. Results and Discussion
3.1. Materials Characterization
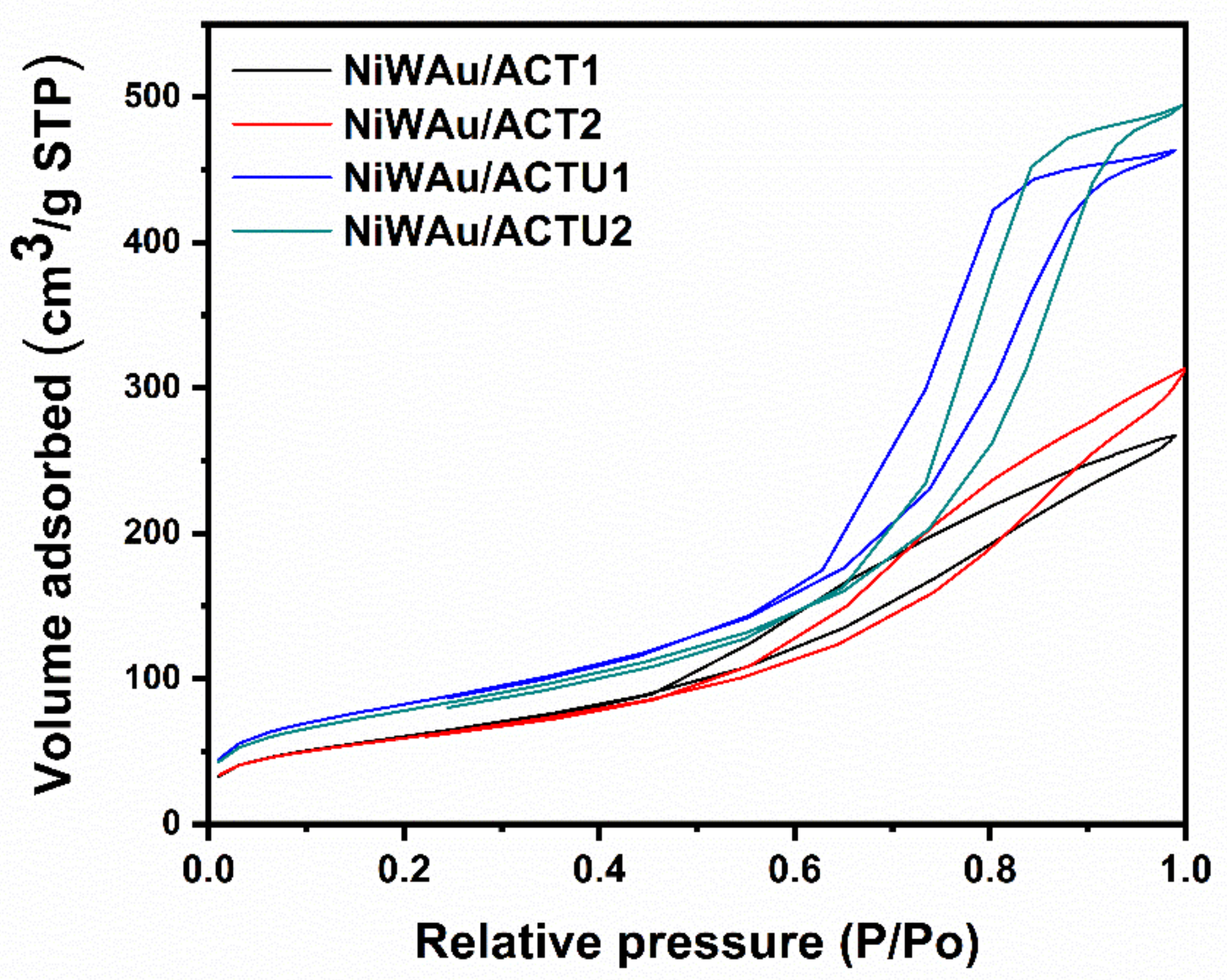
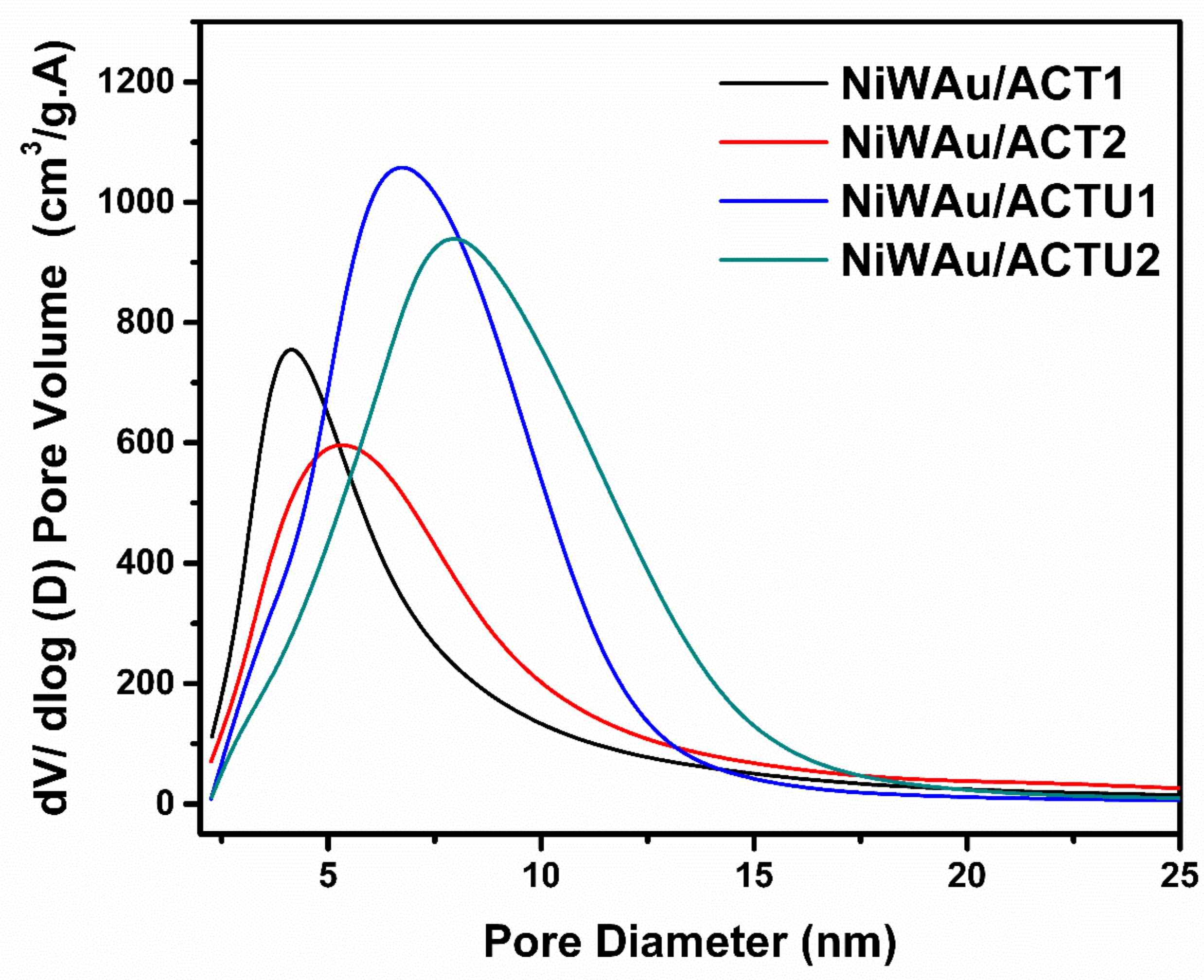
3.1.1. BET Specific Surface Area (SSA)
3.1.2. X-ray Diffraction (XRD)
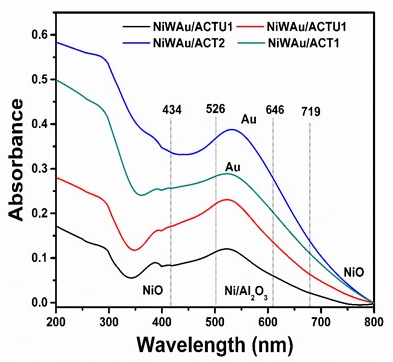
3.1.3. UV-Vis with Diffuse Reflectance of Solids (UV-Vis DRS)
3.1.4. Scanning Electron Microscopy (SEM)
3.1.5. Transmission Electron Microscopy (TEM)
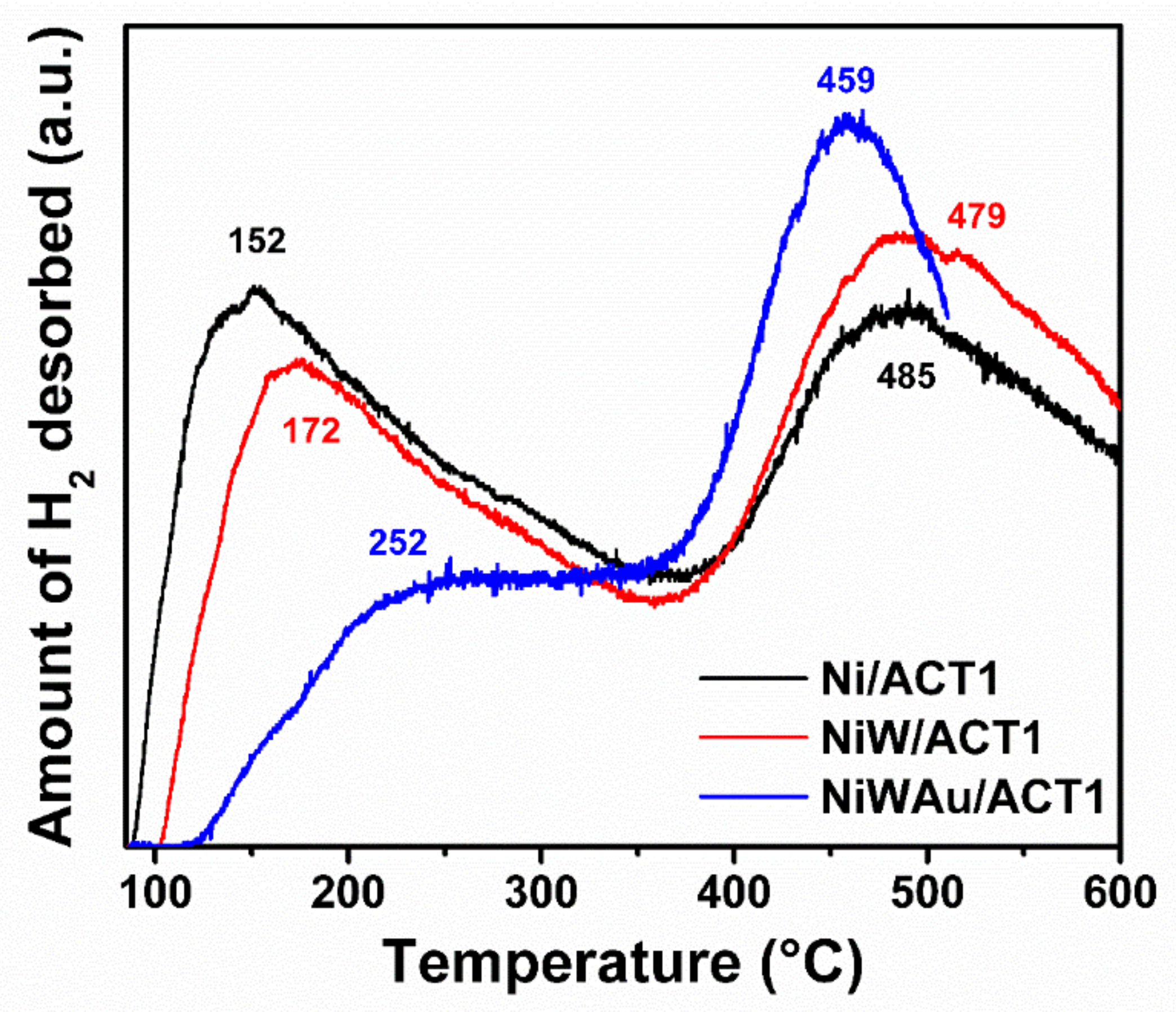
3.1.6. Temperature-Programmed Desorption of Hydrogen (TPD-H2)
3.1.7. Structural and Catalytic Properties of Nanomaterials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jia, C.J.; Schüth, F. Colloidal metal nanoparticles as a component of designed catalyst. Phys. Chem. Chem. Phys. 2011, 13, 2457–2487. [Google Scholar] [CrossRef]
- Khalil, M.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Advanced nanomaterials in oil and gas industry: Design, application and challenges. Appl. Energy 2017, 191, 287–310. [Google Scholar] [CrossRef]
- Mazzieri, V.A.; Grau, J.M.; Vera, C.R.; Yori, J.C.; Parera, J.M.; Pieck, C.L. Pt-Re-Sn/Al2O3 trimetallic catalysts for naphtha reforming processes without presulfiding step. Appl. Catal. A Gen. 2005, 296, 216–221. [Google Scholar] [CrossRef]
- Dong, X.; Zheng, P.; Zheng, A.-G.; Li, H.-F.; Xia, G.-F.; Li, M.-F.; Zheng, R.-Y.; Xu, B.-Q. Noble-metal efficient Pt-Ir-Co/SiO2 catalyst for selective hydrogenolytic ring opening of methylcyclopentane. Catal. Today 2018, 316, 162–170. [Google Scholar] [CrossRef]
- Zhang, H.; Pei, A.; Liao, J.; Ruan, L.; Yang, K.; Wang, J.; Zhu, L.; Chen, B.H. PtRuNi/C novel nanostructures of platinum-ruthenium island-on-Ni/Ni(OH)2 nanoparticles for the selective hydrogenation of quinoline. J. Alloys Compd. 2020, 834, 155203. [Google Scholar] [CrossRef]
- Yurderi, M.; Bulut, A.; Zahmakiran, M.; Kaya, M. Carbon supported trimetallic PdNiAg nanoparticles as highly active, selective and reusable catalyst in the formic acid decomposition. Appl. Catal. B Environ. 2014, 160–161, 514–524. [Google Scholar] [CrossRef]
- Cunha, E.M.; Ribeiro, J.; Kokoh, K.B.; De Andrade, A.R. Preparation, characterization and application of Pt-Ru-Sn/C trimetallic electrocatalysts for ethanol oxidation in direct fuel cell. Int. J. Hydrogen Energy 2011, 36, 11034–11042. [Google Scholar] [CrossRef]
- Jawad, M.; Ali, S.; Waseem, A.; Rabbani, F.; Amin, B.A.Z.; Bilal, M.; Shaikh, A.J. Plasmonic effects and size relation of gold-platinum alloy nanoparticles. Adv. Nano Res. 2019, 7, 167–180. [Google Scholar]
- Yang, L.; Su, J.; Luo, W.; Cheng, G. Strategic synthesis of graphene supported trimetallic Ag-based core-shell nanoparticles toward hydrolytic dehydrogenation of amine boranes. Int. J. Hydrogen Energy 2014, 39, 3360–3370. [Google Scholar] [CrossRef]
- Jin, F.; Fu, Y.; Kong, W.; Wang, J.; Cai, F.; Zhang, J.; Xu, J. Dry reforming of methane over trimetallic NiFeCu alloy catalysts. Chem. Phys. Lett. 2020, 750, 137491. [Google Scholar] [CrossRef]
- Karatas, Y.; Bulut, A.; Yurderi, M.; Ertas, I.E.; Alal, O.; Gulcan, M.; Celebi, M.; Kivrak, H.; Kaya, M.; Zahmarikan, M. PdAu-MnOx nanoparticles supported on amine-functionalized SiO2 for the room temperature dehydrogenation of formic acid in the absence of additives. Appl. Catal. B Environ. 2016, 180, 586–595. [Google Scholar] [CrossRef]
- Lua, A.C.; Wang, H.Y. Hydrogen production by catalytic decomposition of methane over Ni-Cu-Co alloy particles. Appl. Catal. B Environ. 2014, 156–157, 84–93. [Google Scholar] [CrossRef]
- Tsoncheva, T.; Ivanova, R.; Henych, J.; Dimitrov, M.; Kormunda, M.; Kovacheva, D.; Scotti, N.; Santo, V.D.; Štengl, V. Effect of preparation procedure on the formation of nanostructured ceria–zirconia mixed oxide catalysts for ethyl acetate oxidation: Homogeneous precipitation with urea vs. template-assisted hydrothermal synthesis. Appl. Catal. A Gen. 2015, 502, 418–432. [Google Scholar] [CrossRef]
- Roy, B.; Martinez, U.; Loganathan, K.; Datye, A.; Leclerc, C. Effect of preparation methods on the performance of Ni/Al2O3 catalysts for aqueous-phase reforming of ethanol: Part I-catalytic activity. Int. J. Hydrogen Energy 2012, 37, 8143–8153. [Google Scholar] [CrossRef]
- Benito, P.; Gregori, M.; Andreoli, S.; Fornasari, G.; Millefanti, S.; Ospitali, F.; Albonetti, S. Role of the preparation method on properties of Pd/Cu-MCM-41 hydrodechlorinating catalysts. Catal. Today 2014, 235, 134–143. [Google Scholar] [CrossRef]
- Manzoli, M.; Menegazzo, F.; Signoretto, M.; Cruciani, G.; Pinna, F. Effects of synthetic parameters on the catalytic performance of Au/CeO2 for furfural oxidative esterification. J. Catal. 2015, 330, 465–473. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Balamurugan, J.; Vinothkannan, M.; Kim, A.R.; Sengodan, S.; Yoo, D.J. Nitrogen-doped graphene encapsulated FeCoMoS nanoparticles as advanced trifunctional catalyst for water splitting devices and zincair batteries. Appl. Catal. B Environ. 2020, 279, 119381. [Google Scholar] [CrossRef]
- Mendoza-Nieto, J.A.; Robles-Méndez, F.; Klimova, T.E. Support effect on the catalytic performance of trimetallic NiMoW catalysts prepared with citric acid in HDS of dibenzothiophenes. Catal. Today 2015, 250, 47–59. [Google Scholar] [CrossRef]
- Jahel, A.; Avenier, P.; Lacombe, S.; Olivier-Fourcade, J.; Jumas, J.C. Effect of indium in trimetallic Pt/Al2O3SnIn-Cl naphtha-reforming catalysts. J. Catal. 2010, 272, 275–286. [Google Scholar] [CrossRef]
- Bocanegra, S.A.; Castro, A.A.; Scelza, O.A.; de Miguel, S.R. Characterization and catalytic behavior in the n-butane dehydrogenation of trimetallic InPtSn/MgAl2O4 catalysts. Appl. Catal. A Gen. 2007, 333, 49–56. [Google Scholar] [CrossRef]
- Samoilam, P.; Boutzeloit, M.; Benitez, V.; D´Ippolito, S.A.; Especel, C.; Epron, F.; Vera, C.R.; Marécot, P.; Pieck, C.L. Influence of the pretreatment method on the properties of trimetallic Pt-Ir-Ge/Al2O3 prepared by catalytic reduction. Appl. Catal. A Gen. 2007, 332, 37–45. [Google Scholar] [CrossRef]
- Liang, Z.; Xiao, X.; Yu, X.; Huang, X.; Jiang, Y.; Fan, X.; Chen, L. Non-noble trimetallic Cu-Ni-Co nanoparticles supported on metal-organic frameworks as highly efficient catalysts for hydrolysis of ammonia borane. J. Alloys Compd. 2018, 741, 501–508. [Google Scholar] [CrossRef]
- Ruth, K.; Hayes, M.; Burch, R.; Tsubota, S.; Haruta, M. The effects of SO2 on the oxidation of CO and propane on supported Pt and Au catalysts. Appl. Catal. B Environ. 2000, 24, L133–L138. [Google Scholar] [CrossRef]
- Tsubota, S.; Cunningham, D.; Bando, Y.; Haruta, M. Preparation of nanometer gold strongly interacted with TiO2 and the structure sensitivity in low-temperature oxidation of CO. Adv. Pharmacol. 1995, 227–235. [Google Scholar] [CrossRef]
- Haruta, M. Novel catalysis of gold deposited on metal oxides. Catal. Surv. Jpn. 1997, 1, 61–73. [Google Scholar] [CrossRef]
- Haruta, M. Size- and support-dependency in the catalysis of gold. Catal. Today 1997, 36, 153–166. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Huang, K.-M.; Chang, H.-T. Synthesis and characterization of Au core–Au–Ag shell nanoparticles from gold seeds: Impacts of glycine concentration and pH. J. Colloid Interface Sci. 2006, 301, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Bogireddy, N.K.R.; Martinez Gomez, L.; Osorio-Roman, I.; Agarwal, V. Synthesis of gold nanoparticles using Coffea arabica fruit extract. Adv. Nano Res. 2017, 5, 253–260. [Google Scholar]
- Fonseca, J.D.S.L.; Ferreira, H.S.; Bion, N.; Pirault-Roy, L.; Rangel, M.D.C.; Duprez, D.; Epron, F. Cooperative effect between copper and gold on ceria for CO-PROX reaction. Catal. Today 2012, 180, 34–41. [Google Scholar] [CrossRef]
- Aboukaïs, A.; Skaf, M.; Hany, S.; Cousin, R.; Aouad, S.; Labaki, M.; Abi-Aad, E. A comparative study of Cu, Ag and Au doped CeO2 in the total oxidation of volatile organic compounds (VOCs). Mater. Chem. Phys. 2016, 177, 570–576. [Google Scholar] [CrossRef]
- Redina, E.; Kirichenko, O.; Greish, A.; Kucherov, A.; Tkachenko, O.; Kapustin, G.; Mishin, I.; Kustov, L. Preparation of bimetallic gold catalysts by redox reaction on oxide-supported metals for green chemistry applications. Catal. Today 2015, 246, 216–231. [Google Scholar] [CrossRef]
- Kirichenko, O.; Redina, E.; Davshan, N.; Mishin, I.; Kapustin, G.; Brueva, T.; Kustov, L.; Li, W.; Kim, C. Preparation of alumina-supported gold-ruthenium bimetallic catalysts by redox reactions and their activity in preferential CO oxidation. Appl. Catal. B Environ. 2013, 134–135, 123–129. [Google Scholar] [CrossRef]
- Zanella, R.; Delannoy, L.; Louis, C. Mechanism of deposition of gold precursors onto TiO2 during the preparation by cation adsorption and deposition–precipitation with NaOH and urea. Appl. Catal. A Gen. 2005, 291, 62–72. [Google Scholar] [CrossRef]
- Besson, M.; Kallel, A.; Gallezot, P.; Zanella, R.; Louis, C. Gold catalysts supported on titanium oxide for catalytic wet air oxidation of succinic acid. Catal. Commun. 2003, 4, 471–476. [Google Scholar] [CrossRef]
- Acosta, B.; Smolentseva, E.; Beloshapkin, S.; Rangel, R.; Estrada, M.; Fuentes, S.; Simakov, A. Gold supported on ceria nanoparticles and nanotubes. Appl. Catal. A Gen. 2012, 449, 96–104. [Google Scholar] [CrossRef]
- Silahua-Pavón, A.A.; Torres-Torres, G.; Arévalo-Pérez, J.C.; Cervantes-Uribe, A.; Guerra-Que, Z.; Cordero-García, A.; Monteros, A.E.D.L.; Beltramini, J.N. Effect of gold addition by the recharge method on silver supported catalysts in the catalytic wet air oxidation (CWAO) of phenol. RSC Adv. 2019, 9, 11123–11134. [Google Scholar] [CrossRef]
- Lafaye, G.; Micheaud-Especel, C.; Montassier, C.; Marecot, P. Characterization of bimetallic rhodium-germanium catalysts prepared by surface redox reaction. Appl. Catal. A Gen. 2002, 230, 19–30. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Siakavelas, G.; Charisiou, N.D.; Avraam, D.G.; Tzounis, L.; Kousi, K.; Goula, M.A. Comparative study of Ni, Co, Cu supported on γ -alumina catalysts for hydrogen production via. the glycerol steam reforming reaction. Fuel Process. Technol. 2016, 152, 156–175. [Google Scholar] [CrossRef]
- Sepehri, S.; Rezaei, M.; Garbarino, G.; Busca, G. Facile synthesis of a mesoporous alumina and its application as a support of Ni-based autothermal reforming catalysts. Int. J. Hydrogen Energy 2016, 41, 3456–3464. [Google Scholar] [CrossRef]
- Rao, K.N.; Bharali, P.; Thrimurthulu, G.; Reddy, B.M. Supported copper-ceria catalysts for low temperature CO oxidation. Catal. Commun. 2010, 11, 863–866. [Google Scholar] [CrossRef]
- Heracleous, E.; Lee, A.; Wilson, K.; Lemonidou, A. Investigation of Ni-based alumina-supported catalysts for the oxidative dehydrogenation of ethane to ethylene: Structural characterization and reactivity studies. J. Catal. 2005, 231, 159–171. [Google Scholar] [CrossRef]
- Priecel, P.; Kubička, D.; Čapek, L.; Bastl, Z.; Ryšánek, P. The role of Ni species in the deoxygenation of rapeseed oil over NiMo-alumina catalysts. Appl. Catal. A Gen. 2011, 397, 127–137. [Google Scholar] [CrossRef]
- Kaminski, P.; Ziolek, M. Mobility of gold, copper and cerium species in Au, Cu/Ce, Zr-oxides and its impact on total oxidation of methanol. Appl. Catal. B Environ. 2016, 187, 328–341. [Google Scholar] [CrossRef]
- Bai, M.; Xin, H.; Guo, Z.; Guo, D.; Wang, Y.; Zhao, P.; Li, J. α-Alkylation of ketones with primary alcohols driven by visible light and bimetallic gold and palladium nanoparticles supported on transition metal oxide. Appl. Surf. Sci. 2017, 391, 617–626. [Google Scholar] [CrossRef]
- Gaweł, B.; Lambrechts, K.; Øye, G. Preparation and characterization of Au/CeO2–Al2O3 monoliths. Mater. Sci. Eng. B 2012, 177, 575–580. [Google Scholar] [CrossRef]
- Petrović, S.; Milovanović, D.; Salatić, B.; Peruško, D.; Kovač, J.; Dražić, G.; Mitrić, M.; Trtica, M.; Jelenković, B. Composition and structure of NiAu nanoparticles formed by laser ablation of Ni target in Au colloidal solution. Mater. Chem. Phys. 2015, 166, 223–232. [Google Scholar] [CrossRef]
- Reboul, J.; Li, Z.Y.; Yuan, J.; Nakatsuka, K.; Saito, M.; Mori, K.; Yamashita, H.; Xia, Y.; Louis, C. Synthesis of small Ni-core–Au-shell catalytic nanoparticles on TiO2 by galvanic replacement reaction. Nanoscale Adv. 2021, 3, 823–835. [Google Scholar] [CrossRef]
- Liu, L.; Tai, X.; Zhou, X.; Hou, J.; Zhang, Z. Bimetallic Au–Ni alloy nanoparticles in a metal-organic framework (MIL-101) as efficient heterogeneous catalysts for selective oxidation of benzyl alcohol into benzaldehyde. J. Alloys Compd. 2019, 790, 326–336. [Google Scholar] [CrossRef]
- Alotaibi, M.A.; Din, I.U.; Alharthi, A.I.; Bakht, M.; Centi, G.; Shaharun, M.S.; Naeem, A. Green methanol synthesis by catalytic CO2 hydrogenation, deciphering the role of metal-metal interaction. Sustain. Chem. Pharm. 2021, 21, 100420. [Google Scholar] [CrossRef]
- Jiao, J.; Fu, J.; Wei, Y.; Zhao, Z.; Duan, A.; Xu, C.; Li, J.; Song, H.; Zheng, P.; Wang, X.; et al. Al-modified dendritic mesoporous silica nanospheres-supported NiMo catalysts for the hydrodesulfurization of dibenzothiophene: Efficient accessibility of active sites and suitable metal–support interaction. J. Catal. 2017, 356, 269–282. [Google Scholar] [CrossRef]
- Dan, M.; Mihet, M.; Tasnadi-Asztalos, Z.; Imre-Lucaci, A.; Katona, G.; Lazar, M.D. Hydrogen production by ethanol steam reforming on nickel catalysts: Effect of support modification by CeO2 and La2O3. Fuel 2015, 147, 260–268. [Google Scholar] [CrossRef]
- Velu, S.; Gangwal, S. Synthesis of alumina supported nickel nanoparticle catalysts and evaluation of nickel metal dispersions by temperature programmed desorption. Solid State Ion. 2006, 177, 803–811. [Google Scholar] [CrossRef]
- Guerra-Que, Z.; Torres, J.G.T.; Vidal, H.P.; Cuauhtémoc, I.; Monteros, A.E.D.L.; Beltramini, J.; Frías-Márquez, D.M. Silver nanoparticles supported on zirconia–ceria for the catalytic wet air oxidation of methyl tert-butyl ether. RSC Adv. 2017, 7, 3599–3610. [Google Scholar] [CrossRef][Green Version]
- Guerra-Que, Z.; Pérez-Vidal, H.; Torres-Torres, G.; Arévalo-Pérez, J.C.; Pavón, A.A.S.; Cervantes-Uribe, A.; Monteros, A.E.D.L.; Lunagómez-Rocha, M.A. Treatment of phenol by catalytic wet air oxidation: A comparative study of copper and nickel supported on γ-alumina, ceria and γ-alumina–ceria. RSC Adv. 2019, 9, 8463–8479. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, Z.; Chen, Y.; Ma, X. Syngas methanation over Ni/SiO2 catalyst prepared by ammonia-assisted impregnation. Int. J. Hydrogen Energy 2017, 42, 27073–27083. [Google Scholar] [CrossRef]
- Shimizu, K.-I.; Kawachi, H.; Komai, S.-I.; Yoshida, K.; Sasaki, Y.; Satsuma, A. Carbon oxidation with Ag/ceria prepared by self-dispersion of Ag powder into nano-particles. Catal. Today 2011, 175, 93–99. [Google Scholar] [CrossRef]
- Reddy, B.M.; Rao, K.N. Copper promoted ceria-zirconia based bimetallic catalysts for low temperature soot oxidation. Catal. Commun. 2009, 10, 1350–1353. [Google Scholar] [CrossRef]
- Yang, S.; Besson, M.; Descorme, C. Catalytic wet air oxidation of succinic acid over Ru and Pt catalysts supported on CexZr1−xO2 mixed oxides. Appl. Catal. B Environ. 2015, 165, 1–9. [Google Scholar] [CrossRef]
- Wang, J.; Zhu, W.; He, X.; Yang, S. Catalytic wet air oxidation of acetic acid over different ruthenium catalysts. Catal. Commun. 2008, 9, 2163–2167. [Google Scholar] [CrossRef]
- Tao, F.; Yang, S.; Yang, P.; Shi, Z.; Zhou, R. Effects of support property on the catalytic performance of CeO2-ZrO2-CrOx for 1,2-dichloroethane oxidation. J. Rare Earths 2016, 34, 381–389. [Google Scholar] [CrossRef]
- Parvas, M.; Haghighi, M.; Allahyari, S. Catalytic wet air oxidation of phenol over ultrasound-assisted synthesized Ni/CeO2–ZrO2 nanocatalyst used in wastewater treatment. Arab. J. Chem. 2019, 12, 1298–1307. [Google Scholar] [CrossRef]
- Fu, H.; Leitner, N.K.V.; Legube, B. Catalytic ozonation of chlorinated carboxylic acids with Ru/CeO2–TiO2 catalyst in the aqueous system. New J. Chem. 2002, 26, 1662–1666. [Google Scholar] [CrossRef]
- Chen, F.; Ho, P.; Ran, R.; Chen, W.; Si, Z.; Wu, X.; Weng, D.; Huang, Z.; Lee, C. Synergistic effect of CeO2 modified TiO2 photocatalyst on the enhancement of visible light photocatalytic performance. J. Alloys Compd. 2017, 714, 560–566. [Google Scholar] [CrossRef]
- Tomova, D.; Iliev, V.; Eliyas, A.; Rakovsky, S. Promoting the oxidative removal rate of oxalic acid on gold-doped CeO2/TiO2 photocatalysts under UV and visible light irradiation. Sep. Purif. Technol. 2015, 156, 715–723. [Google Scholar] [CrossRef]
- Fukumura, T.; Sambandan, E.; Yamashita, H. Synthesis and VOC degradation ability of a CeO2/WO3 thin-layer visible-light photocatalyst. Mater. Res. Bull. 2017, 94, 493–499. [Google Scholar] [CrossRef]
- Roy, S.; Saroha, A.K. Ceria promoted γ-Al2O3 supported platinum catalyst for catalytic wet air oxidation of oxalic acid: Kinetics and catalyst deactivation. RSC Adv. 2014, 4, 56838–56847. [Google Scholar] [CrossRef]
- Mahofa, E.P.; Narsaiah, T.B.; Chakra, C.S.; Kumar, P. Catalytic removal of soot from diesel engines using CeO2, CeO2-CuO2, and CeO2-Al2O3 nanocomposites. Mater. Today Proc. 2015, 2, 4451–4456. [Google Scholar] [CrossRef]
- Guo, X.; Li, Y.-Y.; Shen, D.-H.; Gan, J.; Tian, M.; Liu, Z.-G. Metalloporphyrins immobilized on core–shell CeO2@SiO2 nanoparticles prepared by a double-coating method for oxidation of diphenyl methane. Appl. Catal. A Gen. 2012, 413–414, 30–35. [Google Scholar] [CrossRef]
- Phanichphant, S.; Nakaruk, A.; Channei, D. Photocatalytic activity of the binary composite CeO2/SiO2 for degradation of dye. Appl. Surf. Sci. 2016, 387, 214–220. [Google Scholar] [CrossRef]
- Punde, S.S.; Tatarchuk, B.J. Pt-CeO2/SiO2 catalyst for CO oxidation in humid air at ambient temperature. Chin. J. Catal. 2017, 38, 475–488. [Google Scholar] [CrossRef]
- Klein, M.; Nadolna, J.; Golabiewska, A.; Mazierski, P.; Klimczuk, T.; Remita, H.; Zaleska-Medynska, A. The effect of metal cluster deposition route on structure and photocatalytic activity of mono- and bimetallic nanoparticles supported on TiO2 by radiolytic method. Appl. Surf. Sci. 2016, 378, 37–48. [Google Scholar] [CrossRef]
- Gong, P.; Xie, J.; Fang, D.; Liu, X.; He, F.; Li, F. Novel heterogeneous denitrification catalyst over a wide temperature range: Synergy between CeO2, ZrO2 and TiO2. Chem. Eng. J. 2019, 356, 598–608. [Google Scholar] [CrossRef]
- Lunagómez-Rocha, M.A.; Del Ángel, G.; Torres-Torres, G.; Cervantes, A.; Vázquez, A.; Arrieta, A.; Beltramini, J.N. Effect of the Pt oxidation state and Ce3+/Ce4+ ratio on the Pt/TiO2-CeO2 catalysts in the phenol degradation by catalytic wet air oxidation (CWAO). Catal. Today 2015, 250, 145–154. [Google Scholar] [CrossRef]
- Monteros, A.E.D.L.; Lafaye, G.; Cervantes, A.; Del Angel, G.; Barbier, J., Jr.; Torres, J.G.T. Catalytic wet air oxidation of phenol over metal catalyst (Ru,Pt) supported on TiO2-CeO2 oxides. Catal. Today 2015, 258, 564–569. [Google Scholar] [CrossRef]
- Izquierdo-Colorado, A.; Torres-Torres, G.; Gamboa-Rodríguez, M.T.; Silahua-Pavón, A.A.; Arévalo-Pérez, J.C.; Cervantes-Uribe, A.; Cordero-García, A.; Beltramini, J.N. Catalytic wet air oxidation (CWAO) of phenol in a fixed bed reactor using supported Ru and Ru-Au catalysts: Effect of gold and Ce loading. ChemistrySelect 2019, 4, 1275–1284. [Google Scholar] [CrossRef]
- García-Hernández, L.E.; Frías-Márquez, D.M.; Pacheco-Sosa, J.G.; Cervantes-Uribe, A.; Arévalo-Pérez, J.C.; Pérez-Vidal, H.; Silahua-Pavón, A.A.; Lunagómez-Rocha, M.A.; Torres-Torres, J.G. 2-Chlorophenol degradation by catalytic wetair oxidation using copper supported on TiO2-CeO2-ZrO2. Water Sci. Technol. 2019, 80, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Córdova-Pérez, G.E.; Torres-Torres, G.; Ortíz-Chi, F.; Godavarthi, S.; Silahua-Pavón, A.A.; Izquierdo-Colorado, A.; Da Costa, P.; Hernández-Como, N.; Alemán, M.; Espinosa-González, C.F. Effect of acid-basic sites ratio on the catalytic activity to obtain 5-HMF from glucose using Al2O3-TiO2-W catalysts. ChemistrySelect 2018, 3, 12854–12864. [Google Scholar] [CrossRef]
- Atanda, L.; Silahua, A.; Mukundan, S.; Shrotri, A.; Torres, J.G.T.; Beltramini, J. Catalytic behaviour of TiO2–ZrO2 binary oxide synthesized by sol–gel process for glucose conversion to 5-hydroxymethylfurfural. RSC Adv. 2015, 5, 80346–80352. [Google Scholar] [CrossRef]
- Silahua-Pavón, A.A.; Espinosa-González, C.G.; Ortiz-Chi, F.; Pacheco-Sosa, J.G.; Pérez-Vidal, H.; Arévalo-Pérez, J.C.; Godavarthi, S.; Torres-Torres, J.G. Production of 5-HMF from glucose using TiO2-ZrO2 catalysts: Effect of the sol-gel synthesis additive. Catal. Commun. 2019, 129, 105723. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.; Peng, C.; Shi, J.; Wang, Q.; He, L.; Shi, L. Enhanced activity and stability of copper oxide/γ-alumina catalyst in catalytic wet-air oxidation: Critical roles of cerium incorporation. Appl. Surf. Sci. 2018, 436, 981–988. [Google Scholar] [CrossRef]
- Li, N.; Descorme, C.; Besson, M. Catalytic wet air oxidation of 2-chlorophenol over Ru loaded CexZr1−xO2 solid solutions. Appl. Catal. B Environ. 2007, 76, 92–100. [Google Scholar] [CrossRef]
- Barbierjr, J.; Delanoë, F.; Jabouille, F.; Duprez, D.; Blanchard, G.; Isnard, P. Total oxidation of acetic acid in aqueous solutions over noble metal catalysts. J. Catal. 1998, 177, 378–385. [Google Scholar] [CrossRef]
- Kundakovic, L.; Flytzani-Stephanopoulos, M. Cu- and Ag-modified cerium oxide catalysts for methane oxidation. J. Catal. 1998, 179, 203–221. [Google Scholar] [CrossRef]
- Arena, F.; Italiano, C.; Raneri, A.; Saja, C. Mechanistic and kinetic insights into the wet air oxidation of phenol with oxygen (CWAO) by homogeneous and heterogeneous transition-metal catalysts. Appl. Catal. B Environ. 2010, 99, 321–328. [Google Scholar] [CrossRef]
- Vittenet, J.; Aboussaoud, W.; Mendret, J.; Pic, J.-S.; Debellefontaine, H.; Lesage, N.; Faucher, K.; Manero, M.-H.; Thibault-Starzyk, F.; Leclerc, H.; et al. Brosillon, catalytic ozonation with γ-Al2O3 to enhance the degradation of refractory organics in water. Appl. Catal. A Gen. 2015, 504, 519–532. [Google Scholar] [CrossRef]
- Massa, A.; Hernández, S.; Ansaloni, S.; Castellino, M.; Russo, N.; Fino, D. Enhanced electrochemical oxidation of phenol over manganese oxides under mild wet air oxidation conditions. Electrochim. Acta 2018, 273, 53–62. [Google Scholar] [CrossRef]
- Zhou, S.; Qian, Z.; Sun, T.; Xu, J.; Xia, C. Catalytic wet peroxide oxidation of phenol over Cu-Ni-Al hydrotalcite. Appl. Clay Sci. 2011, 53, 627–633. [Google Scholar] [CrossRef]
- Keav, S.; de los Monteros, A.E.; Barbier, J.; Duprez, D. Wet air oxidation of phenol over Pt and Ru catalysts supported on cerium-based oxides: Resistance to fouling and kinetic modelling. Appl. Catal. B Environ. 2014, 150–151, 402–410. [Google Scholar] [CrossRef]


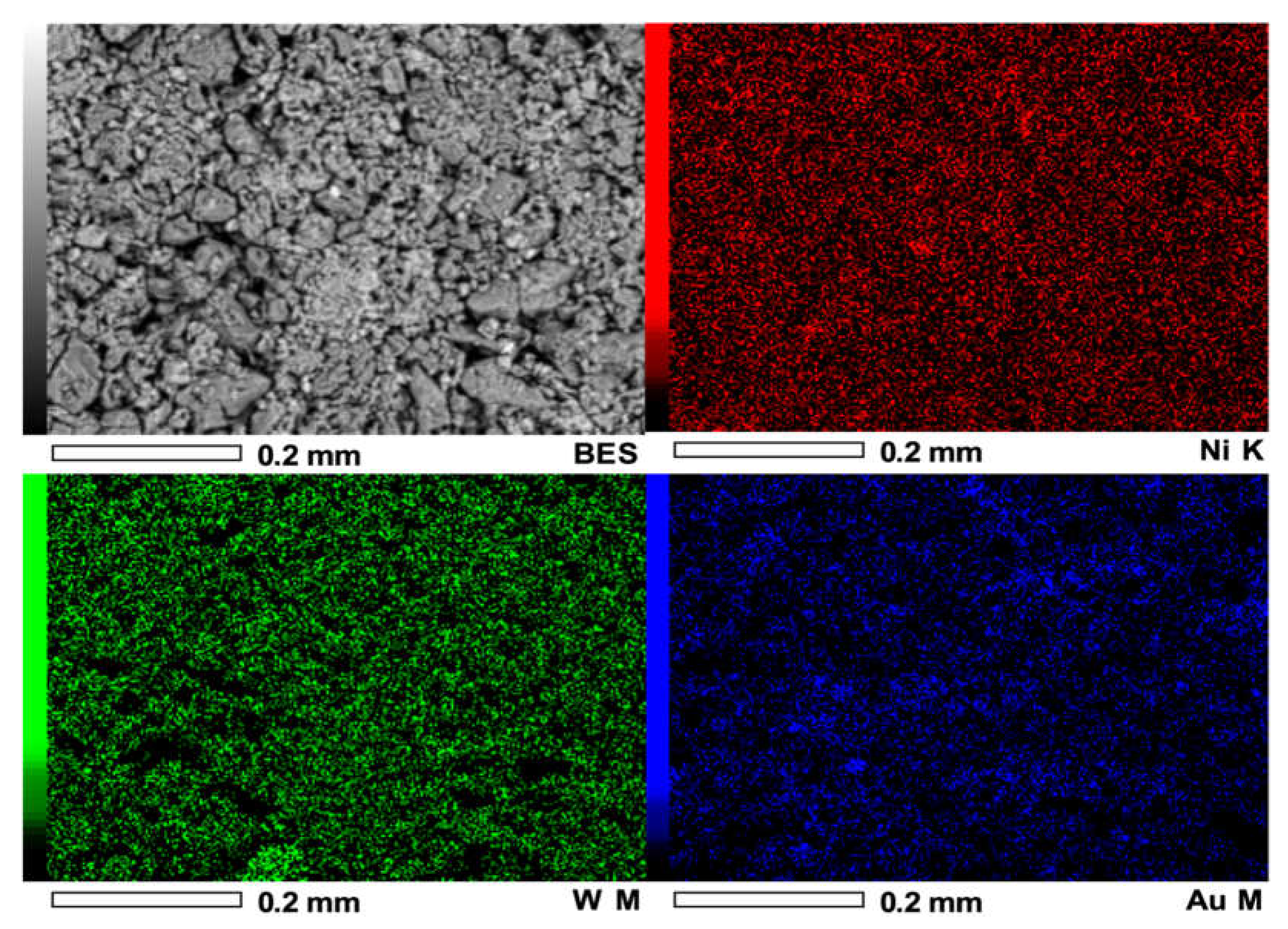
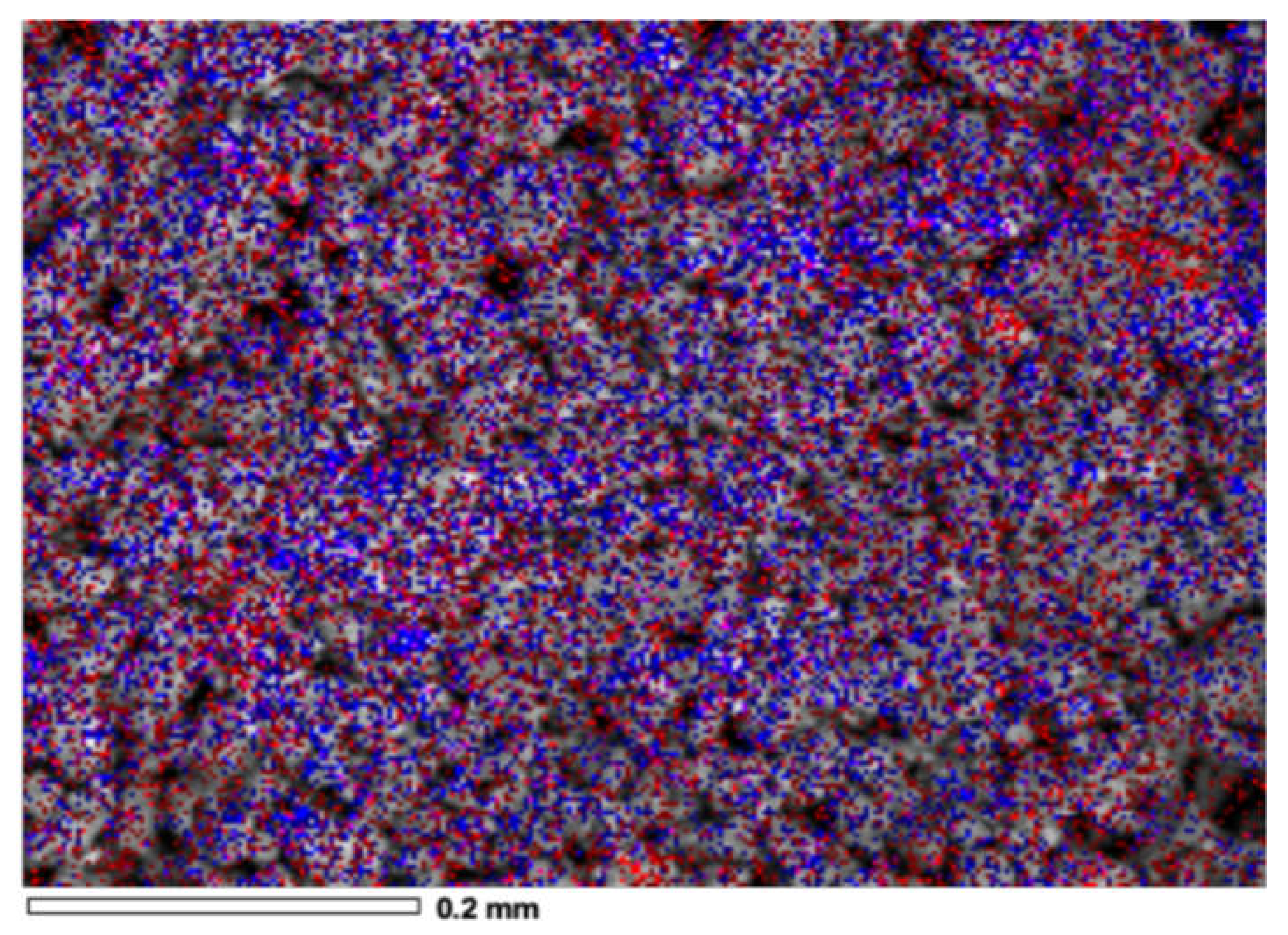
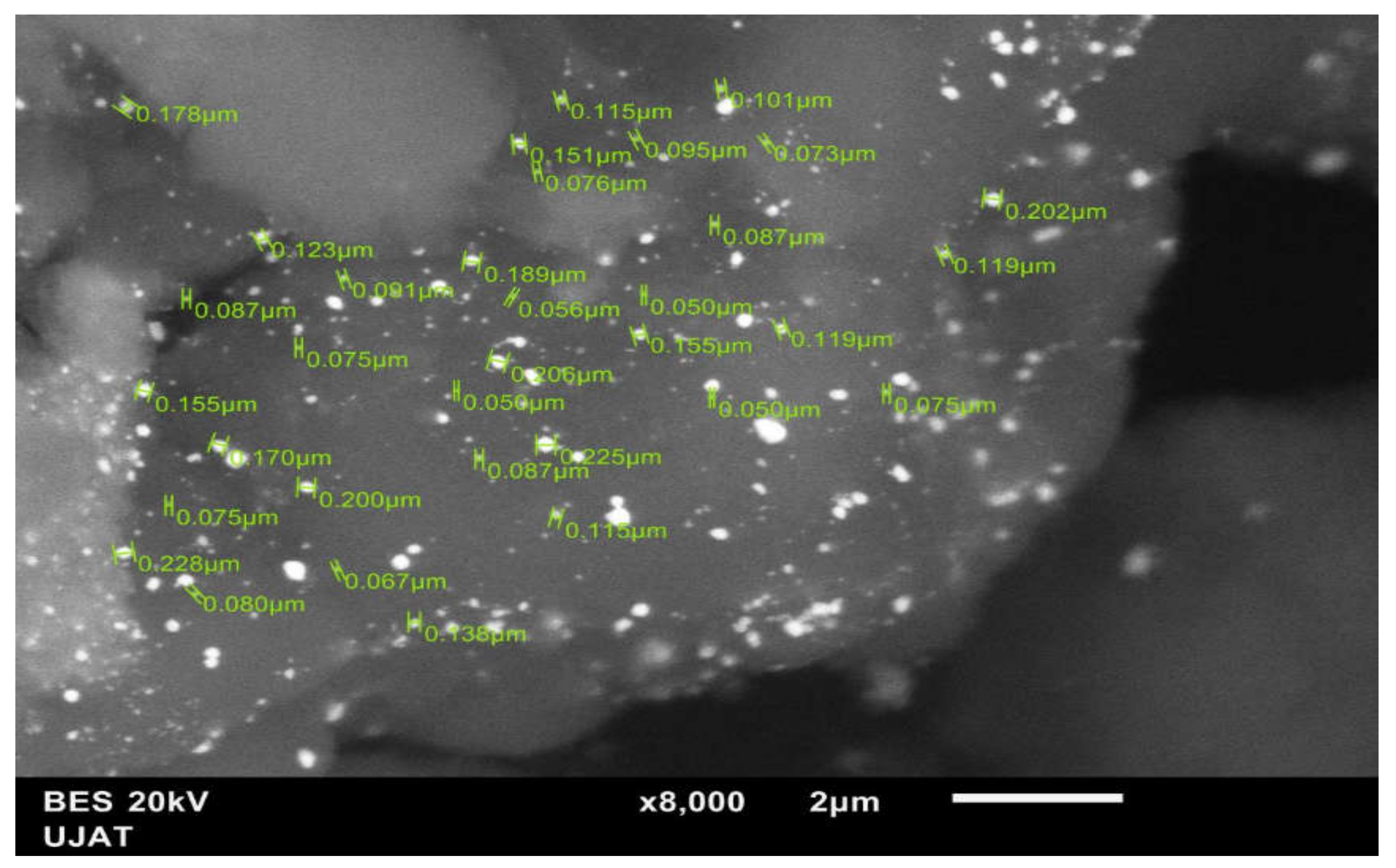

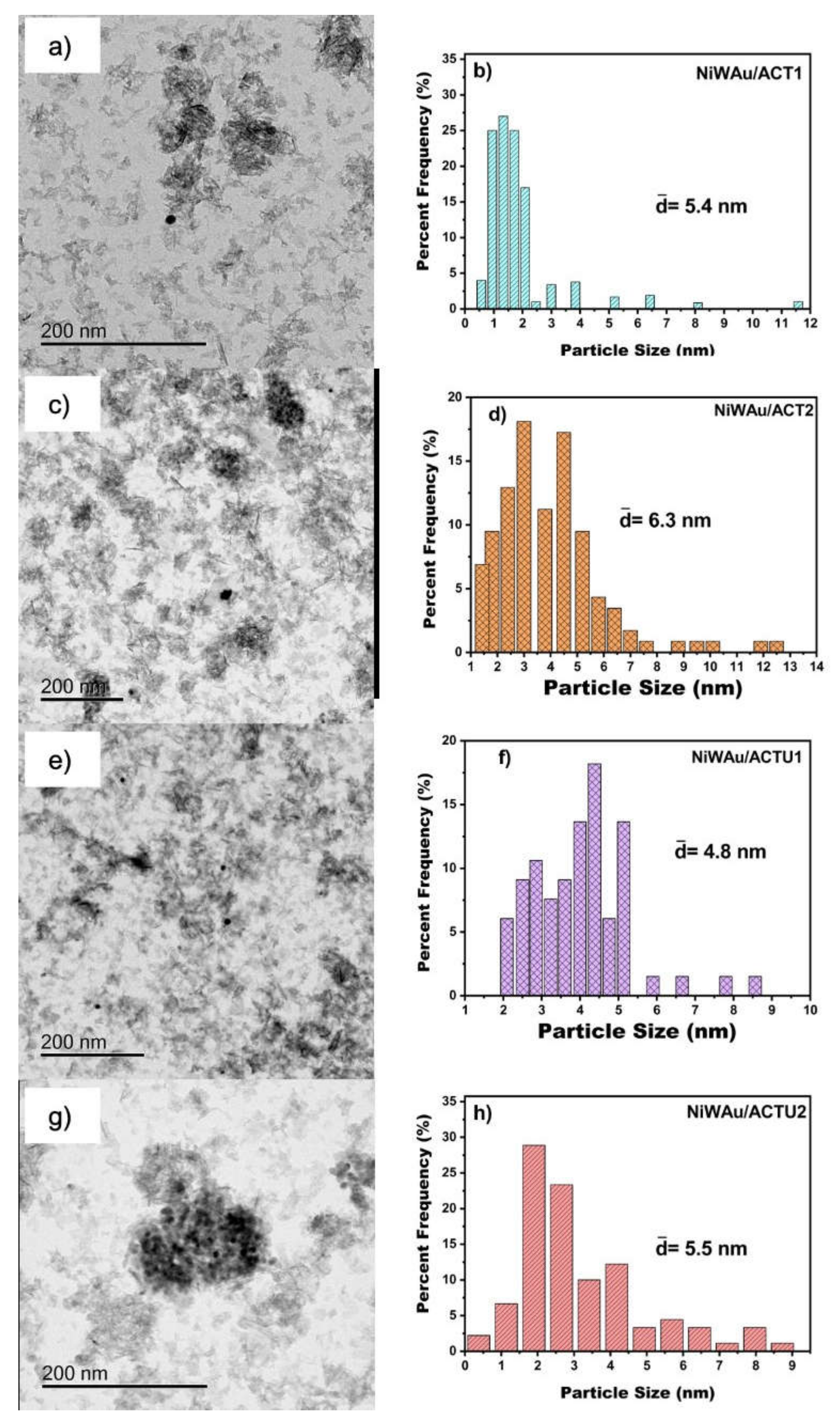
| Method | Weight Percentage | Material | Code | |
|---|---|---|---|---|
| Nickel (Ni) | Tungsten (W) | |||
| Wet impregnation | 5% | 2.5% | NiW/Al2O3-CeO2 –TiO2 90% 1% 9% | NiW/ACT1 |
| NiW/Al2O3-CeO2 –TiO2 94% 1% 5% | NiW/ACT2 | |||
| Ultrasound impregnation | 5% | 2.5% | NiW/Al2O3-CeO2 –TiO2 90% 1% 9% | NiW/ACTU1 |
| NiW/Al2O3-CeO2 –TiO2 94% 1% 5% | NiW/ACTU2 | |||
| Recharge | Gold (Au) | 2.5% | NiWAu/ACT1 | NiWAu/ACTU1 |
| NiWAu/ACT2 | NiWAu/ACTU2 | |||
| Materials | BET SSA (m2/g) | Materials | BET SSA (m2/g) |
|---|---|---|---|
| ACT1 | 382 | Ni/ACTU1 | 263 |
| ACT2 | 367 | Ni/ACTU2 | 277 |
| Ni/ACT1 | 233 | NiW/ACTU1 | 214 |
| Ni/ACT2 | 225 | NiW/ACTU2 | 233 |
| NiW/ACT1 | 218 | NiWAu/ACTU1 | 290 |
| NiW/ACT2 | 179 | NiWAu/ACTU2 | 280 |
| NiWAu/ACT1 | 220 | ||
| NiWAu/ACT2 | 215 |
| Materials | Average Au Particle Size by DRX (nm) | Average Au Particle Size by TEM (nm) |
|---|---|---|
| NiWAu/ACT1 | 4.2 | 5.4 |
| NiWAu/ACT2 | 4.0 | 6.3 |
| NiWAu/ACTU1 | 3.8 | 4.8 |
| NiWAu/ACTU2 | 5.4 | 5.5 |
| NiWAu/ACT1 | NiWAu/ACTU1 | ||
|---|---|---|---|
| Chemical Elements | ms% | Chemical Elements | ms% |
| O | 45.8 | O | 44.3 |
| Al | 36.6 | Al | 43.5 |
| Ti | 7.0 | Ti | 5.3 |
| Ni | 5.0 | Ni | 1.1 |
| Ce | 1.0 | Ce | 2.2 |
| W | 2.3 | W | 2.2 |
| Au | 2.3 | Au | 1.4 |
| Total | 100 | Total | 100 |
| Metal | SG (m2//g) | ρ (g/cm3) |
|---|---|---|
| Ni | 654 | 8.90 |
| W | 753 | 19.35 |
| Au | 266 | 19.32 |
| Sample | BET Area (m2/g) | Average Au Particle Size (nm) a | HTC ((μmol H2/gcat) | TPD-H2 (H/M = 1 μmol H2/gcat) | % D (H/M) | MCS (nm) b |
|---|---|---|---|---|---|---|
| NiW/ACT1 | 214 | - | 0.85 | 0.60 | 70 | 1.5 |
| NiW/ACT2 | 179 | - | 0.85 | 0.63 | 74 | 1.4 |
| NiW/ACTU1 | 218 | - | 2.47 | 0.65 | 26 | 3.3 |
| NiW/ACTU2 | 179 | - | 2.47 | 0.55 | 22 | 3.9 |
| NiWAu/ACT1 | 220 | 4.2 | 0.99 | 0.46 | 46 | 1.8 |
| NiWAu/ACT2 | 215 | 4.0 | 0.99 | 0.50 | 50 | 1.7 |
| NiWAu/ACTU1 | 290 | 3.8 | 0.99 | 0.33 | 33 | 2.6 |
| NiWAu/ACTU2 | 280 | 5.4 | 0.99 | 0.41 | 41 | 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortez-Elizalde, J.; Cuauhtémoc-López, I.; Guerra-Que, Z.; Espinosa de los Monteros, A.E.; Lunagómez-Rocha, M.A.; Silahua-Pavón, A.A.; Arévalo-Pérez, J.C.; Cordero-García, A.; Cervantes-Uribe, A.; Torres-Torres, J.G. Chemical and Structural Changes by Gold Addition Using Recharge Method in NiW/Al2O3-CeO2-TiO2 Nanomaterials. Materials 2021, 14, 5470. https://doi.org/10.3390/ma14195470
Cortez-Elizalde J, Cuauhtémoc-López I, Guerra-Que Z, Espinosa de los Monteros AE, Lunagómez-Rocha MA, Silahua-Pavón AA, Arévalo-Pérez JC, Cordero-García A, Cervantes-Uribe A, Torres-Torres JG. Chemical and Structural Changes by Gold Addition Using Recharge Method in NiW/Al2O3-CeO2-TiO2 Nanomaterials. Materials. 2021; 14(19):5470. https://doi.org/10.3390/ma14195470
Chicago/Turabian StyleCortez-Elizalde, Jorge, Ignacio Cuauhtémoc-López, Zenaida Guerra-Que, Alejandra Elvira Espinosa de los Monteros, Ma. Antonia Lunagómez-Rocha, Adib Abiu Silahua-Pavón, Juan Carlos Arévalo-Pérez, Adrián Cordero-García, Adrián Cervantes-Uribe, and José Gilberto Torres-Torres. 2021. "Chemical and Structural Changes by Gold Addition Using Recharge Method in NiW/Al2O3-CeO2-TiO2 Nanomaterials" Materials 14, no. 19: 5470. https://doi.org/10.3390/ma14195470
APA StyleCortez-Elizalde, J., Cuauhtémoc-López, I., Guerra-Que, Z., Espinosa de los Monteros, A. E., Lunagómez-Rocha, M. A., Silahua-Pavón, A. A., Arévalo-Pérez, J. C., Cordero-García, A., Cervantes-Uribe, A., & Torres-Torres, J. G. (2021). Chemical and Structural Changes by Gold Addition Using Recharge Method in NiW/Al2O3-CeO2-TiO2 Nanomaterials. Materials, 14(19), 5470. https://doi.org/10.3390/ma14195470






