Helium Bubbles and Blistering in a Nanolayered Metal/Hydride Composite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanolayered Composite Preparation
2.2. In-Situ Ion Implantation
2.3. Ex-Situ Ion Implantation
2.4. Microstructure Characterization
3. Results
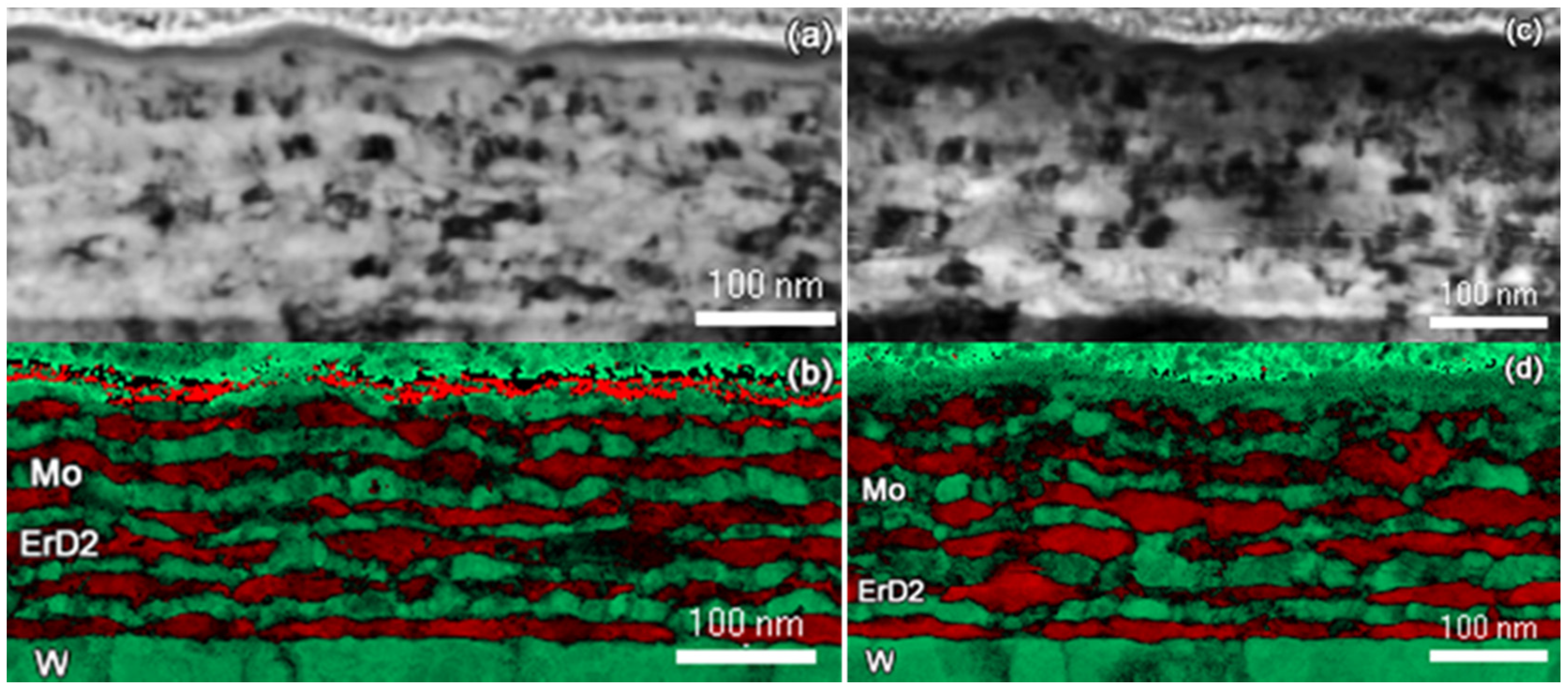
3.1. Nanolayer Structure before and after Hydriding
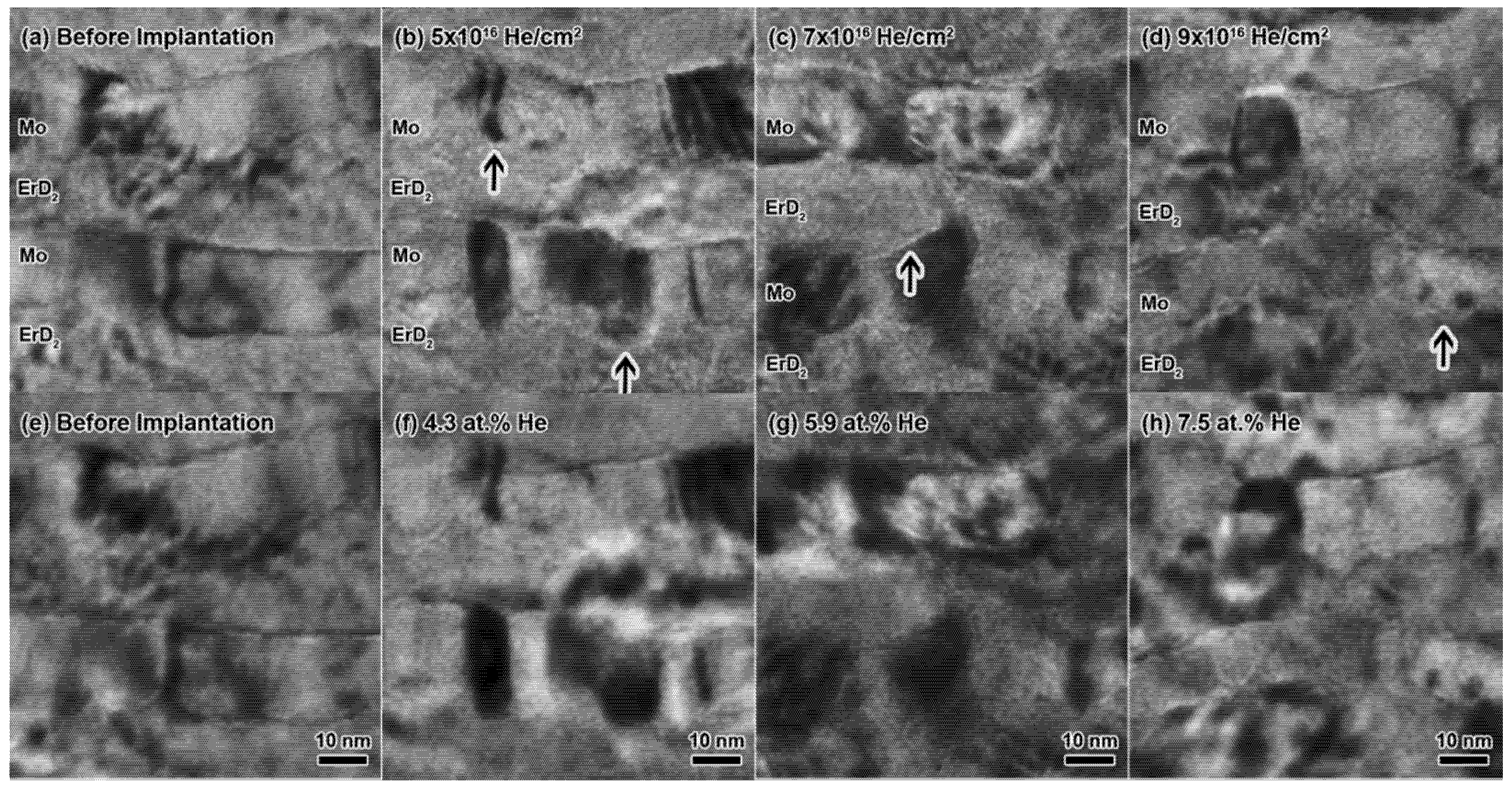
3.2. Helium Bubble Nucleation
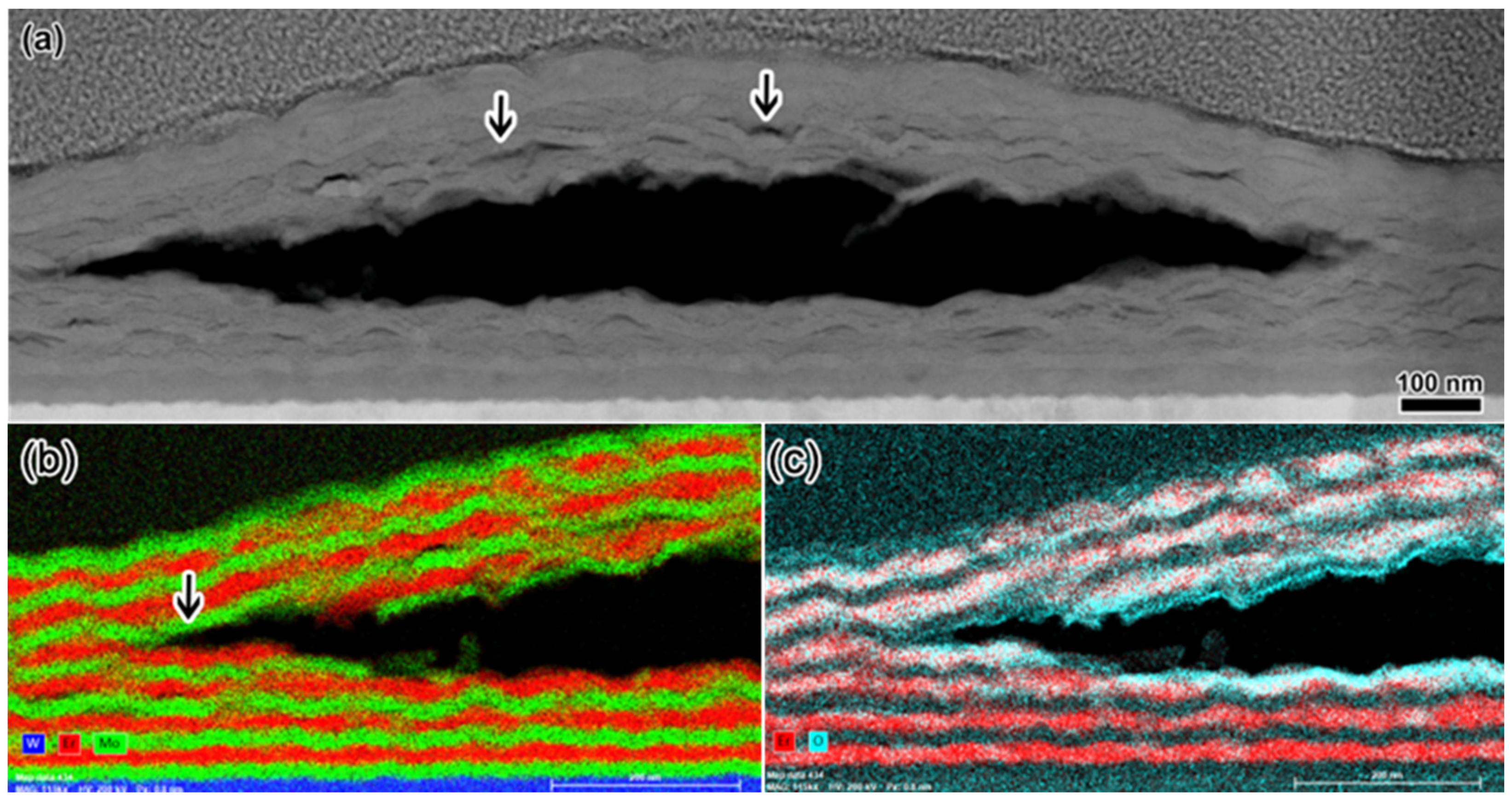
3.3. Bubble Growth and Blistering at High Concentration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mueller, W.M.; Blackledge, J.P.; Libowitz, G.G. Metal Hydrides; Academic Press: New York, NY, USA, 1968. [Google Scholar]
- Trinkhaus, H.; Singh, B.N. Helium accumulation in metals during irradiation—Where do we stand? J. Nucl. Mater. 2003, 323, 229–242. [Google Scholar] [CrossRef]
- Li, S.H.; Li, J.T.; Han, W.Z. Radiation-induced helium bubbles in metals. Materials 2019, 12, 1036. [Google Scholar] [CrossRef] [Green Version]
- Donnelly, S.E.; Evans, J.H. Fundamental Aspects of Inert Gases in Solids; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Schober, T.; Farrell, K. Helium Bubbles in α-Ti and Ti Tritide Arising from Tritium Decay: A TEM Study. J. Nucl. Mater. 1989, 168, 171–177. [Google Scholar] [CrossRef]
- Snow, C.S.; Brewer, L.N.; Gelles, D.S.; Rodriguez, M.A.; Kotula, P.G.; Banks, J.C.; Mangan, M.A.; Browning, J.F. Helium release and microstructural changes in Er(D,T)2−x3Hex films. J. Nucl. Mater. 2008, 374, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Snow, C.S.; Browning, J.F.; Bond, G.M.; Rodriguez, M.A.; Knapp, J.A. 3He bubble evolution in ErT2: A survey of experimental results. J. Nucl. Mater. 2014, 453, 296–306. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.H.; van Veen, A.; Caspers, L.M. Formation of heium platelets in molybdenum. Nature 1981, 291, 310–312. [Google Scholar] [CrossRef]
- Ullmaier, H. The influence of helium on the bulk properties of fusion reactor structural materials. Nucl. Fusion 1984, 24, 1039. [Google Scholar] [CrossRef]
- Ullmaier, H.; Trinkaus, H. Helium in metals: Effect on mechanical properties. Mater. Sci. Forum 1992, 97–99, 451–472. [Google Scholar] [CrossRef]
- Beavis, L.C.; Miglionico, C.J. Structural behavior of metal tritide films. J. Less Common Met. 1972, 27, 201–211. [Google Scholar] [CrossRef]
- Blewer, R.S. Surface damage and topography of erbium metal films implanted with helium to high fluences. Radiat. Eff. 1973, 19, 243–248. [Google Scholar] [CrossRef]
- Blewer, R.S.; Maurin, J.K. Dimensional expansion and surface microstructure in helium implanted erbium and erbium hydride films. J. Nucl. Mater. 1972, 44, 260–278. [Google Scholar] [CrossRef]
- Knapp, J.A.; Browning, J.F.; Bond, G.M. Evolution of mechanical properties in ErT2 thin films. J. Appl. Phys. 2009, 105, 053501. [Google Scholar] [CrossRef]
- Lifshitz, Y.; Cheifetz, E. Helium-implanted titanium hydride: Influence of initial structure on implantation-induced properties. J. Less Common Met. 1984, 104, 349–359. [Google Scholar] [CrossRef]
- Allen, F.I.; Hosemann, P.; Balooch, M. Key Mechanistic Features of Swelling Blister of Helium-Ion-Irradiated Tungsten. Scr. Mater. 2020, 178, 256–260. [Google Scholar] [CrossRef] [Green Version]
- Evans, J.H. A mechanism of surface blistering on metals irradiated with helium ions. J. Nucl. Mater. 1976, 61, 1–7. [Google Scholar] [CrossRef]
- Evans, J.H. The role of implanted gas and lateral stress in blister formation mechanisms. J. Nucl. Mater. 1978, 76–77, 228–234. [Google Scholar] [CrossRef]
- Evans, J.H. Blister lid thickness measurements—A discussion in terms of the interbubble fracture model of blister formation. J. Nucl. Mater. 1980, 93–94, 745–749. [Google Scholar] [CrossRef]
- Evans, J.H.; van Veen, A.; De Hosson, J.T.M.; Bullough, R.; Willis, J.R. The trapping of helium at low angle tilt boundary in molybdenum. J. Nucl. Mater. 1984, 125, 298–303. [Google Scholar] [CrossRef]
- Liu, S.; Dai, S.; Sang, C.; Sun, J.; Stirner, T.; Wang, D. Molecular dynamics simulation of the formation, growth and bursting of bubbles in tungsten exposed to high fluxes of lower energy deuterium. J. Nucl. Mater. 2015, 463, 363–366. [Google Scholar] [CrossRef]
- Martynenko, Y. The theory of blister formation. Radiat. Eff. 1979, 45, 93–101. [Google Scholar] [CrossRef]
- Shu, W.M.; Wakai, E.; Yamanishi, T. Blister bursting and deuterium bursting release from tungsten exposed to high fluences of high flux and low energy deuterium plasma. Nucl. Fusion 2007, 47, 201–209. [Google Scholar] [CrossRef]
- Ackland, G. Controlling radiation damage. Science 2010, 327, 1587–1588. [Google Scholar] [CrossRef]
- Bai, X.M.; Voter, A.F.; Hoagland, R.G.; Nastasi, M.; Uberuaga, B.P. Efficient annealing of radiation damage near grain boundaries via interstitial emission. Science 2010, 327, 1631–1634. [Google Scholar] [CrossRef]
- Beyerlein, I.J.; Demkowicz, M.J.; Misra, A. Defect-interface interactions. Prog. Mater. Sci. 2015, 74, 125–210. [Google Scholar] [CrossRef] [Green Version]
- Demkowicz, M.J.; Misra, A.; Caro, A. The role of interface structure in controlling high helium concentrations. Curr. Opin. Solid State Mater. Sci. 2012, 16, 101–108. [Google Scholar] [CrossRef] [Green Version]
- El-Atwani, O.; Hattar, K.; Hinks, J.; Greaves, G.; Harilal, S.S.; Hassanein, A. Helium bubble formation in ultrafine and nanocrystalline tungsten under different extreme conditions. J. Nucl. Mater. 2015, 458, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Lucas, A.A. Helium in metals. Phys. B + C 1984, 127, 225–239. [Google Scholar]
- Demkowicz, M.J.; Bhattacharyya, D.; Usov, I.; Wang, Y.Q.; Nastasi, M.; Misra, A. The effect of excess atomic volume on He bubble formation at fcc-bcc interfaces. Appl. Phys. Lett. 2010, 97, 161903. [Google Scholar] [CrossRef] [Green Version]
- Gao, R.; Jin, M.; Han, F.; Wang, B.; Wang, X.; Fang, Q.; Dong, Y.; Sun, C.; Shao, L.; Li, M.; et al. Superconducting Cu/Nb nanolaminate by coded accumulative roll bonding and its helium damage characteristics. Acta Mater. 2020, 197, 212–223. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Anderoglu, O.; Wang, H.; Swadener, J.G.; Hochbauer, T.; Misra, A.; Hoagland, R.G. Nanostructured Cu/Nb multilayers subjected to helium ion-irradiation. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2007, 261, 1129–1132. [Google Scholar] [CrossRef]
- Hattar, K.; Demkowicz, M.J.; Misra, A.; Robertson, I.; Hoagland, R.G. Arrest of He bubble growth in Cu-Nb multilayer nanocomposites. Scr. Mater. 2008, 58, 541–544. [Google Scholar] [CrossRef]
- Misra, A.; Demkowicz, M.J.; Zhang, X.; Hoagland, R.G. The radiation damage tolerance of ultra-high strength nanolayered composites. J. Nucl. Mater. 2007, 59, 62–65. [Google Scholar] [CrossRef]
- Bhattacharyya, D.; Demkowicz, M.J.; Wang, Y.-Q.; Baumer, R.E.; Nastasi, M.; Misra, A. A Transmission Electron Microscopy Study of the Effect of Interfaces on Bubble Formation in He-Implanted Cu-Nb Multilayers. Microsc. Microanal. 2012, 18, 152–161. [Google Scholar] [CrossRef]
- Gao, R.; Jin, M.; Li, Q.-J.; So, K.P.; Zhang, L.; Wang, X.; Fang, Q.; Sun, C.; Shao, L.; Li, J. Hybrid diffusive-displacive helium outgassing in Cu/Nb multilayer composites. Scr. Mater. 2021, 194, 113706. [Google Scholar] [CrossRef]
- Li, N.; Nastasi, M.; Misra, A. Defect structures and hardening mechanisms in high dose helium ion implanted Cu and Cu/Nb multilayer thin films. Int. J. Plast. 2012, 32–33, 1–16. [Google Scholar] [CrossRef]
- Zhang, L.F.; Gao, R.; Hou, J.; Zhao, B.L.; Sun, M.; Hao, T.; Xie, Z.M.; Liu, R.; Wang, X.P.; Fang, Q.F.; et al. Study on thermal stability of irradiation response of copper/iron nano-multilayer composite fabricated by cross accumulative roll bonding. J. Nucl. Mater. 2021, 543, 152548. [Google Scholar] [CrossRef]
- Fu, E.G.; Wang, H.; Carter, J.; Shao, L.; Wang, Y.Q.; Zhang, X. Fluence-dependent radiation damage in helium (He) ion-irradiated Cu/V multilayers. Philos. Mag. 2013, 93, 883–898. [Google Scholar] [CrossRef]
- Chen, D.; Li, N.; Yuryev, D.; Wen, J.; Baldwin, K.; Demkowicz, M.J.; Wang, Y.Q. Imaging the in-plane distribution of helium precipitates at a Cu/V interface. Mater. Res. Lett. 2017, 5, 335–342. [Google Scholar] [CrossRef]
- Fu, E.G.; Misra, A.; Wang, H.; Shao, L.; Zhang, X. Interace enabled defects reduction in helium ion irradiated Cu/V nanolayers. J. Nucl. Mater. 2010, 407, 178–188. [Google Scholar] [CrossRef]
- Li, N.; Martin, M.S.; Anderoglu, O.; Misra, A.; Shao, L.; Wang, H.; Zhang, X. He ion irradiation damage in Al/Nb multilayers. J. Appl. Phys. 2009, 105, 123522. [Google Scholar] [CrossRef]
- Chen, S.; Liu, B.; Lin, L.; Jiao, G. Microstructural development and helium bubble formation in Cu/W(Re) nanometer multilayer films irradiated by He+ ion. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 354, 244–248. [Google Scholar] [CrossRef]
- Callisti, M.; Karlik, M.; Polcar, T. Bubbles formation in helium ion irradiated Cu/W multilayer nanocomposites: Effects on structure and mechanical properties. J. Nucl. Mater. 2016, 473, 18–27. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Yang, T.; Xue, J.; Yan, S.; Zhou, S.; Wang, Y.; Kwok, D.T.K.; Chu, P.K.; Zhang, Y. Radiation tolerance of Cu/W multilayered nanocomposites. J. Nucl. Mater. 2011, 413, 11–15. [Google Scholar] [CrossRef]
- Anderoglu, O.; Zhou, M.J.; Zhang, J.; Wang, Y.Q.; Maloy, S.A.; Baldwin, J.K.; Misra, A. He+ ion irradiation response of Fe-TiO2 mutlilayers. J. Nucl. Mater. 2013, 435, 96–101. [Google Scholar] [CrossRef]
- Li, N.; Fu, E.G.; Wang, H.; Carter, J.J.; Shao, L.; Maloy, S.A.; Misra, A.; Zhang, X. He ion irradiation damage in Fe/W nanolayer films. J. Nucl. Mater. 2009, 389, 233–238. [Google Scholar] [CrossRef]
- Zhang, H.; Ren, F.; Wang, Y.Q.; Hong, M.; Xiao, X.; Qin, W.; Jiang, C. In situ TEM observation of helium bubble evolution in V/Ag multilayer during annealing. J. Nucl. Mater. 2015, 467, 537–543. [Google Scholar] [CrossRef] [Green Version]
- Hattar, K.; Bufford, D.C.; Buller, D.L. Concurrent in situ ion irradiation transmission electron microscope. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2014, 338, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.A.; Bufford, D.C.; Muntifering, B.R.; Senor, D.; Steckbeck, M.; Davis, J.; Doyle, B.; Buller, D.; Hattar, K. In situ TEM multi-beam ion irradiation as a technique for elucidating synergistic radiation effects. Materials 2017, 10, 1148. [Google Scholar] [CrossRef] [Green Version]
- Ziegler, J.F.; Ziegler, M.D.; Biersack, J.P. SRIM-The stopping and range of ions in matter. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2010, 268, 1818–1823. [Google Scholar] [CrossRef] [Green Version]
- Schneider, C.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Lasser, R. Tritium and Helium-3 in Metals; Springer Science and Business Media: New York, NY, USA, 2013. [Google Scholar]
- Bonnet, J.E.; Daou, J.N. Rare-earth dihydride compounds: Lattice thermal expansion and investigation of the thermal dissociation. J. Appl. Phys. 1977, 48, 964–968. [Google Scholar] [CrossRef]
- Wang, Z.W.; Li, Z.Y.; Park, S.J.; Abdela, A.; Tang, D.; Palmer, R.E. Quantitative Z-contrast imaging in the scanning transmission electron microscope with size-selected clusters. Phys. Rev. B 2011, 84, 073408. [Google Scholar] [CrossRef]
- Oveisi, E.; Spadaro, M.C.; Rotunno, E.; Grillo, V.; Hébert, C. Insights into image contrast from dislocations in ADF-STEM. Ultramicroscopy 2019, 200, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Robinson, D.B.; Sugar, J.D.; Barr, C.M.; Wang, Y.; Snow, C.S.; Hattar, K. Microstructural effects of high dose helium implantation in ErD2. 2021; to be published. [Google Scholar]
- Erhart, P. A first-principles study of helium storage in oxides and at oxide-iron interfaces. J. Appl. Phys. 2012, 111, 113502. [Google Scholar] [CrossRef] [Green Version]
- Danielson, T.; Tea, E.; Hin, C. Investigation of helium at a Y2Ti2O7 nanocluster embedded in a BCC Fe matrix. Phys. Chem. Chem. Phys. 2016, 18, 30128–30134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; Parish, C.M.; Powers, K.A.; Miller, M.K. Helium solubility and helium bubble formation in a nanostructured ferritic alloy. J. Nucl. Mater. 2014, 445, 165–174. [Google Scholar] [CrossRef]
- Foiles, S.M.; Battaile, C.C. Helium Trapping at Erbium Oxide Precipitates in Erbium Hydride; Sandia National Laboratories: Albuquerque, NM, USA, 2016. [Google Scholar]
- Johnson, P.B.; Armstrong, T.R. In-situ detection of radiation blistering and dynamic deuterium depth profiling during D+ bombardment of solids. Nucl. Instrum. Methods 1978, 148, 85–92. [Google Scholar] [CrossRef]
- Evans, J.H. An interbubble fracture mechanism of blister formation on helium-irradiated metals. J. Nucl. Mater. 1977, 68, 129–140. [Google Scholar] [CrossRef]



| He Fluence (cm−2) | ErD2 Layers (nm) | Mo Layers (nm) | Interfaces (nm) |
|---|---|---|---|
| 5 × 1016 | 0.8 ± 0.2 | 0.9 ± 0.2 | 1.9 ± 1.0 |
| 7 × 1016 | 0.9 ± 0.2 | 1.0 ± 0.2 | 2.1 ± 1.0 |
| 9 × 1016 | 1.1 ± 0.2 | 1.0 ± 0.2 | 2.7 ± 2.2 |
| He Fluence (cm−2) | ErD2 Layers (cm−2) | Mo Layers (cm−2) |
|---|---|---|
| 5 × 1016 | 3.6 ± 1.3 × 1012 | 4.1 ± 1.0 × 1012 |
| 7 × 1016 | 6.7 ± 3.6 × 1012 | 5.3 ± 2.6 × 1012 |
| 9 × 1016 | 7.6 ± 0.61 × 1012 | 7.4 ± 1.0 × 1012 |
| Average Bubble Diameter (nm) | ||||
|---|---|---|---|---|
| He fluence (cm−2) | 7 × 1016 | 1 × 1017 | 1.5 × 1017 | 2.5 × 1017 |
| ErD2 | 1.6 ± 0.5 | 1.4 ± 0.4 | 1.5 ± 0.5 | 1.7 ± 0.4 |
| Mo | 1.7 ± 0.4 | 1.5 ± 0.3 | 1.6 ± 0.4 | 1.6 ± 0.4 |
| Interfaces | 1.4 ± 0.3 | 1.6 ± 0.4 | 2.0 ± 0.5 | 2.9 ± 0.9 |
| Bubble Density (cm−2) | ||||
|---|---|---|---|---|
| He fluence (cm−2) | 7 × 1016 | 1 × 1017 | 1.5 × 1017 | 2.5 × 1017 |
| ErD2 | 2.1 × 1012 | 1.0 × 1012 | 0.8 × 1012 | 0.7 × 1012 |
| Mo | 1.9 × 1012 | 1.3 × 1012 | 1.2 × 1012 | 0.8 × 1012 |
| Interfaces | n/a | n/a | n/a | n/a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, C.A.; Lang, E.; Kotula, P.G.; Goeke, R.; Snow, C.S.; Wang, Y.; Hattar, K. Helium Bubbles and Blistering in a Nanolayered Metal/Hydride Composite. Materials 2021, 14, 5393. https://doi.org/10.3390/ma14185393
Taylor CA, Lang E, Kotula PG, Goeke R, Snow CS, Wang Y, Hattar K. Helium Bubbles and Blistering in a Nanolayered Metal/Hydride Composite. Materials. 2021; 14(18):5393. https://doi.org/10.3390/ma14185393
Chicago/Turabian StyleTaylor, Caitlin A., Eric Lang, Paul G. Kotula, Ronald Goeke, Clark S. Snow, Yongqiang Wang, and Khalid Hattar. 2021. "Helium Bubbles and Blistering in a Nanolayered Metal/Hydride Composite" Materials 14, no. 18: 5393. https://doi.org/10.3390/ma14185393
APA StyleTaylor, C. A., Lang, E., Kotula, P. G., Goeke, R., Snow, C. S., Wang, Y., & Hattar, K. (2021). Helium Bubbles and Blistering in a Nanolayered Metal/Hydride Composite. Materials, 14(18), 5393. https://doi.org/10.3390/ma14185393






