The Effect of Ca Dopant on the Electrical and Dielectric Properties of BaTi4O9 Sintered Ceramics
Abstract
:1. Introduction
2. Experimental Method
3. Results and Discussions
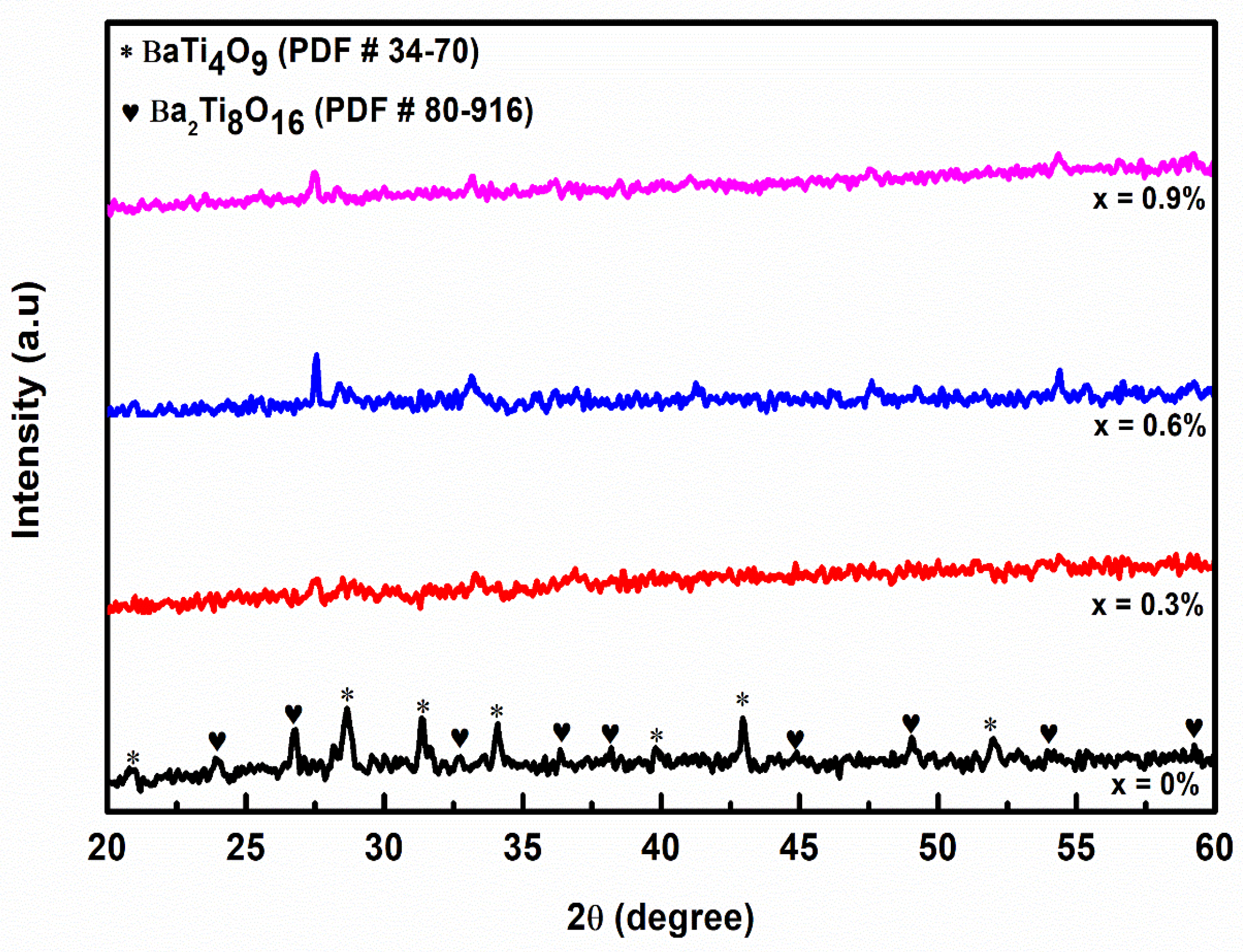
3.1. Phase Analysis
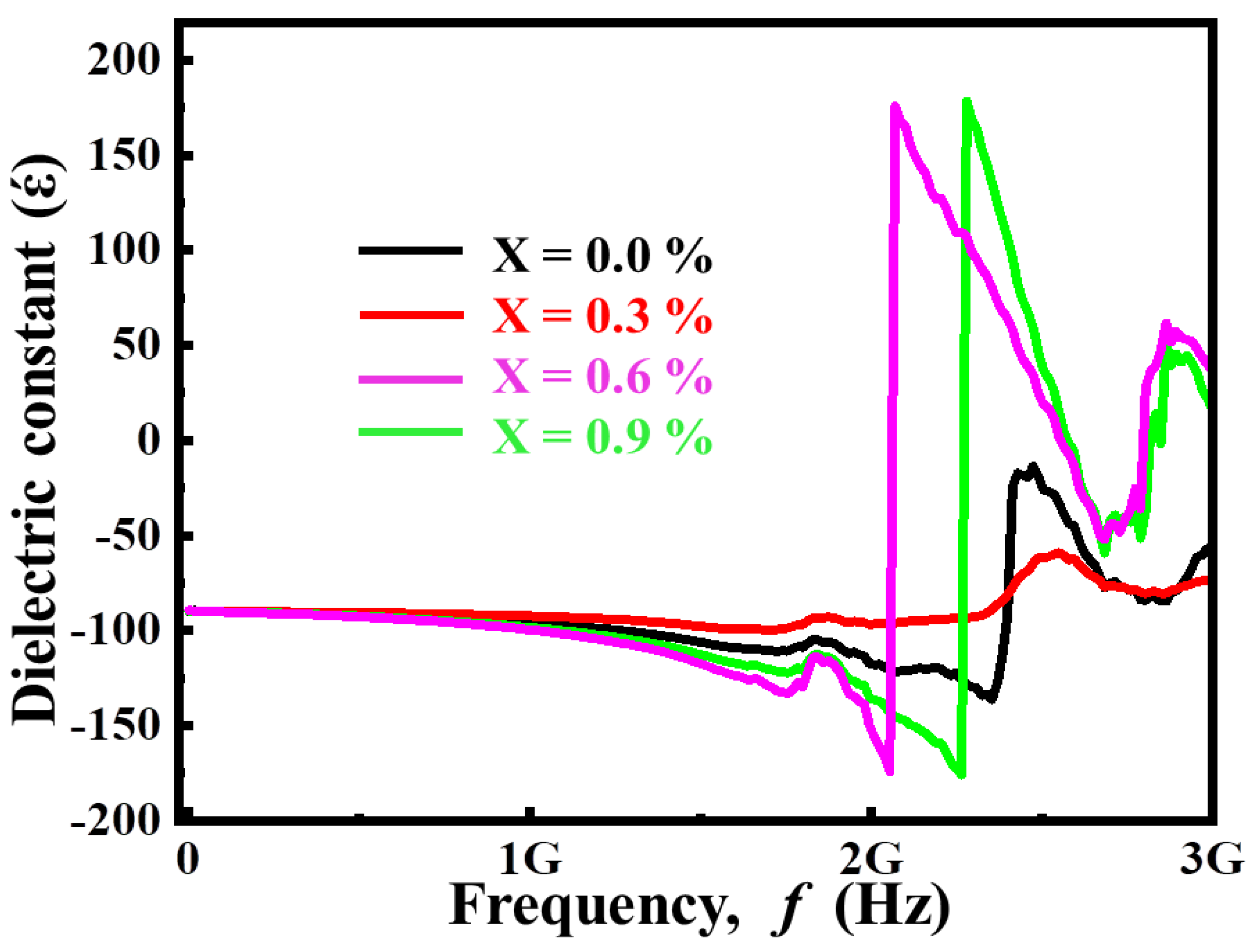
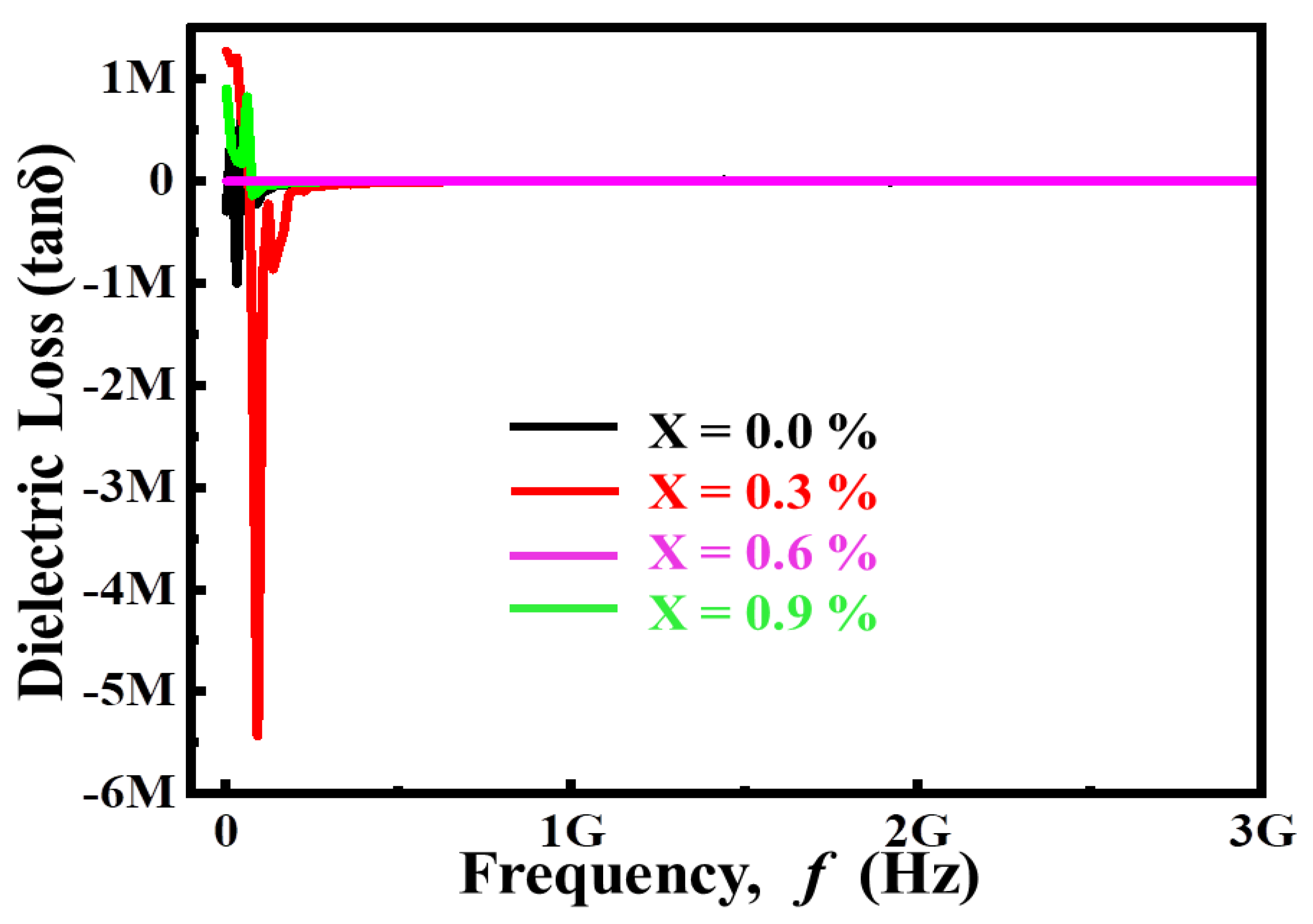
3.2. Dielectric Characteristics
3.2.1. Complex Dielectric Constant
3.2.2. Conductivity Analysis
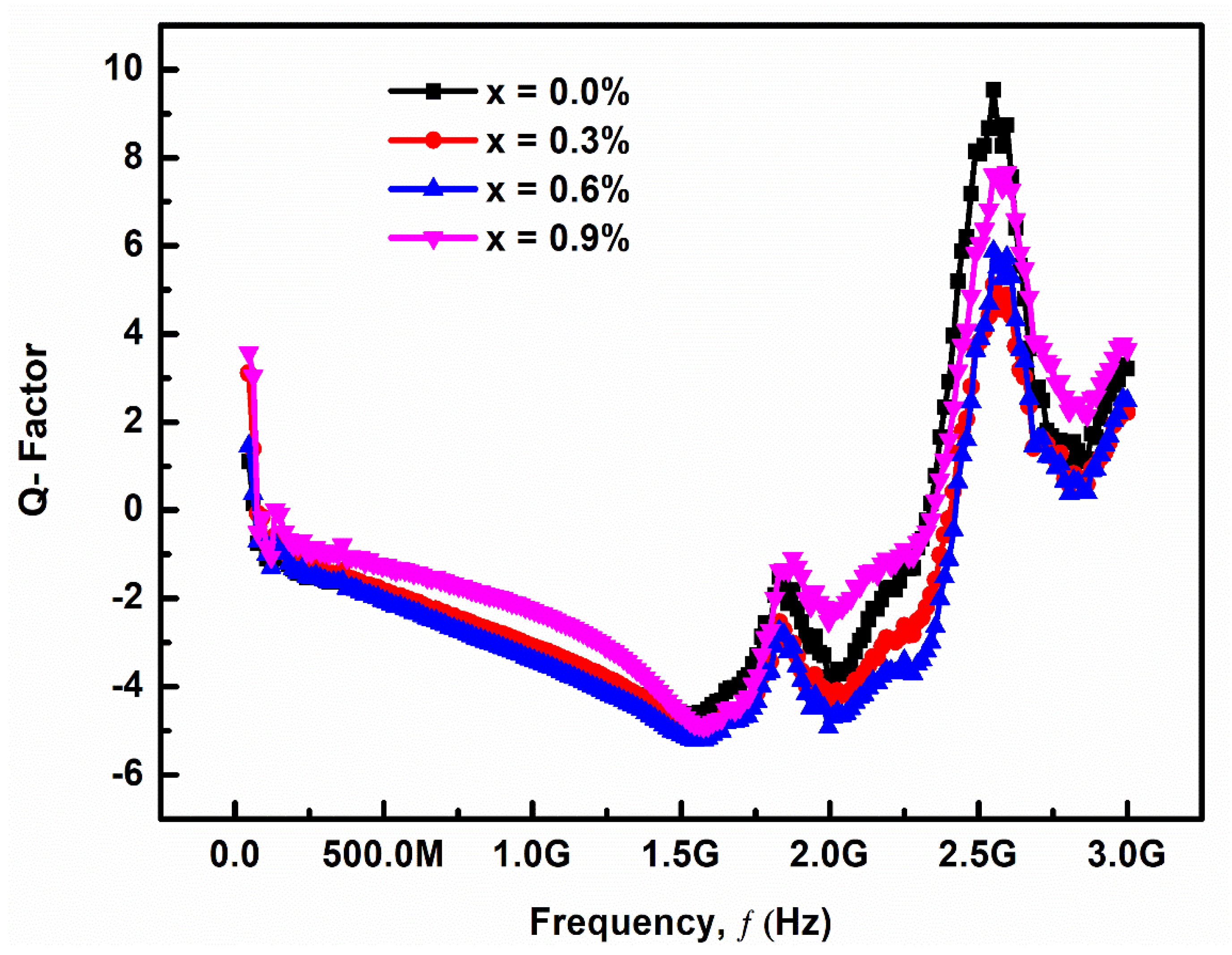
3.2.3. Quality Factor
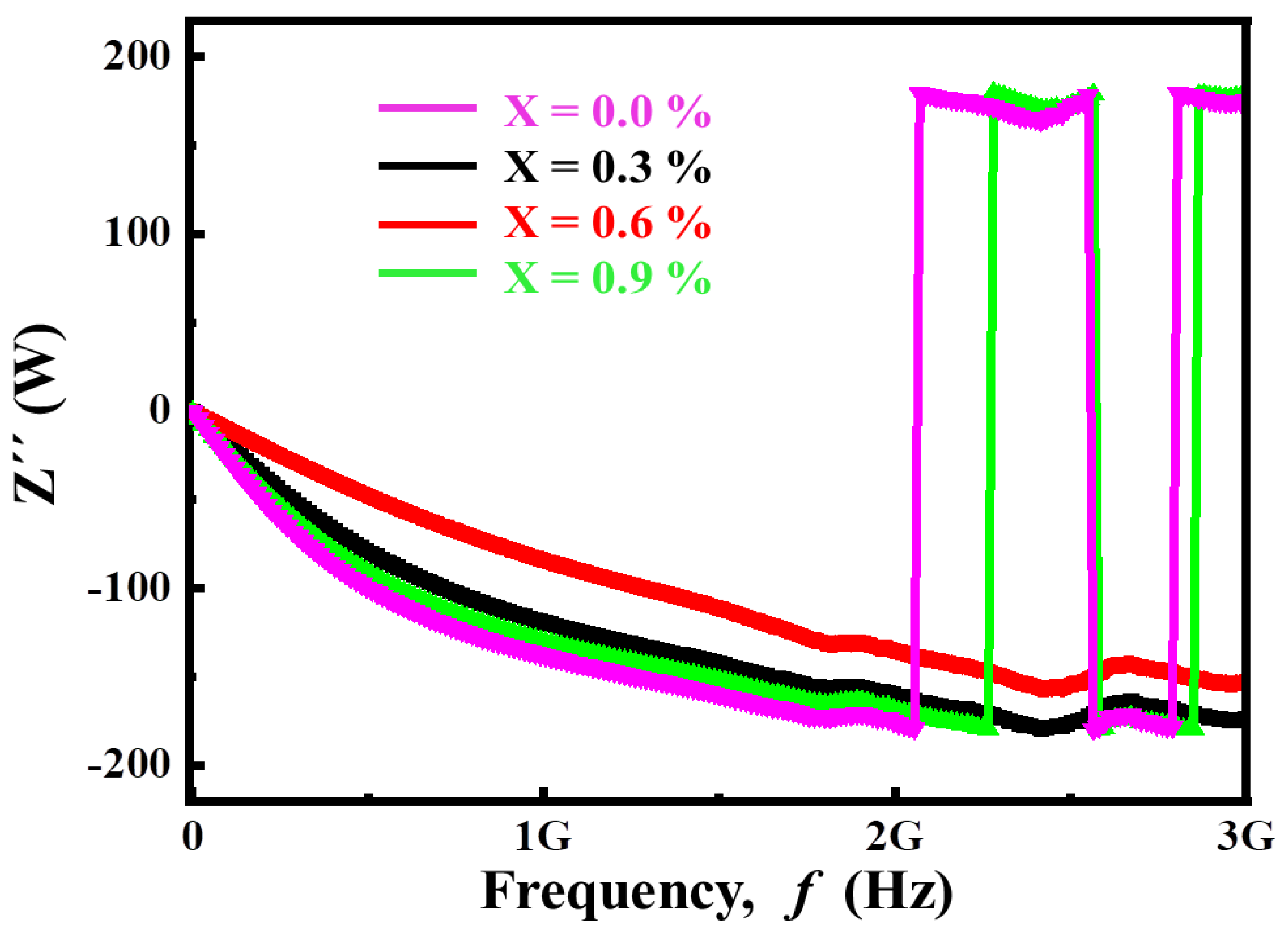
3.2.4. Complex Impedance Analysis
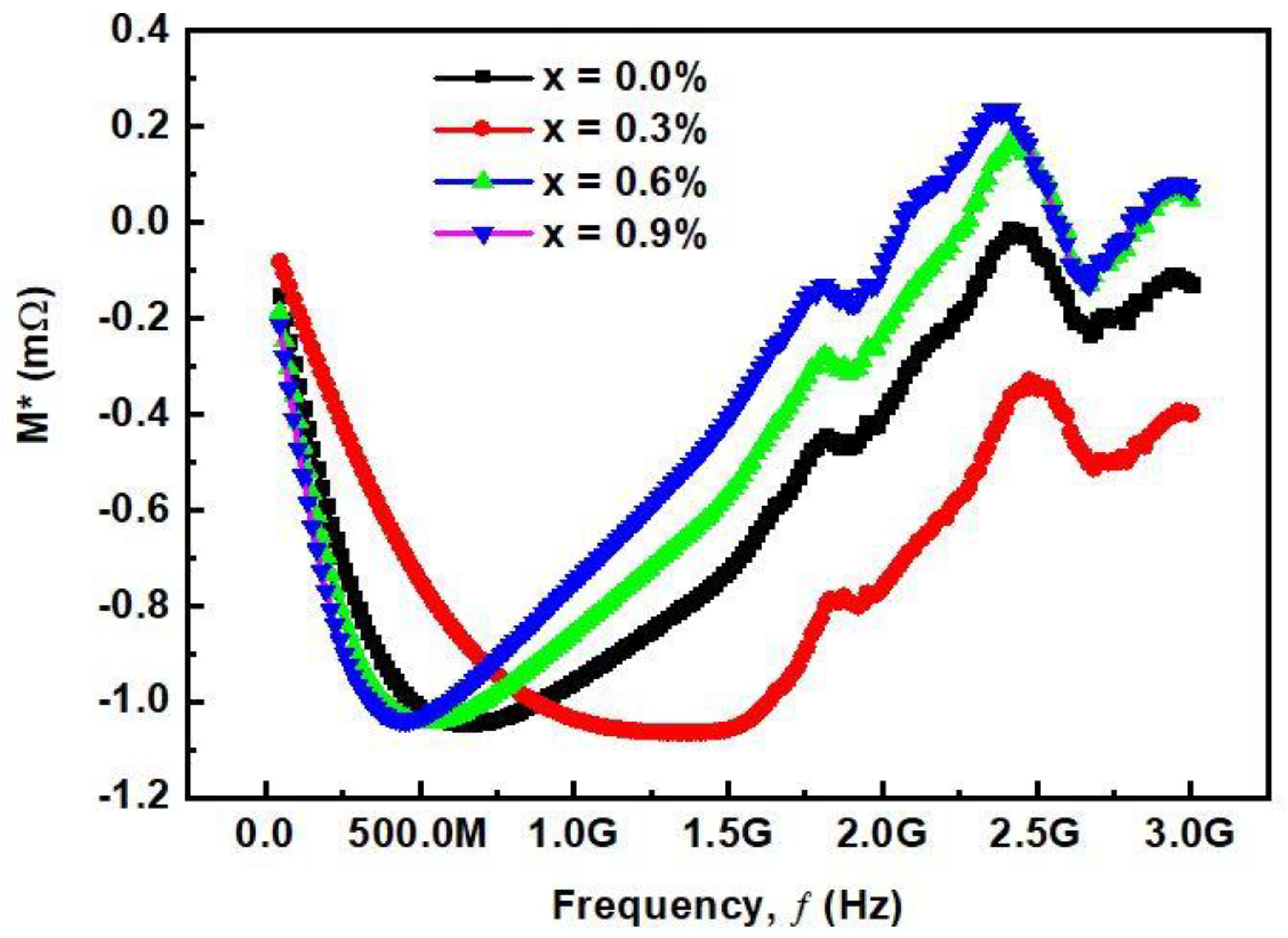
3.2.5. Complex Modulus Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jameel, M.H.; Hamzah, M.Q.; Arif, M.; Agam, B. Synthesis and Characterizations of Co-Doped TiO2 Nanoparticles Via Co-Precipitation Method. Solid State Technol. 2020, 63, 267–277. [Google Scholar]
- Arif, M. Co-precipitation Method in Synthesis and Characterization of BiFeO3 Doped with Various Concentrations of Mn Nanoparticles for Electronic Device Applications. Test Eng. Manag. 2020, 83, 22302–22309. [Google Scholar]
- Ma, H.; Zhang, W.; Kong, X.; Uemura, S.; Kusunose, T.; Feng, Q. BaTi4O9 mesocrystal: Topochemical synthesis, fabrication of ceramics, and relaxor ferroelectric behavior. J. Alloy. Compd. 2019, 777, 335–343. [Google Scholar] [CrossRef]
- Luo, T.; He, L.; Yang, H.; Yu, H. Phase evolution and microwave dielectric properties of BaTi4O9 ceramics prepared by reaction sintering method. Int. J. Appl. Ceram. Technol. 2019, 16, 146–151. [Google Scholar] [CrossRef] [Green Version]
- Anokhin, A.S.; Es’kov, A.V.; Pakhomov, O.V.; Semenov, A.A.; Lähderanta, E. Electro-Caloric Effect and Dielectric Properties in Ferroelectric Ceramics Based on Solid Solution of Barium-Calcium Titanate; IOP Publishing: Bristol, UK, 2019. [Google Scholar]
- Boujelben, F.; Bahri, F.; Boudaya, C.; Maalej, A.; Khemakhem, H.; Simon, A.; Maglione, M. Effect of Ni doped BaTiO3 on the dielectric properties in the Ba (Ni1/3Nb2/3) xTi1− xO3 solid solution. J. Alloy. Compd. 2009, 481, 559–562. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Tseng, Y.-W.; Huang, C.-L. Improved high Q value of (1−x)Ca(Mg1/3Ta2/3)O3–xCa0.8Sm0.4/3TiO3 solid solution with zero temperature coefficient of resonant frequency. J. Alloy. Compd. 2010, 494, 205–209. [Google Scholar] [CrossRef]
- Bin, Y.; Shih, C.; Liu, S. Dielectric properties of low Zr-substituted BaTi4O9 at microwave frequencies. J. Mater. Sci. Mater. Electron. 2019, 30, 5567–5572. [Google Scholar]
- Zaman, A.; Uddin, S.; Mehboob, N.; Ali, A. Structural investigation and improvement of microwave dielectric properties in Ca (HfxTi1−x) O3 ceramics. Phys. Scr. 2020, 96, 025701. [Google Scholar] [CrossRef]
- Ali, A.; Uddin, S.; Zaman, A.; Ahmad, A.; Iqbal, Z. Effect of Zr on the properties of layered structured Ba (Ti1−x Zrx)4O9 perovskites. Adv. Appl. Ceram. 2020, 119, 482–486. [Google Scholar] [CrossRef]
- Gyüre-garami, B. Development of Time Domain Techniques in Microwave Spectroscopy. 2020. Available online: http://dept.phy.bme.hu/phd/gyure_balazs/Gyure-Garami_thesis.pdf (accessed on 16 September 2021).
- Harold, L.; Fay, D.; Theodore, D. Michelson spectrometer system. Syst. Asp. Electro-Opt. 1979, 187, 141–147. [Google Scholar]
- Ali, A.; Uddin, S.; Iqbal, Z.; Lal, M.; Jameel, M.H.; Zaman, A.; Ahmad, A.; Khan, W. Synthesis and characterizations of (Ba1−xCax)Ti4O9, 0 ≤ x ≤ 0.9 ceramics. J. Mater. Res. Technol. 2021, 11, 1828–1833. [Google Scholar] [CrossRef]
- Cullity, B.D. Elements of X-ray Diffractions; Addison-Wesley: Reading, MA, USA, 1978. [Google Scholar]
- Zaman, A.; Uddin, S.; Mehboob, N. Synthesis and Microwave Dielectric Characterization of Ca1−xSrxTiO3, Low-Loss Ceramics. Iran. J. Sci. Technol. Trans. A Sci. 2021, 45, 367–371. [Google Scholar] [CrossRef]
- Motta, F.; Marques, A.; Espinosa, J.; Pizani, P.S.; Longo, E.; Varela, J. Room temperature photoluminescence of BCT prepared by Complex Polymerization Method. Curr. Appl. Phys. 2010, 10, 16–20. [Google Scholar] [CrossRef]
- Neese, B.; Chu, B.; Lu, S.G.; Wang, Y.; Furman, E.; Zhang, Q.M. Large electrocaloric effect in ferroelectric polymers near room temperature. Sci. 2008, 321, 821–823. [Google Scholar] [CrossRef]
- Tian, H.; Wang, Y.; Miao, J.; Chan, H.; Choy, C. Preparation and characterization of hafnium doped barium titanate ceramics. J. Alloy. Compd. 2007, 431, 197–202. [Google Scholar] [CrossRef]
- Valant, M. Progress in Materials Science Electrocaloric materials for future solid-state refrigeration technologies. Prog. Mater. Sci. 2012, 57, 980–1009. [Google Scholar] [CrossRef]
- Yang, M.; Wei, S.; Su, S.L.; Chao, M.; Guo, J.; Wang, H.; Tang, X.; Jiao, Y.; Liang, E. The influence of ZrMgMo3O12 on the dielectric properties of BaTi4O9. Ceram. Int. 2020, 46, 10250–10255. [Google Scholar] [CrossRef]
- Abbas, M.; Ullah, R.; Ullah, K.; Sultana, F.; Mahmood, A.; Mateen, A.; Zhang, Y.; Ali, A.; Althubeiti, K.; Mushtaq, M.; et al. Structural, optical, electrical and dielectric properties of (Sr1−xMgx)(Sn0.5Ti0.5)O3 (X= 0.00, 0.25, 0.50, 0.75) ceramics via solid state route. Ceram. Int. 2021, 47, 30129–30136. [Google Scholar] [CrossRef]
- Murugesan, C.; Ugendar, K.; Okrasa, L.; Shen, J.; Chandrasekaran, G. Zinc substitution effect on the structural, spectroscopic and electrical properties of nanocrystalline MnFe2O4 spinel ferrite. Ceram. Int. 2021, 47, 1672–1685. [Google Scholar] [CrossRef]
- Lal, M.; Shandilya, M.; Rai, R.; Ranjan, A.; Sharma, S.; Valente, M. Study of structural, electrical and magnetic properties of 1−x(Ba0.96Ca0.04TiO3)-x(BiFeO3) ceramics composites. J. Mater. Sci. Mater. Electron. 2018, 29, 13984–14002. [Google Scholar] [CrossRef]
- Hamzah, M.Q.; bin Roslan, M.S.; Zaid, H.M.; Agam, M.A. Effect study of H2O2 and THF on the preparation and characterization of polystyrene/TiO2 nanomaterials. Solid State Technol. 2020, 63, 3347–3358. [Google Scholar]
- Sebastian, M.T. Dielectric Materials for Wireless Communication; Elsevier: Oxford, UK, 2008; pp. 12–14. [Google Scholar]


| X | Calcination Temperature | Sintering Temperature | ρexp (g/cm3) | ρx (g/cm3) | ρre (%) | εr | (at 3 GHz) | Tan(δ) |
|---|---|---|---|---|---|---|---|---|
| 0 | 900 °C/3 h | 1000 °C/4 h | 4.402 | 4.71 | 74.4 | 36.62 | 5669.75 | 0.00017 |
| 0.3 | 900 °C/3 h | 1000 °C/4 h | 4.593 | 5.95 | 76.28 | 37.88 | 6595.05 | 0.00015 |
| 0.6 | 900 °C/3 h | 1000 °C/4 h | 4.433 | 3.95 | 76.65 | 38.13 | 6732.67 | 0.00014 |
| 0.9 | 900 °C/3 h | 1000 °C/4 h | 4.691 | 4.23 | 77.85 | 77.85 | 58.6139 | 0.00017 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Jameel, M.H.; Uddin, S.; Zaman, A.; Iqbal, Z.; Gul, Q.; Sultana, F.; Mushtaq, M.; Althubeiti, K.; Ullah, R. The Effect of Ca Dopant on the Electrical and Dielectric Properties of BaTi4O9 Sintered Ceramics. Materials 2021, 14, 5375. https://doi.org/10.3390/ma14185375
Ali A, Jameel MH, Uddin S, Zaman A, Iqbal Z, Gul Q, Sultana F, Mushtaq M, Althubeiti K, Ullah R. The Effect of Ca Dopant on the Electrical and Dielectric Properties of BaTi4O9 Sintered Ceramics. Materials. 2021; 14(18):5375. https://doi.org/10.3390/ma14185375
Chicago/Turabian StyleAli, Asad, Muhammad Hasnain Jameel, Sarir Uddin, Abid Zaman, Zafar Iqbal, Qeemat Gul, Fozia Sultana, Muhammad Mushtaq, Khaled Althubeiti, and Rafi Ullah. 2021. "The Effect of Ca Dopant on the Electrical and Dielectric Properties of BaTi4O9 Sintered Ceramics" Materials 14, no. 18: 5375. https://doi.org/10.3390/ma14185375
APA StyleAli, A., Jameel, M. H., Uddin, S., Zaman, A., Iqbal, Z., Gul, Q., Sultana, F., Mushtaq, M., Althubeiti, K., & Ullah, R. (2021). The Effect of Ca Dopant on the Electrical and Dielectric Properties of BaTi4O9 Sintered Ceramics. Materials, 14(18), 5375. https://doi.org/10.3390/ma14185375







