Peculiarities of Aluminum Anodization in AHAs-Based Electrolytes: Case Study of the Anodization in Glycolic Acid Solution
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jani, A.M.M.; Losic, D.; Voelcker, N.H. Nanoporous anodic aluminium oxide: Advances in surface engineering and emerging applications. Prog. Mater. Sci. 2013, 58, 636–704. [Google Scholar] [CrossRef]
- Lee, W.; Park, S.-J. Porous Anodic Aluminum Oxide: Anodization and Templated Synthesis of Functional Nanostructures. Chem. Rev. 2014, 114, 7487–7556. [Google Scholar] [CrossRef]
- Cai, Y.; Chen, D.; Li, N.; Xu, Q.; Li, H.; He, J.; Lu, J. A smart membrane with antifouling capability and switchable oil wettability for high-efficiency oil/water emulsions separation. J. Membr. Sci. 2018, 555, 69–77. [Google Scholar] [CrossRef]
- Santos, A.; Kumeria, T.; Losic, D. Nanoporous anodic aluminum oxide for chemical sensing and biosensors. TrAC Trends Anal. Chem. 2013, 44, 25–38. [Google Scholar] [CrossRef]
- Banerjee, P.; Perez, I.; Henn-Lecordier, L.; Lee, S.B.; Rubloff, G.W. Nanotubular metal-insulator capacitor arrays for energy storage. Nat. Nanotechnol. 2009, 4, 292–296. [Google Scholar] [CrossRef]
- Yasui, K.; Nishio, K.; Masuda, H. Fabrication of nanocomposites by filing nanoholes in highly ordered anodic porous alumina by vacuum deposition of metal. Jpn. J. Appl. Phys. 2005, 44, L1181–L1183. [Google Scholar] [CrossRef]
- Ruiz-Clavijo, A.; Caballero-Calero, O.; Martín-González, M.S. Revisiting anodic alumina templates: From fabrication to applications. Nanoscale 2021, 13, 2227–2265. [Google Scholar] [CrossRef]
- Domagalski, J.T.; Xifre-Perez, E.; Marsal, L.F. Recent Advances in Nanoporous Anodic Alumina: Principles, Engineering, and Applications. Nanomaterials 2021, 11, 430. [Google Scholar] [CrossRef]
- Ono, S.; Saito, M.; Asoh, H. Self-Ordering of Anodic Porous Alumina Induced by Local Current Concentration: Burning. Electrochem. Solid-State Lett. 2004, 7, B21–B24. [Google Scholar] [CrossRef]
- Ono, S.; Saito, M.; Ishiguro, M.; Asoh, H. Controlling Factor of Self-Ordering of Anodic Porous Alumina. J. Electrochem. Soc. 2004, 151, B473–B478. [Google Scholar] [CrossRef]
- Ono, S.; Saito, M.; Asoh, H. Self-ordering of anodic porous alumina formed in organic acid electrolytes. Electrochim. Acta 2005, 51, 827–833. [Google Scholar] [CrossRef]
- Friedman, A.L.; Brittain, D.; Menon, L. Roles of pH and acid type in the anodic growth of porous alumina. J. Chem. Phys. 2007, 127, 154717. [Google Scholar] [CrossRef]
- Su, Z.; Zhou, W.; Jiang, F.; Hong, M. Anodic formation of nanoporous and nanotubular metal oxides. J. Mater. Chem. 2012, 22, 535–544. [Google Scholar] [CrossRef]
- Li, Y.; Ling, Z.Y.; Chen, S.S.; Wang, J.C. Fabrication of novel porous anodic alumina membranes by two-step hard anodization. Nanotechnology 2008, 19, 225604. [Google Scholar] [CrossRef]
- Li, A.-P.; Müller, F.; Birner, A.; Nielsch, K.; Gösele, U. Hexagonal pore arrays with a 50–420 nm interpore distance formed by self-organization in anodic alumina. J. Appl. Phys. 1998, 84, 6023–6026. [Google Scholar] [CrossRef] [Green Version]
- Qin, X.; Zhang, J.; Meng, X.; Deng, C.; Zhang, L.; Ding, G.; Zeng, H.; Xu, X. Preparation and analysis of anodic aluminum oxide films with continuously tunable interpore distances. Appl. Surf. Sci. 2015, 328, 459–465. [Google Scholar] [CrossRef]
- Albella, J.M.; Montero, I.; Martinez-Duart, J.M. A theory of avalanche breakdown during anodic oxidation. Electrochim. Acta 1987, 32, 255–258. [Google Scholar] [CrossRef]
- Li, Y.; Ling, Z.Y.; Hu, X.; Liu, Y.S.; Chang, Y. Investigation of intrinsic mechanisms of aluminium anodization processes by analyzing the current density. RSC Adv. 2012, 2, 5164–5171. [Google Scholar]
- Michalska-Domańska, M.; Norek, M.; Stępniowski, W.J.; Budner, B. Fabrication of high quality anodic aluminum oxide (AAO) on low purity aluminum—A comparative study with the AAO produced on high purity aluminum. Electrochim. Acta 2013, 105, 424–432. [Google Scholar] [CrossRef]
- Zhao, N.-Q.; Jiang, X.-X.; Shi, C.-S.; Li, J.-J.; Zhao, Z.-G.; Du, X.-W. Effects of anodizing conditions on anodic alumina structure. J. Mater. Sci. 2007, 42, 3878–3882. [Google Scholar] [CrossRef]
- Nielsch, K.; Choi, J.; Schwirn, K.; Wehrspohn, A.R.B.; Gösele, U. Self-ordering Regimes of Porous Alumina: The 10 Porosity Rule. Nano Lett. 2002, 2, 677–680. [Google Scholar] [CrossRef]
- Masuda, H.; Yada, K.; Osaka, A. Self-Ordering of Cell Configuration of Anodic Porous Alumina with Large-Size Pores in Phosphoric Acid Solution. Jpn. J. Appl. Phys. 1998, 37, L1340–L1342. [Google Scholar] [CrossRef]
- Martell, A.E.; Smith, R.M. Critical Stability Constants; Plenum Press: New York, NY, USA, 1976; Volume 1–4. [Google Scholar]
- Kikuchi, T.; Nakajima, D.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Porous Aluminum Oxide Formed by Anodizing in Various Electrolyte Species. Curr. Nanosci. 2015, 11, 560–571. [Google Scholar] [CrossRef]
- Kikuchi, T.; Yamamoto, T.; Natsui, S.; Suzuki, R.O. Fabrication of Anodic Porous Alumina by Squaric Acid Anodizing. Electrochim. Acta 2014, 123, 14–22. [Google Scholar] [CrossRef] [Green Version]
- Kikuchi, T.; Nishinaga, O.; Natsiu, S.; Suzuki, R.O. Fabrication of anodic nanoporous alumina via acetylenedicarboxylic acid anodizing. ECS Electrochem. Lett. 2014, 3, C25–C28. [Google Scholar] [CrossRef]
- Vorarat, S.; Aromdee, C.; Podokmai, Y. Determination of alpha hydroxyl in fruits by capillary electrophoresis. Anal. Sci. 2002, 18, 893–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.-C.; Yang, J.-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef] [Green Version]
- Eden, M.; Bates, R.G. Resolution of the dissociation constants of d,l-malic acid from 0-degrees-c to 50-degrees-c. J. Res. Natl. Inst. Stand. Technol. 1959, 62, 161. [Google Scholar] [CrossRef]
- Quitmann, H.; Fan, R.; Czermak, P. Acidic Organic Compounds in Beverage, Food, and Feed Production. Adv. Biochem. Eng. Biotechnol. 2014, 143, 91–141. [Google Scholar]
- Theron, M.M.; Lues, J.F.R. Organic Acids and Meat Preservation: A Review. Food Rev. Int. 2007, 23, 141–158. [Google Scholar] [CrossRef]
- Brown, T.E.; Lemay, H.E.; Bursten, B.E.; Murphy, C.; Woodward, P.M. Chemistry: The Central Science, 11th ed.; Pearson Prentice Hall: New York, NY, USA, 2009; pp. 689–715. [Google Scholar]
- Mirzoev, R.A.; Davydov, A.D.; Vystupov, S.I.; Kabanova, T.B. Conditions for self-ordering of porous structure of anodic aluminum oxide in weak and strong acids. Electrochim. Acta 2018, 294, 276–285. [Google Scholar] [CrossRef]
- Poznyak, A.; Pligovka, A.; Laryn, T.; Salerno, M. Porous Alumina Films Fabricated by Reduced Temperature Sulfuric Acid Anodizing: Morphology, Composition and Volumetric Growth. Materials 2021, 14, 767. [Google Scholar] [CrossRef]
- Bellemare, J.; Sirois, F.; Menard, D. Fabrication of Micrometer-Scale Self-Organized Pore Arrays in Anodic Alumina. J. Electrochem. Soc. 2014, 161, E75–E80. [Google Scholar] [CrossRef]
- Bellemare, J.; Carignan, L.-P.; Sirois, F.; Ménard, D. Etching the Oxide Barrier of Micrometer-Scale Self-Organized Porous Anodic Alumina Membranes. J. Electrochem. Soc. 2015, 162, E47–E50. [Google Scholar] [CrossRef] [Green Version]
- Katsuta, Y.; Yasumori, A.; Wada, K.; Kurashima, K.; Suehara, S.; Inoue, S. Three-dimensionally nanostructured alumina film on glass substrate: Anodization of glass surface. J. Non-Cryst. Solids 2008, 354, 451–455. [Google Scholar] [CrossRef]
- Wang, Q.; Long, Y.; Sun, B. Fabrication of highly ordered porous anodic alumina membrane with ultra-large pore intervals in ethylene glycol-modified citric acid solution. J. Porous Mater. 2013, 20, 785–788. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, Y.; Li, J.; Li, Y.; Zhang, Z.; Feng, C.; Sun, R. Fabrication of Self-Ordered Alumina Films with Large Interpore Distance by Janus Anodization in Citric Acid. Sci. Rep. 2016, 6, 39165. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wen, Y.; Li, J.; Lu, J.; Li, Y.; Yang, Y.; Feng, C.; Hao, C.; Zhang, Z.; Hu, J.; et al. Pore Nucleation Mechanism of Self-Ordered Alumina with Large Period in Stable Anodization in Citric Acid. J. Electrochem. Soc. 2018, 165, E311–E317. [Google Scholar] [CrossRef]
- Sulka, G.D. Highly ordered anodic porous alumina formation by self-organized anodizing. In Nanostructured Materials in Electrochemistry, 1st ed.; Eftekhari, A., Ed.; Wiley-VCH: Weinheim, Germany, 2008; pp. 1–116. [Google Scholar]
- Chu, S.-Z.; Wada, K.; Inoue, S.; Isogai, M.; Katsuta, Y.; Yasumori, A. Large-Scale Fabrication of Ordered Nanoporous Alumina Films with Arbitrary Pore Intervals by Critical-Potential Anodization. J. Electrochem. Soc. 2006, 153, B384–B391. [Google Scholar] [CrossRef]
- Kikuchi, T.; Yamamoto, T.; Suzuki, R.O. Growth behavior of anodic porous alumina formed in malic acid solution. Appl. Surf. Sci. 2013, 284, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Zajączkowska, L.; Siemiaszko, D.; Norek, M. Towards self-organized anodization of aluminum in malic acid solutions—new aspects of anodization n the organic acid. Materials 2020, 13, 3899. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gomez-Rodriguez, J.M.; Colchero, J.; Gomez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Zaraska, L.; Sulka, G.; Jaskuła, M. Anodic alumina membranes with defined pore diameters and thicknesses obtained by adjusting the anodizing duration and pore opening/widening time. J. Solid State Electrochem. 2011, 15, 2427–2436. [Google Scholar] [CrossRef] [Green Version]
- Zaraska, L.; Sulka, G.; Szeremeta, J.; Jaskuła, M. Porous anodic alumina formed by anodization of aluminium alloy (AA1050) and high purity aluminum. Electrochim. Acta 2010, 55, 4377–4386. [Google Scholar] [CrossRef]
- Kushwaha, M. A comparative Study of Different Electrolytes for Obtaining Thick and Well-ordered nano-porous Anodic Aluminium Oxide (AAO) Films. Procedia Mater. Sci. 2014, 5, 1266–1273. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Li, Y.; Li, Z.; Li, S.; Shen, L.; Hu, X.; Ling, Z. Ultra-slow growth rate: Accurate control of the thickness of porous anodic aluminum oxide films. Electrochem. Commun. 2019, 109, 106602. [Google Scholar] [CrossRef]
- Buijnsters, J.G.; Zhong, R.; Tsyntsaru, N.; Celis, J.-P. Surface wettability of macroporous anodized aluminium oxide. ACS Appl. Mater. Interfaces 2013, 5, 3224–3233. [Google Scholar] [CrossRef]
- Kant, K.; Low, S.P.; Marshal, A.; Shapter, J.G.; Losic, D. Nanopore Gradients on Porous Aluminum Oxide Generated by Nonuniform Anodization of Aluminum. ACS Appl. Mater. Interfaces 2010, 2, 3447–3454. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, D.; Liu, Q.; Wang, Q.; Yu, H.; Zhu, X.; Song, Y. Bipolar Electrochemical Anodization Route for the Fabrication of Porous Anodic Alumina with Nanopore Gradients. Langmuir 2021, 37, 4340–4346. [Google Scholar] [CrossRef]
- Pashchanka, M.; Schneider, J.J. Experimental validation of the novel theory explaining self-organization in porous anodic alumina films. Phys. Chem. Chem. Phys. 2013, 15, 7070. [Google Scholar] [CrossRef] [PubMed]
- Nishinaga, O.; Kikuchi, T.; Natsui, S.; Suzuki, R.O. Rapid fabrication of self-ordered porous alumina with 10-/sub-10-nm-scale nanostructures by selenic acid anodizing. Sci. Rep. 2013, 3, 2748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, T.; Nishinaga, O.; Natsui, S.; Suzuki, R.O. Self-Ordering Behavior of Anodic Porous Alumina via Selenic Acid Anodizing. Electrochim. Acta 2014, 137, 728–735. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Vergara, S.; Skeldon, P.; Thompson, G.; Habazaki, H. Stress generated porosity in anodic alumina formed in sulphuric acid electrolyte. Corros. Sci. 2007, 49, 3772–3782. [Google Scholar] [CrossRef]
- Zhu, X.-F.; Song, Y.; Liu, L.; Wang, C.-Y.; Zheng, J.; Jia, H.-B.; Wang, X.-L. Electronic currents and the formation of nanopores in porous anodic alumina. Nanotechnology 2009, 20, 475303. [Google Scholar] [CrossRef] [PubMed]
- Venemana, F.R.; Van Koningsveld, H.; Peters, J.A.; Van Bekkum, H. Co-operative hydrogen bonding with short O—O distances in a binuclear AlIII- glycolate complex. J. Chem. Soc. Chem. Commun. 1990, 9, 699–700. [Google Scholar] [CrossRef]
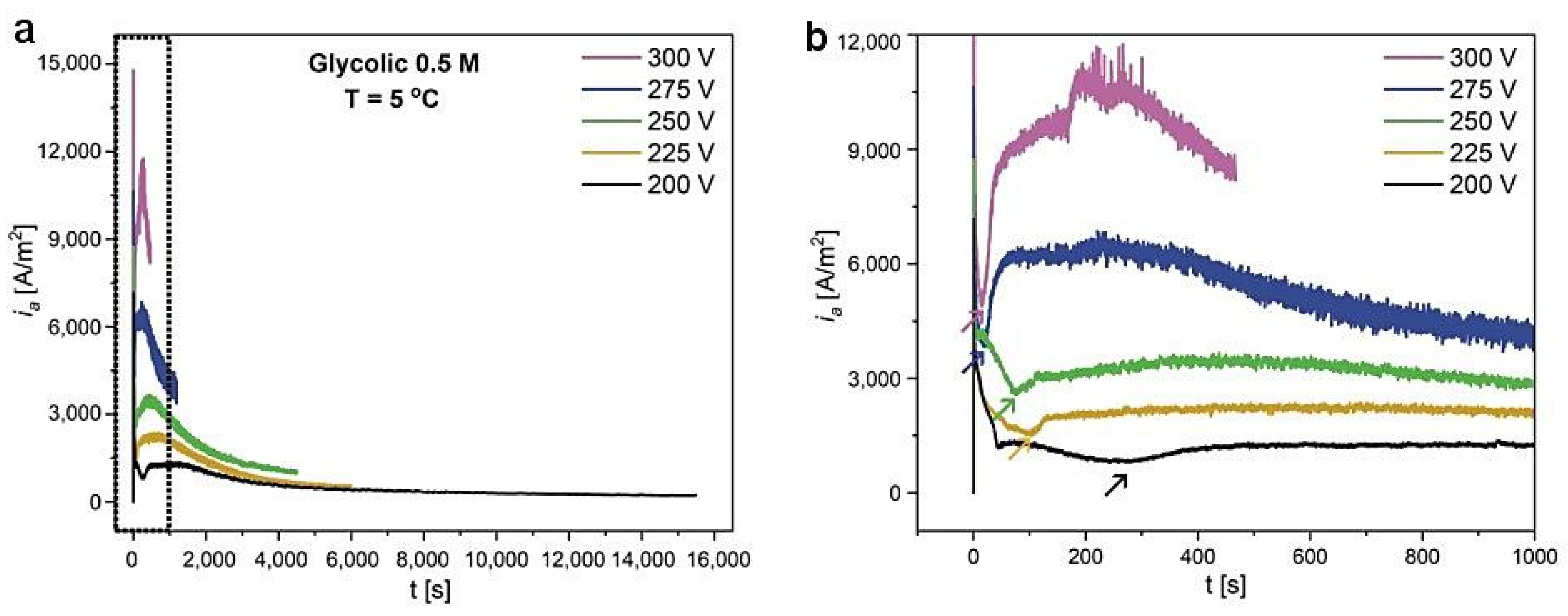
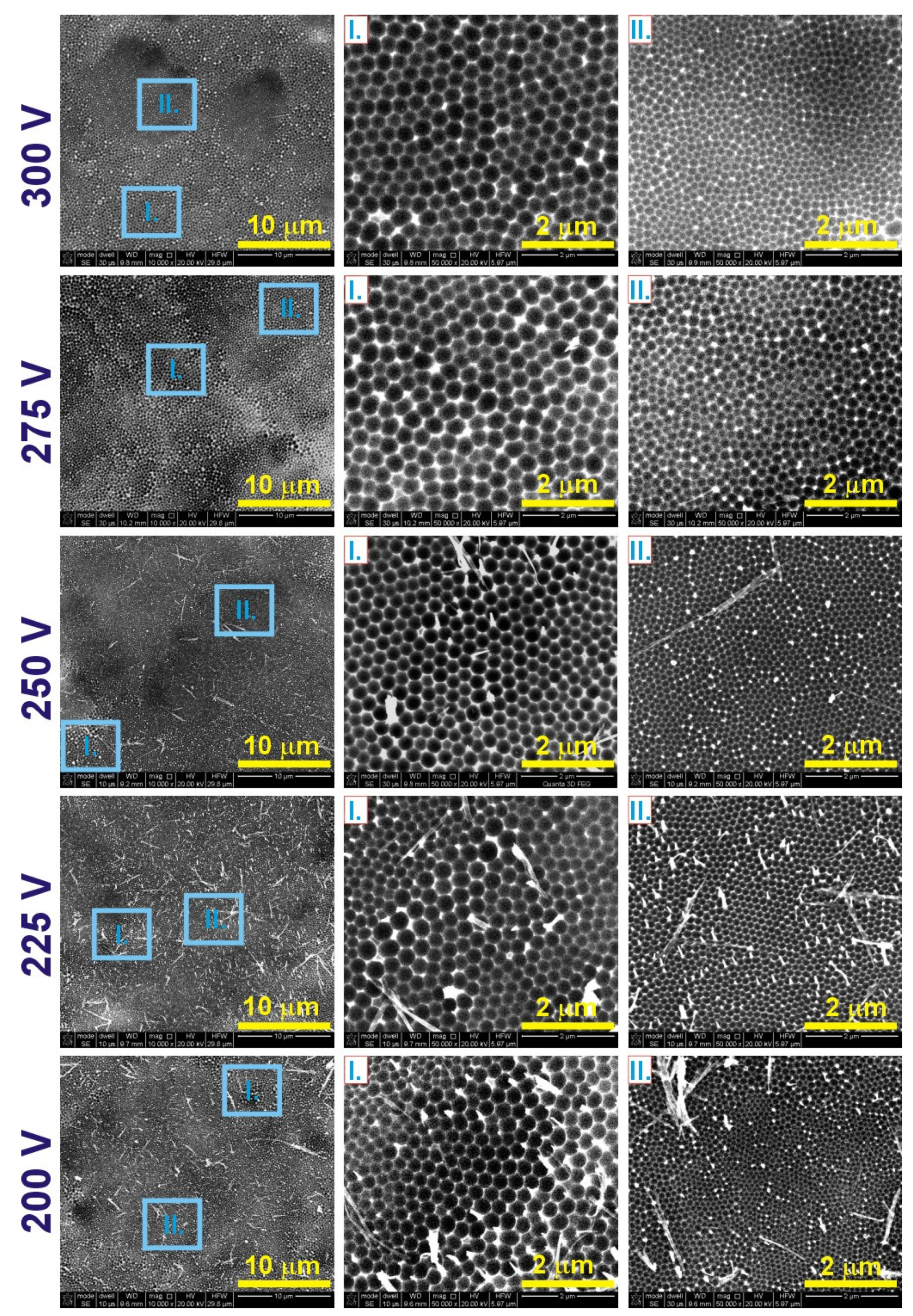
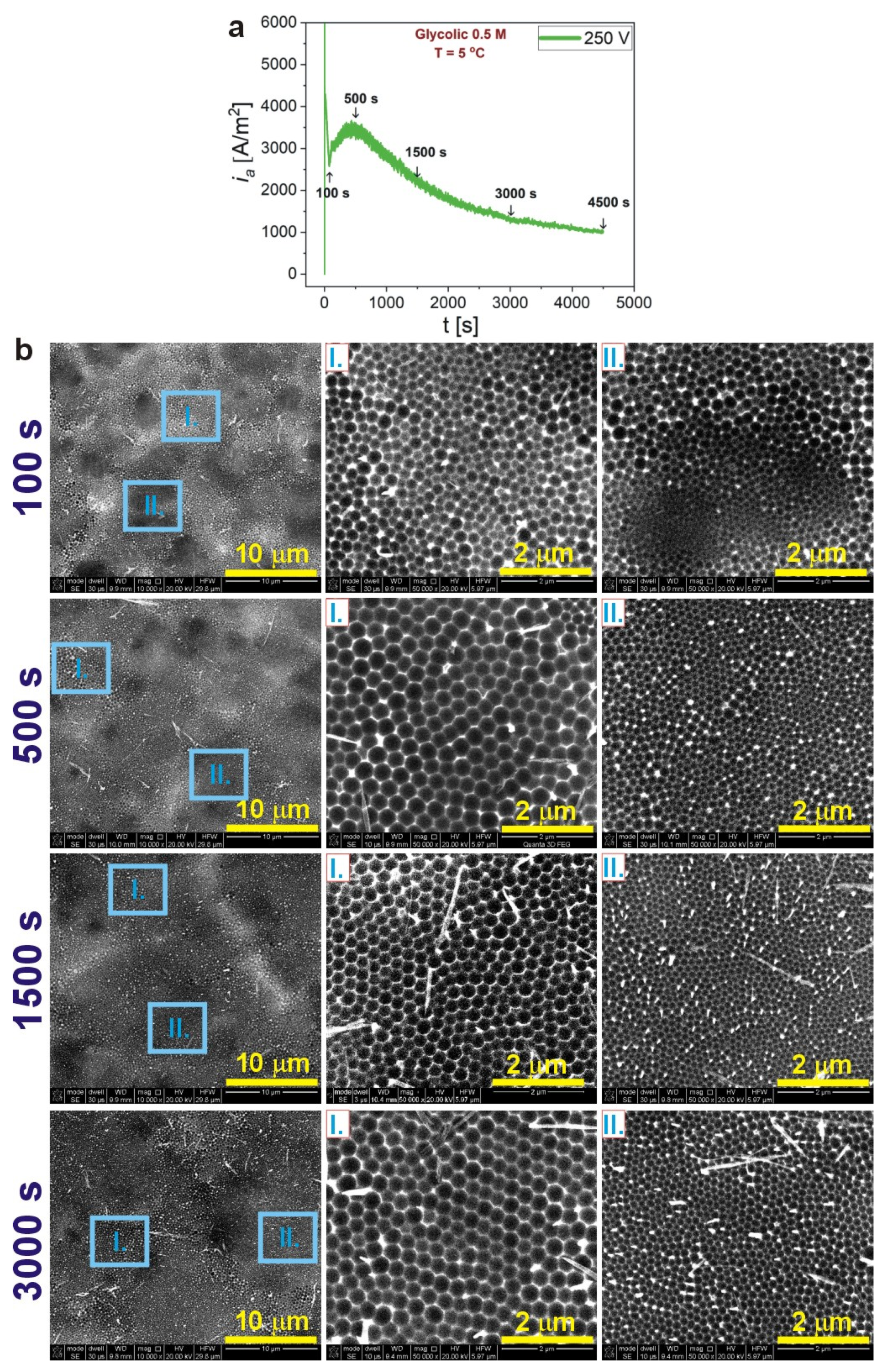

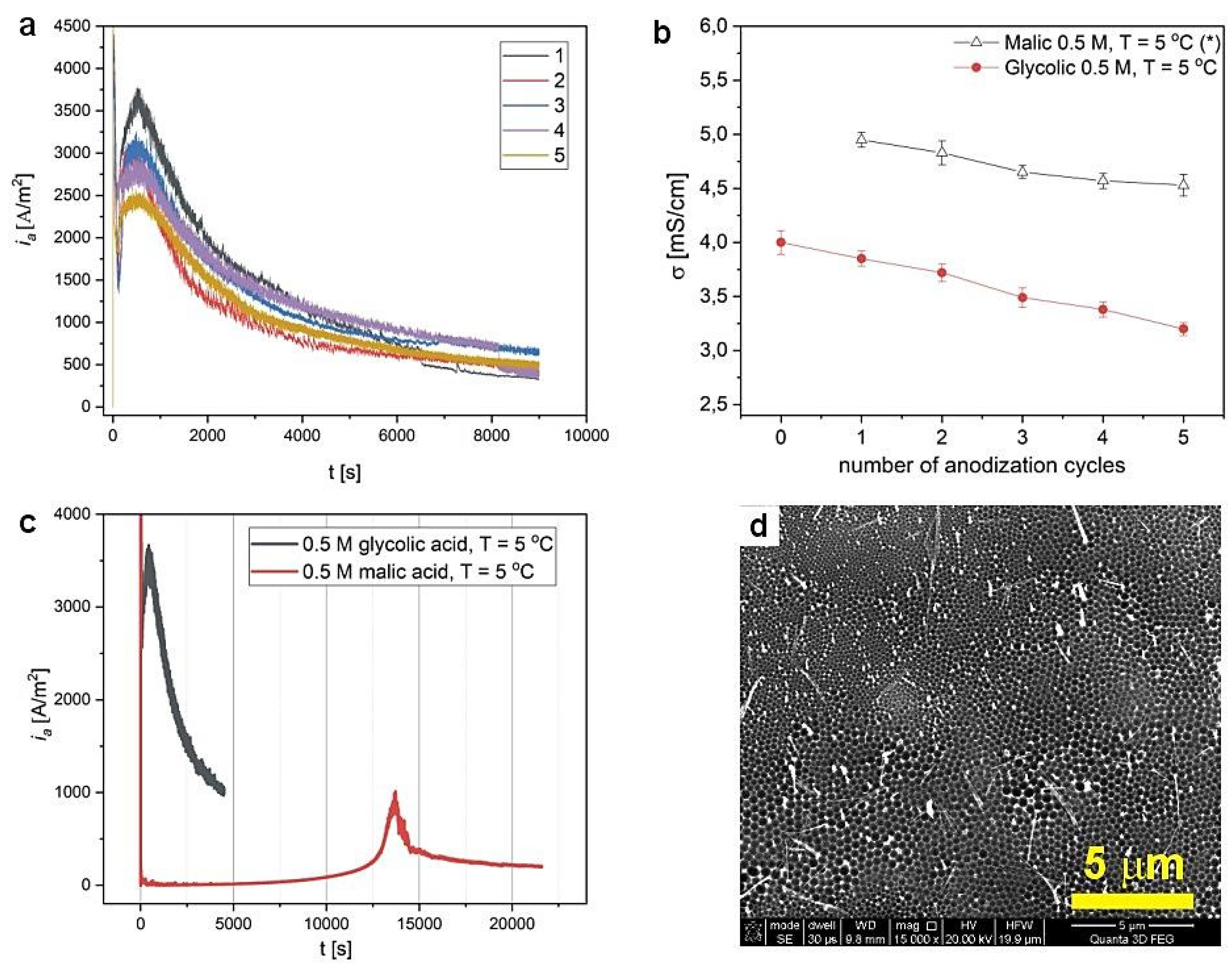
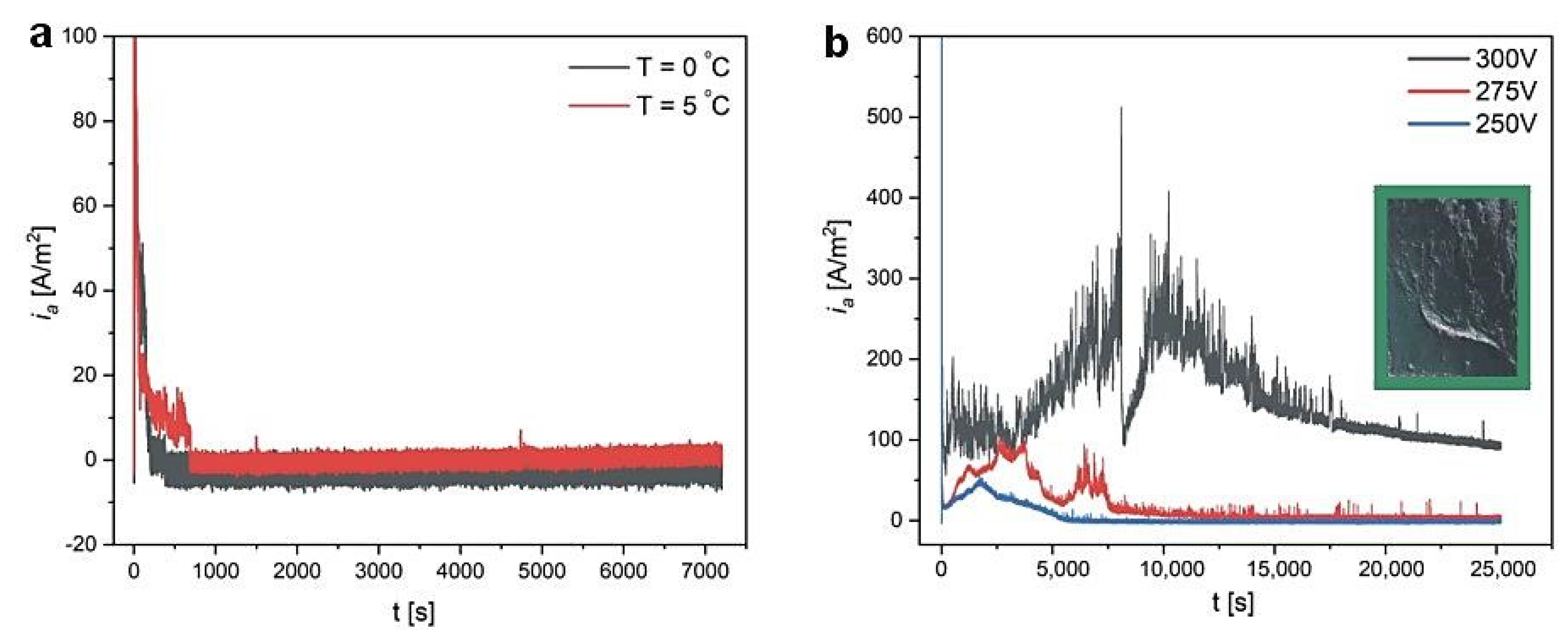
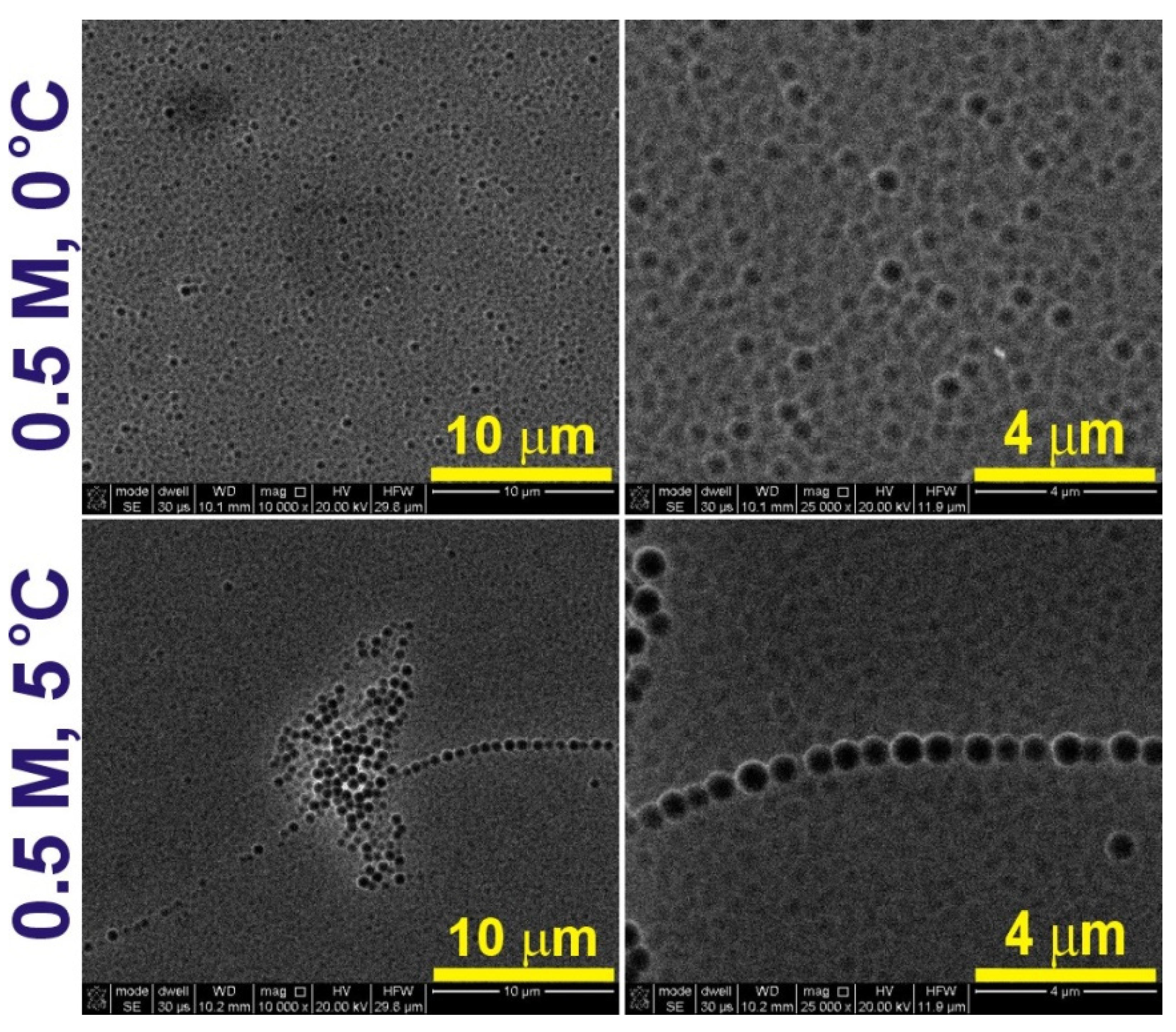
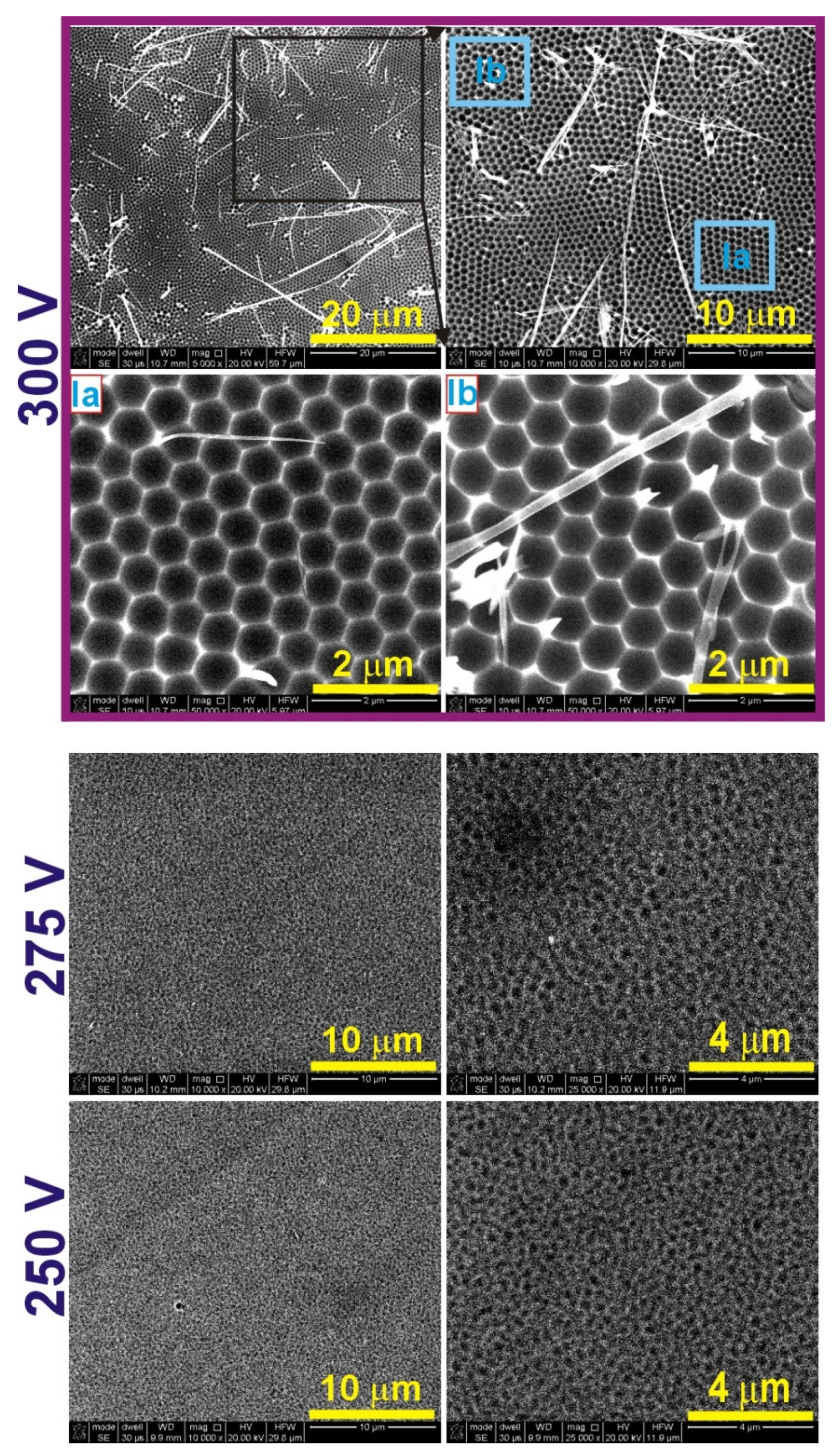
| 300 V | 275 V | 250 V | 225 V | 200 V | |
|---|---|---|---|---|---|
| Dc (nm) | I. 397 ± 39 II. 193 ± 41 | I. 408 ± 38 II. 202 ± 30 | I. 369 ± 22 II. 136 ± 26 | I. 305 ± 49 II. 145 ± 18 | I. 295 ± 29 II. 116 ± 18 |
| Electrolyte, Type of the Process | Anodizing Voltage (V) | Anodizing Temperature (°C) | Anodizing Time (h) | AAO Thickness (μm) | Refs |
|---|---|---|---|---|---|
| 0.3 M sulfuric acid, MA | 25/25/25 | 1/0/1 | 10/4/1 | ~40/~15/~10 | [47]/[48]/[49] |
| 0.3 M oxalic acid, MA | 40/40/40 | 1/0/17 | 16/4/1 | ~30/~10/~15 | [47]/[48]/[49] |
| 0.3 M oxalic acid:ethanol = 1:1, v/v, MA | 40 | 0 | 24 | ~12 | [50] |
| 0.3 M oxalic acid, HA | 140 | 1 | 1 | ~80 | [46] |
| 2 wt% phosphoric acid, MA | 175 | 0 | 30 | ~55 | [47] |
| 1 wt% phosphoric acid, MA | 195 | 0 | 1 | ~2.5 | [51] |
| 0.1 M phosphoric acid, MA | 195 | 12 | 1 | ~6 | [49] |
| 1.5 M citric acid, JA | 400 | 0 | 1 | ~50 | [39] |
| 0.5 M malic acid, JA | 250 | 5 | 6 | ~162 | [44] |
| 0.5 M glycolic acid, JA | 250 | 5 | 1 | ~142 | This work |
| Glycolic 0.5 M 250 V | Malic 0.5 M 250 V | Citric 1.5 M 300 V | |
|---|---|---|---|
| Dc (nm) | I. 369 ± 22 II. 136 ± 26 | 527 ± 12 (*) | Ia. 605 ± 20 Ib. 677 ± 55 |
| σ (mS/cm) | |||
|---|---|---|---|
| 0.5 M | 0 °C | fresh | 7.42 ± 0.10 |
| 300 V | 7.39 ± 0.09 | ||
| 5 °C | 300 V | 7.24 ± 0.07 | |
| 1.5 M | 5 °C | fresh | 8.09 ± 0.08 |
| 300 V | 8.15 ± 0.08 | ||
| 275 V | 8.12 ± 0.06 | ||
| 250 V | 8.13 ± 0.11 | ||
| Name (Molecular Mass (g/mol)) | Molecular Structure | pKa T~25 °C | Conductivity (mS/cm) | Concentration (M) | Umax (V) |
|---|---|---|---|---|---|
| Citric acid (192.12) | 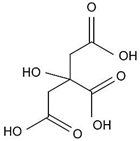 | 3.1 (COOH) 4.8 (COOH) 6.4 (COOH) | 8.15 ± 0.08 | 1.5 | 400 V * 300 V |
| Malic acid (134.08) | 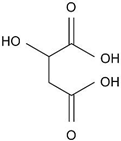 | 3.5 (COOH) 5.1 (COOH) | 4.95 ± 0.07 ** | 0.5 ** | 250 V ** |
| Glycolic acid (76.05) | 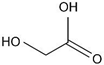 | 3.8 (COOH) | 3.85 ± 0.07 | 0.5 | 250–225 V |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zajączkowska, L.; Norek, M. Peculiarities of Aluminum Anodization in AHAs-Based Electrolytes: Case Study of the Anodization in Glycolic Acid Solution. Materials 2021, 14, 5362. https://doi.org/10.3390/ma14185362
Zajączkowska L, Norek M. Peculiarities of Aluminum Anodization in AHAs-Based Electrolytes: Case Study of the Anodization in Glycolic Acid Solution. Materials. 2021; 14(18):5362. https://doi.org/10.3390/ma14185362
Chicago/Turabian StyleZajączkowska, Lidia, and Małgorzata Norek. 2021. "Peculiarities of Aluminum Anodization in AHAs-Based Electrolytes: Case Study of the Anodization in Glycolic Acid Solution" Materials 14, no. 18: 5362. https://doi.org/10.3390/ma14185362
APA StyleZajączkowska, L., & Norek, M. (2021). Peculiarities of Aluminum Anodization in AHAs-Based Electrolytes: Case Study of the Anodization in Glycolic Acid Solution. Materials, 14(18), 5362. https://doi.org/10.3390/ma14185362







