Kinetics, Thermodynamics and Equilibrium Studies for Gold Recovery from Diluted Waste Solution
Abstract
:1. Introduction
2. Materials and Methods
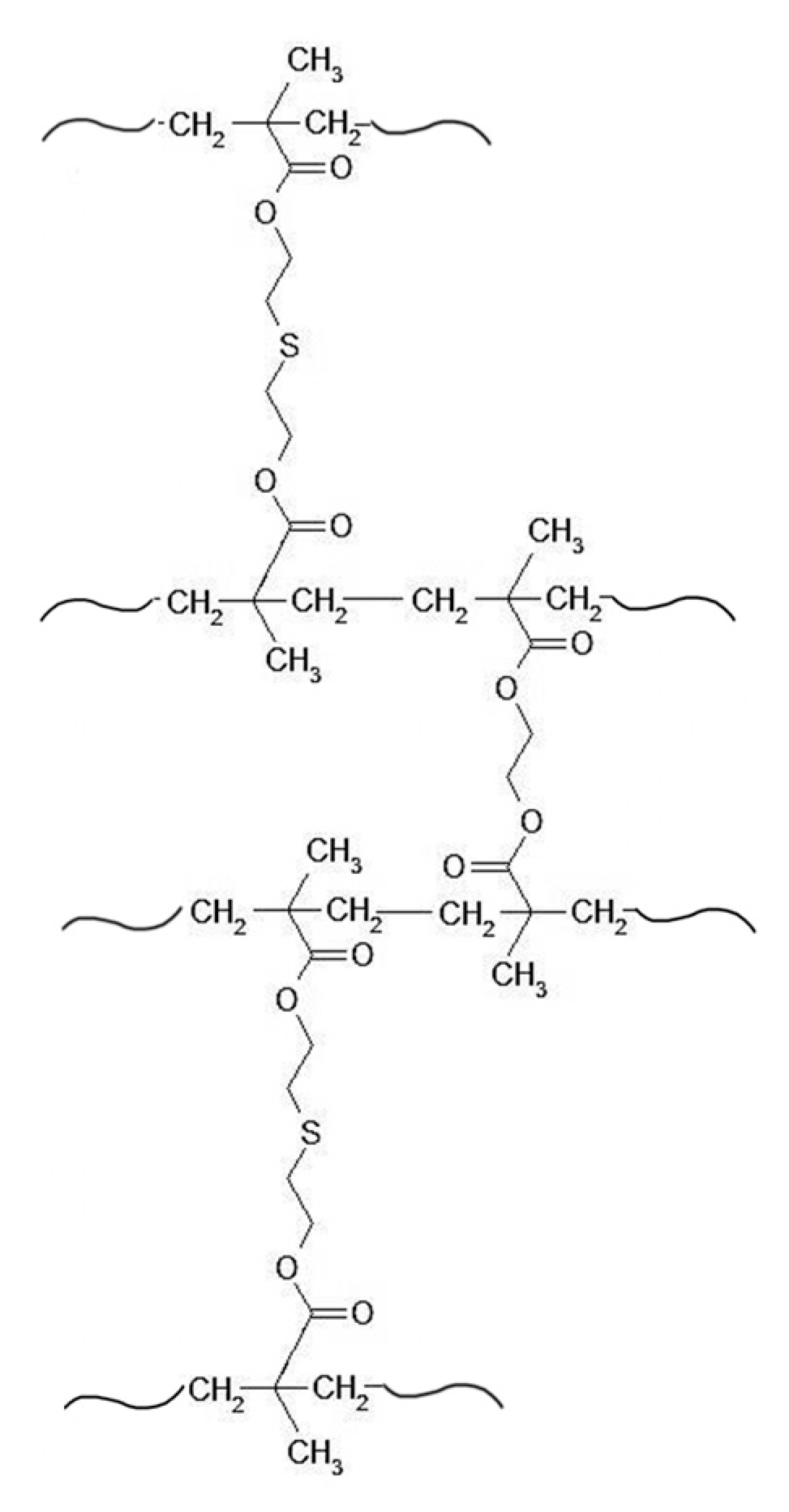
2.1. Preparation of Polymeric Sorbent
2.2. Characterization of Polymeric Sorbent
2.3. Sorption Studies
3. Results and Discussion
3.1. Characterization of Polymeric Sorbent
3.1.1. Physico-Chemical Characteristics
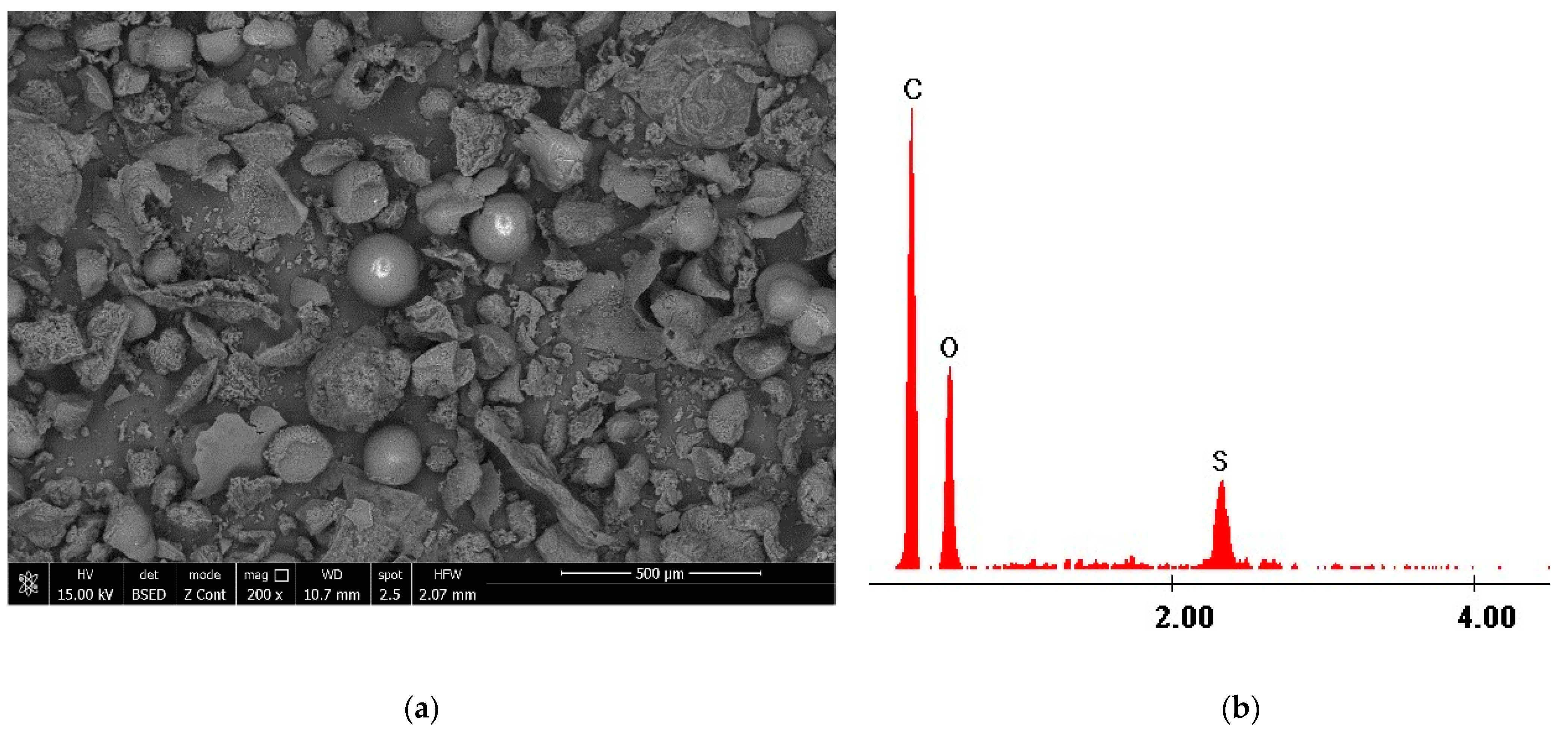
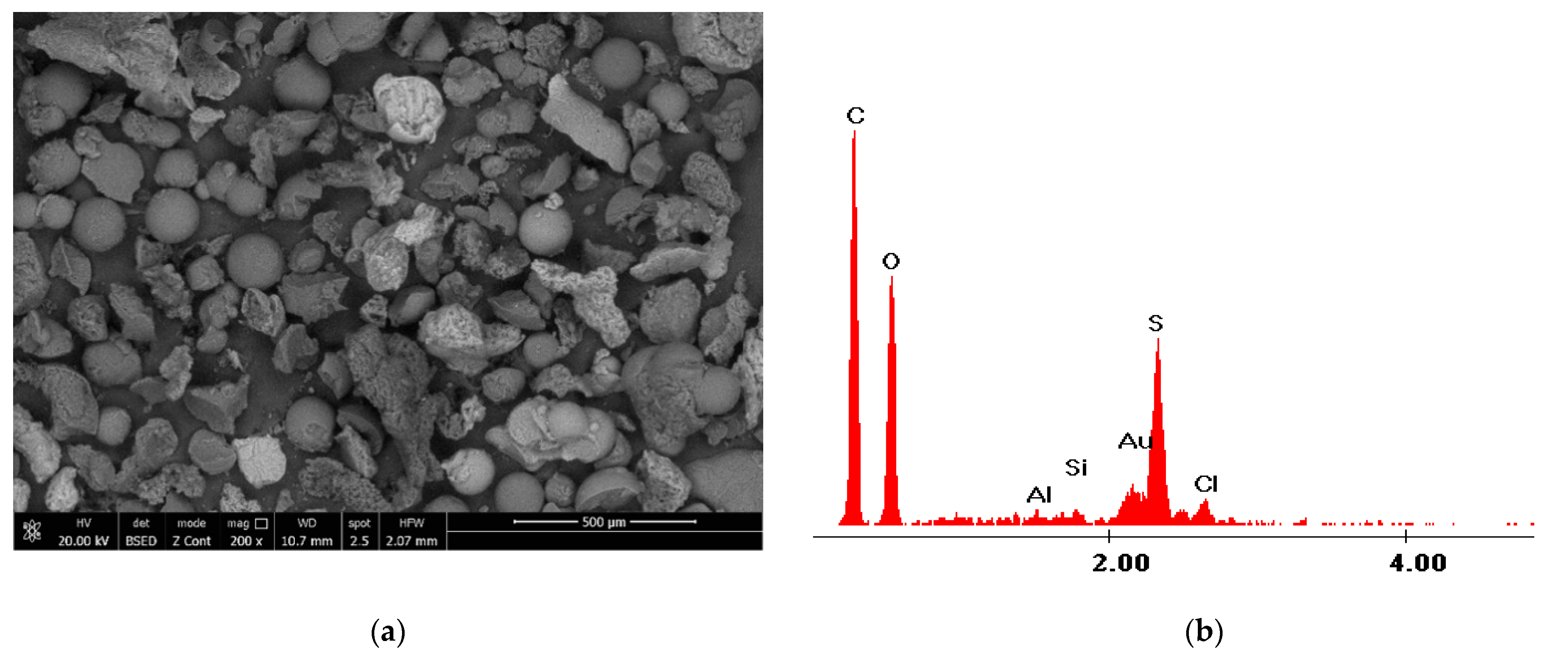
3.1.2. Scanning Electron Microscopy, SEM, and Energy Dispersive X-ray Diffraction, EDX
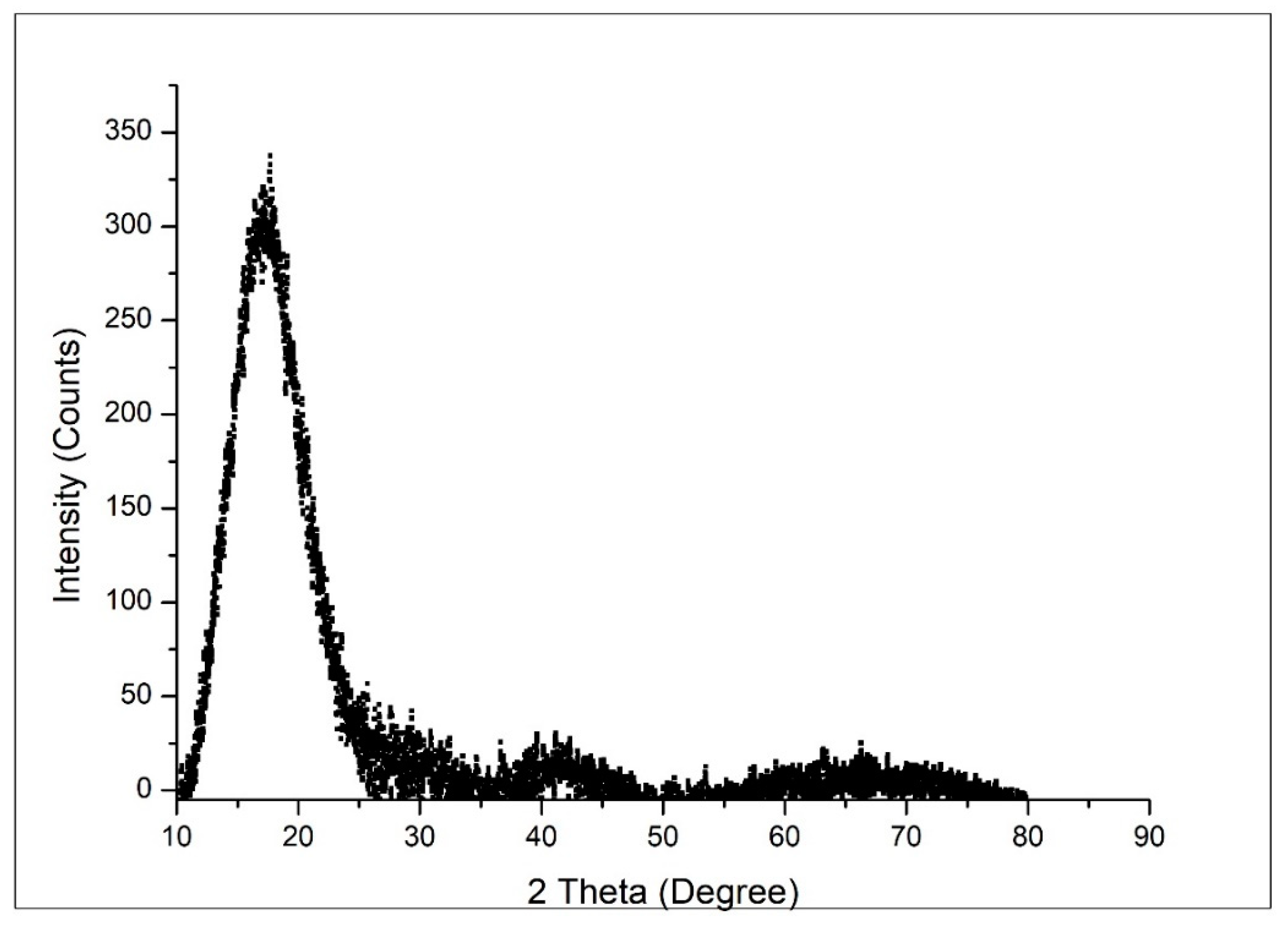
3.1.3. X-ray Diffraction, XRD
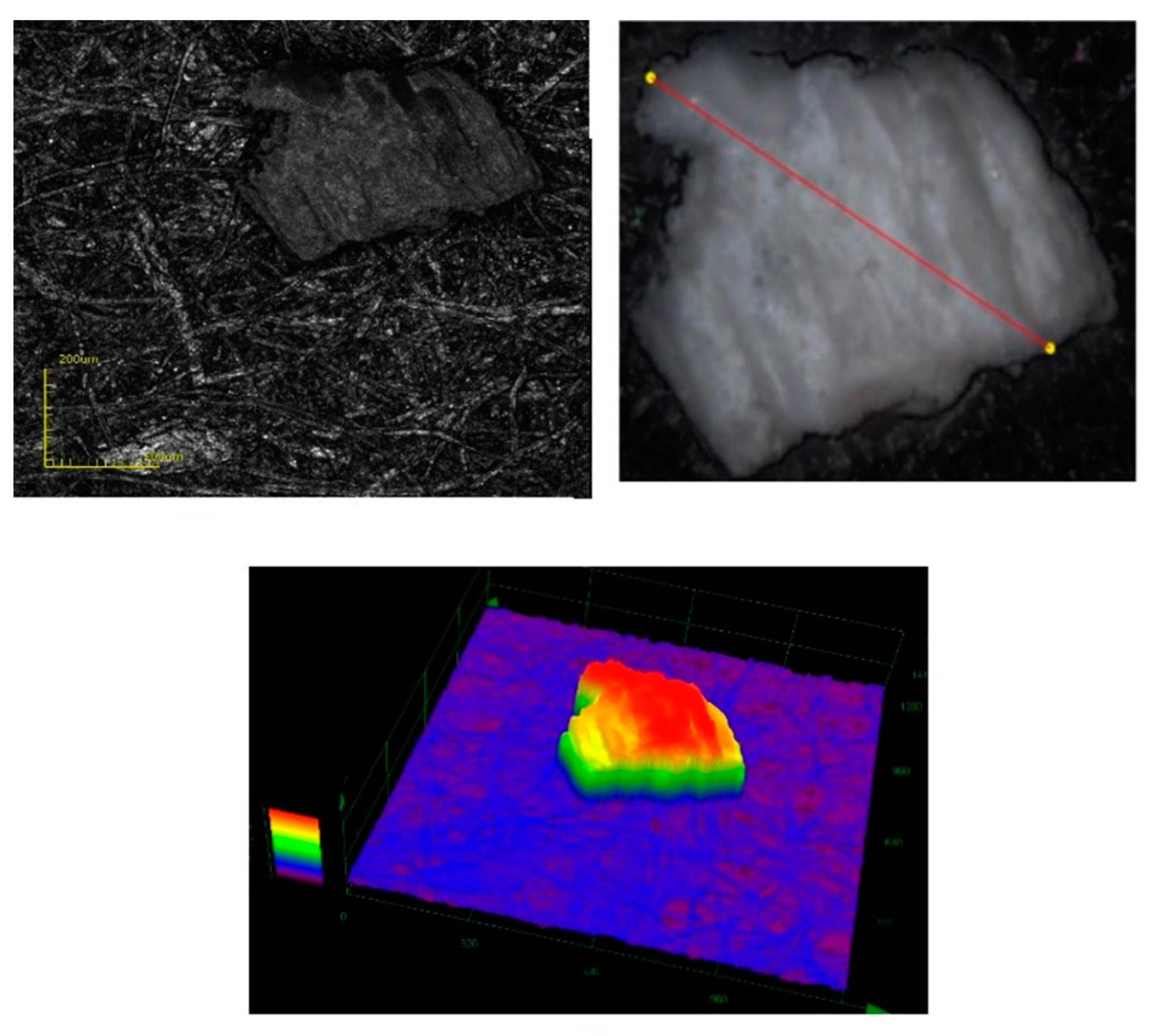
3.1.4. Confocal Laser Scanning Microscopy, CLSM
3.2. Sorption Studies
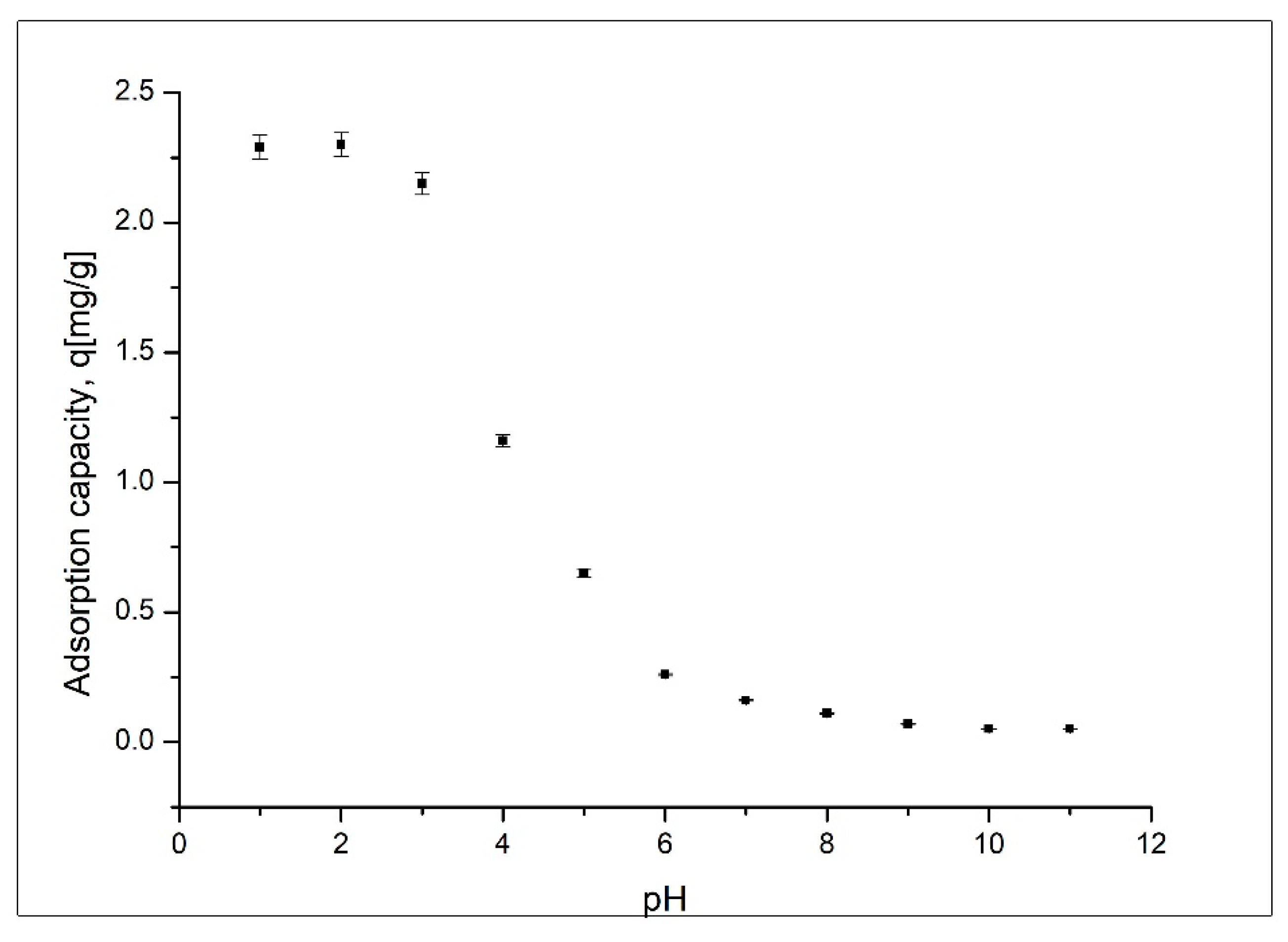
Effect of pH
3.3. Kinetic Studies
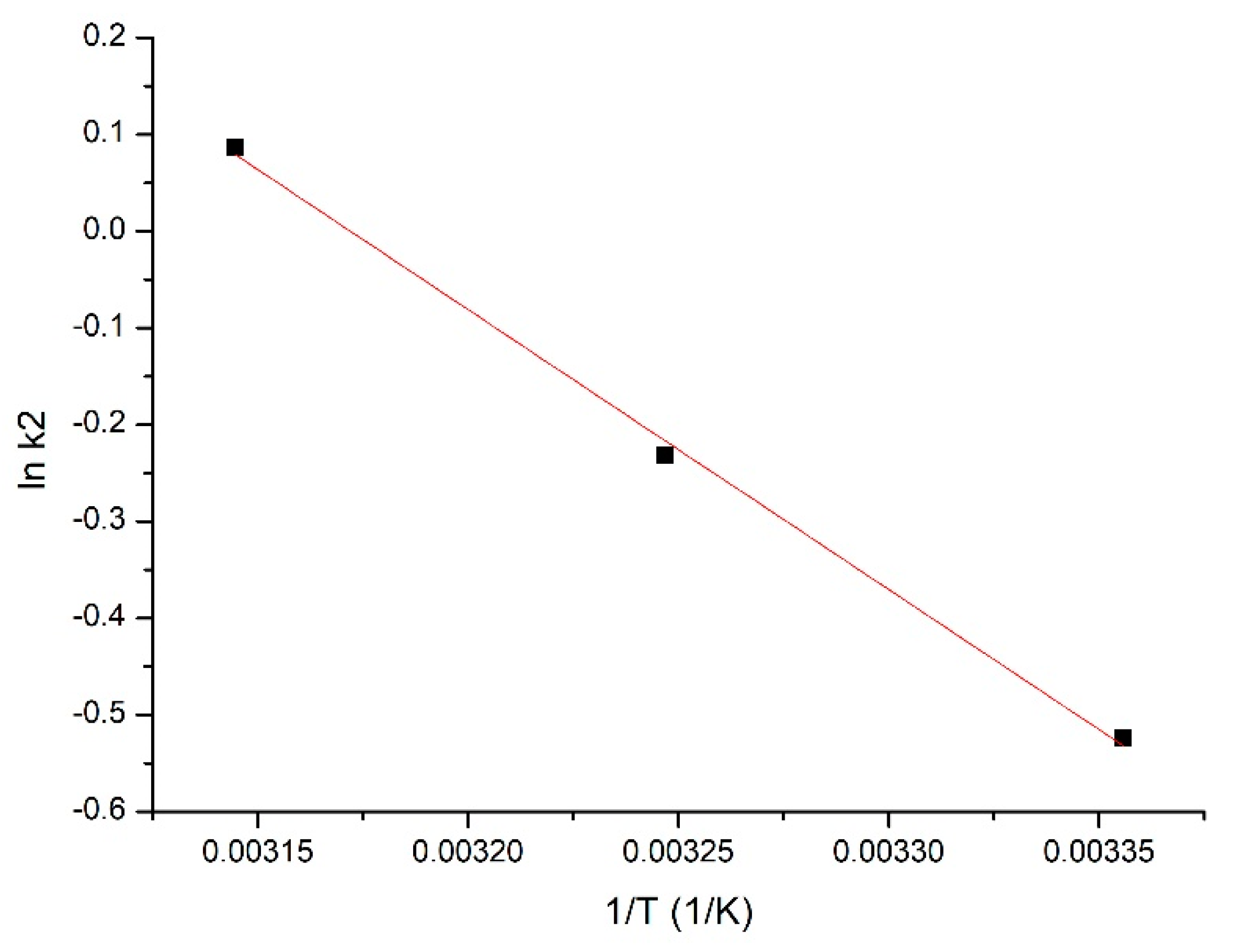
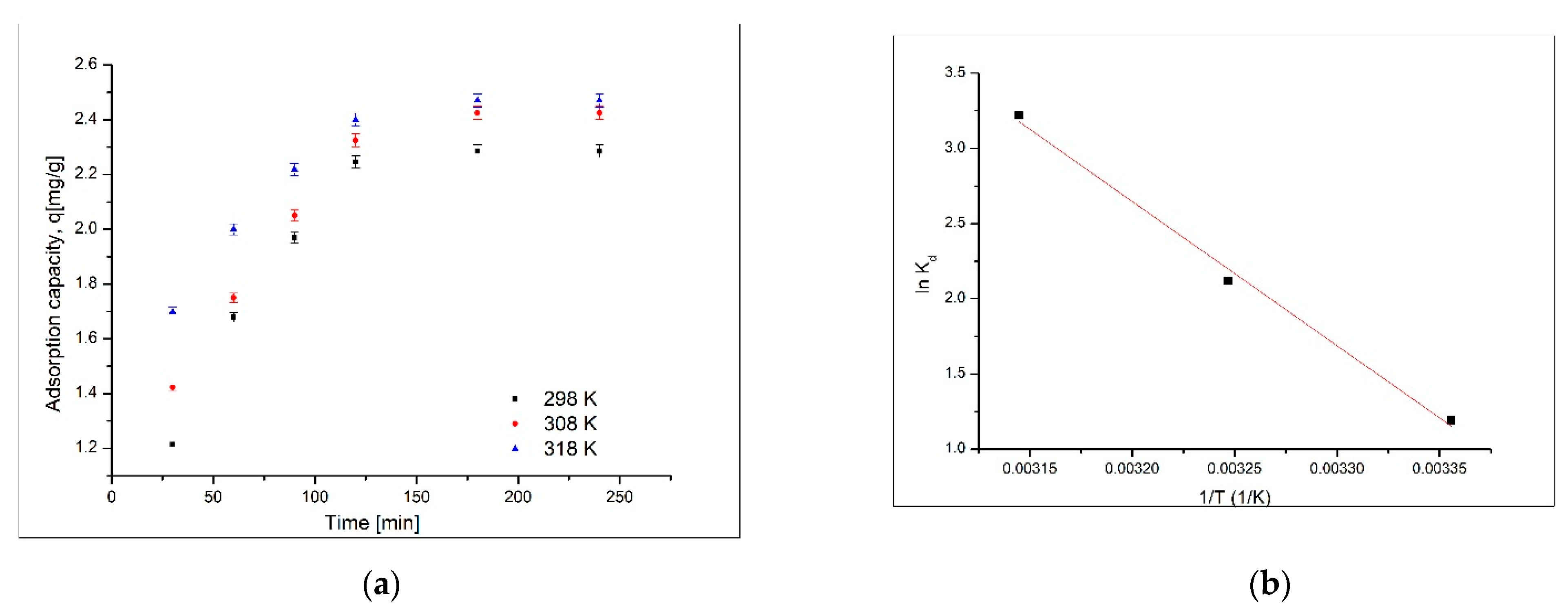
3.4. Thermodynamic Study
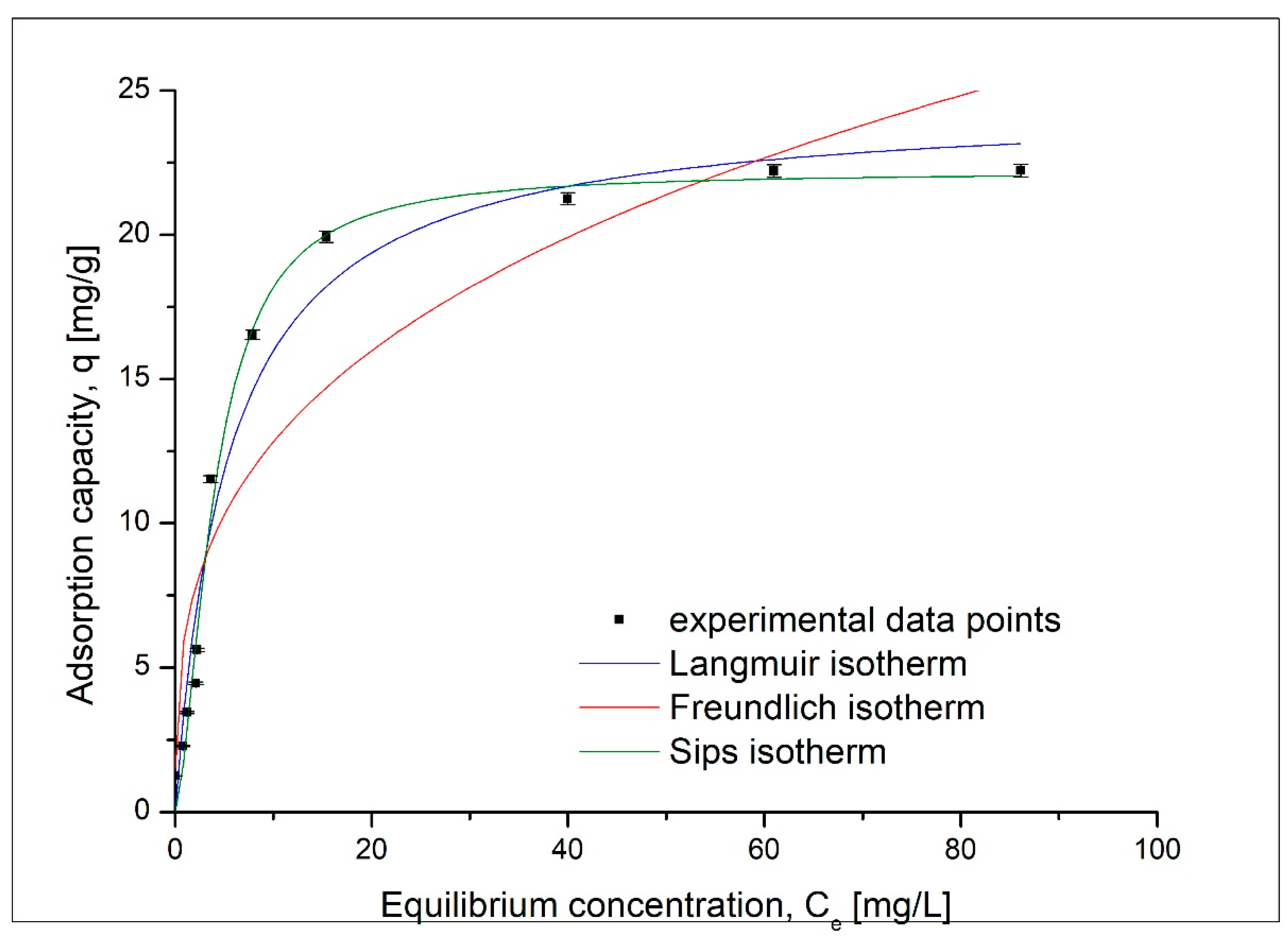
3.5. Effect of Initial Concentration Equilibrium Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mihailescu, M.; Negrea, A.; Ciopec, M.; Negrea, P.; Duteanu, N.; Grozav, I.; Svera, P.; Vancea, C.; Barbulescu, A.; Dumitriu, C.S. Full Factorial Design for Gold Recovery from Industrial Solutions. Toxics 2021, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Mihailescu, M.; Negrea, A.; Ciopec, M.; Davidescu, C.M.; Negrea, P.; Duteanu, N.; Rusu, G. Gold (III) adsorption from dilute waste solutions onto Amberlite XAD7 resin modified with L-glutamic acid. Sci. Rep. 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Negrea, A.; Mihailescu, M.; Mosoarca, G.; Ciopec, M.; Duteanu, N.; Negrea, P.; Minzatu, V. Estimation on Fixed-Bed Column Parameters of Breakthrough Behaviors for Gold Recovery by Adsorption onto Modified/Functionalized Amberlite XAD7. Int. J. Environ. Res. Public Health 2020, 17, 14. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freundlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Sips, R. On the Structure of a Catalyst Surface. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Ciopec, M.; Davidescu, C.M.; Negrea, A.; Grozav, I.; Lupa, L.; Muntean, C.; Negrea, P.; Popa, A. Statististical optimization of chromium ions adsorption on dehpa-impregnated amberlite XAD7. Environ. Eng. Manag. J. 2012, 11, 525–531. [Google Scholar] [CrossRef]
- Ronka, S. New sulfur-containing polymeric sorbents based on 2,2′-thiobisethanol dimethacrylate. Pure Appl. Chem. 2019, 91, 409–420. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [Green Version]
- Ho, Y.S.; Mckay, G. The kinetics of sorption of basic dyes from aqueous solution by sphagnum moss peat. Can. J. Chem. Eng. 1998, 76, 822–827. [Google Scholar] [CrossRef]
- Atkins, P.; de Paula, J. Atkins’ Physical Chemistry; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Ronka, S.; Targońska, S. Gold(III) ions sorption on sulfur-containing polymeric sorbent based on 2,2‘-thiobisethanol dimethacrylate. Sep. Sci. Technol. 2020, 55, 2158–2169. [Google Scholar] [CrossRef]
- Kucuker, M.A.; Wieczorek, N.; Kuchta, K.; Copty, N.K. Biosorption of neodymium on Chlorella vulgaris in aqueous solution obtained from hard disk drive magnets. PLoS ONE 2017, 12, 13. [Google Scholar] [CrossRef]
- Esposito, A.; Pagnanelli, F.; Veglio, F. pH-related equilibria models for biosorption in single metal systems. Chem. Eng. Sci. 2002, 57, 307–313. [Google Scholar] [CrossRef]
- Aksu, Z.; Isoglu, I.A. Removal of copper(II) ions from aqueous solution by biosorption onto agricultural waste sugar beet pulp. Process Biochem. 2005, 40, 3031–3044. [Google Scholar] [CrossRef]
- Fujiwara, K.; Ramesh, A.; Maki, T.; Hasegawa, H.; Ueda, K. Adsorption of platinum (IV), palladium (II) and gold (III) from aqueous solutions onto l-lysine modified crosslinked chitosan resin. J. Hazard. Mater. 2007, 146, 39–50. [Google Scholar] [CrossRef]
- Palmieri, M.C.; Volesky, B.; Garcia, O. Biosorption of lanthanum using Sargassum fluitans in batch system. Hydrometallurgy 2002, 67, 31–36. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Sathishkumar, M.; Balasubramanian, R. Interaction of rare earth elements with a brown marine alga in multi-component solutions. Desalination 2011, 265, 54–59. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, B.Z.; Shi, X.C.; Chen, Y. Boron removal from brine by XSC-700. J. Cent. South Univ. 2012, 19, 2768–2773. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, F.; Cheng, W.P.; Wang, J.C.; Ma, J.J. Adsorption Equilibrium and Kinetics of the Removal of Ammoniacal Nitrogen by Zeolite X/Activated Carbon Composite Synthesized from Elutrilithe. J. Chem. 2017, 2017, 9. [Google Scholar] [CrossRef]
- Unit 3-Physical Properties & Intermolecular Interactions Elaboration-Enthalpy, Entropy and Free Energy. Available online: https://www.chem.uwec.edu/Chem150_Resources/content/elaborations/unitxxx/unit3-b-energy.html (accessed on 20 November 2019).
- Zhang, S.; Kano, N.; Mishima, K.; Okawa, H. Adsorption and Desorption Mechanisms of Rare Earth Elements (REEs) by Layered Double Hydroxide (LDH) Modified with Chelating Agents. Appl. Sci. 2019, 9, 4805. [Google Scholar] [CrossRef] [Green Version]
- Feng, S.; Du, X.; Bat-Amgalan, M.; Zhang, H.; Miyamoto, N.; Kano, N. Adsorption of REEs from Aqueous Solution by EDTA-Chitosan Modified with Zeolite Imidazole Framework (ZIF-8). Int. J. Mol. Sci. 2021, 22, 3447. [Google Scholar] [CrossRef]
- Duong, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998; Volume 2. [Google Scholar]
- Hang, C.Z.; Hu, B.; Jiang, Z.C.; Zhang, N. Simultaneous on-line preconcentration and determination of trace metals in environmental samples using a modified nanometer-sized alumina packed micro-column by flow injection combined with ICP-OES. Talanta 2007, 71, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liang, P. Determination of gold by nanometer titanium dioxide immobilized on silica gel packed microcolumn and flame atomic absorption spectrometry in geological and water samples. Anal. Chim. Acta 2007, 604, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Senturk, H.B.; Gundogdu, A.; Bulut, V.N.; Duran, C.; Soylak, M.; Elci, L.; Tufekci, M. Separation and enrichment of gold(III) from environmental samples prior to its flame atomic absorption spectrometric determination. J. Hazard. Mater. 2007, 149, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Pu, Q.S.; Su, Z.X.; Hu, Z.H.; Chang, X.J.; Yang, M. 2-mercaptobenzothiazole-bonded silica gel as selective adsorbent for preconcentration of gold, platinum and palladium prior to their simultaneous inductively coupled plasma optical emission spectrometric determination. J. Anal. At. Spectrom. 1998, 13, 249–253. [Google Scholar] [CrossRef]
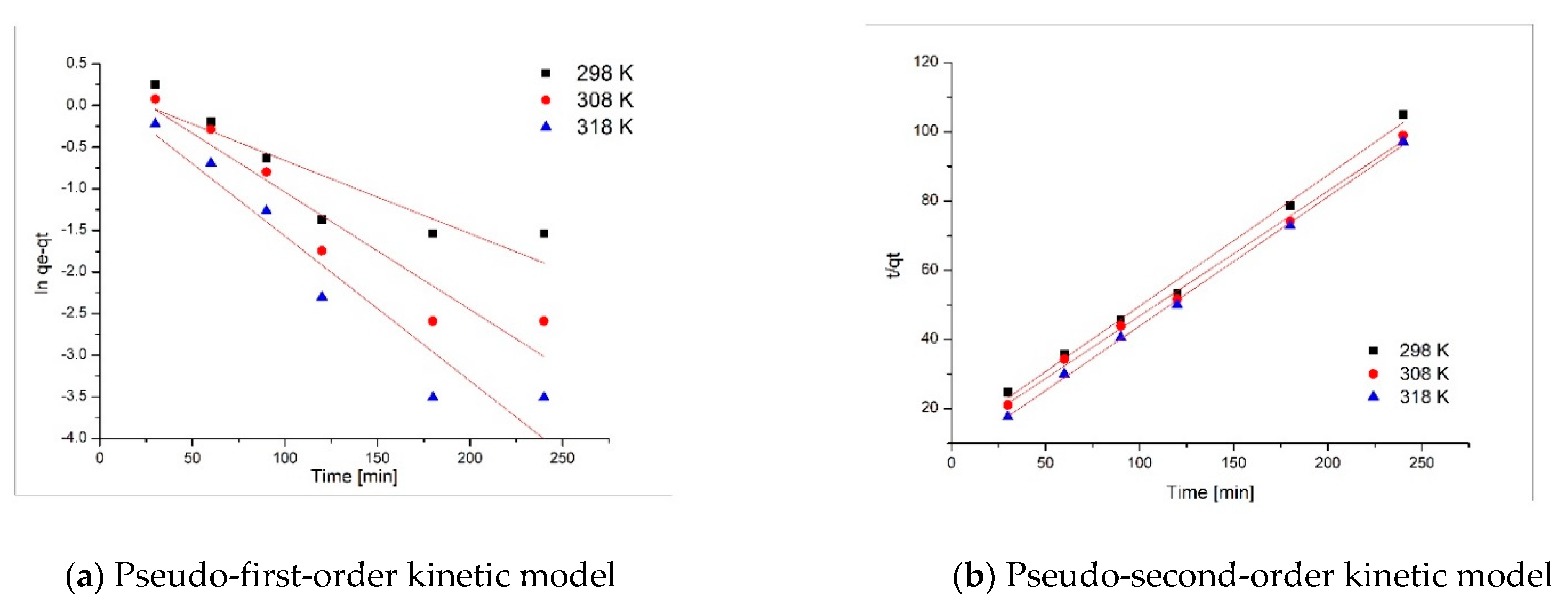

| Pseudo-First Order | ||||
| Temperature (K) | qe,exp (mg·g−1) | k1 (min−1) | qe,calc (mg·g−1) | R2 |
| 298 | 2.28 | 0.0088 | 1.24 | 0.8121 |
| 308 | 2.42 | 0.0141 | 1.45 | 0.9107 |
| 318 | 2.47 | 0.0174 | 1.18 | 0.924 |
| Pseudo-Second Order | ||||
| Temperature (K) | qe,exp (mg·g−1) | k2 (g mg−1·min−1) | qe,calc (mg·g−1) | R2 |
| 298 | 2.28 | 0.591 | 2.63 | 0.9942 |
| 308 | 2.42 | 0.793 | 2.76 | 0.9963 |
| 318 | 2.47 | 1.090 | 2.67 | 0.9989 |
| Material | Activated Energy, Ea kJ/mol | R2 |
|---|---|---|
| (coP-TEDMA/EGDMA) | 24.1 | 0.9982 |
| ΔHº (kJ/mol) | ΔSº (J/mol·K) | ΔGº (kJ/mol) | R2 | ||
|---|---|---|---|---|---|
| 79.7 | 2.72 | 298 K | 308 K | 318 K | 0.9955 |
| −2.8 | −5.6 | −8.4 | |||
| Langmuir Isotherm | |||
| qm,exp (mg/g) | KL (L/mg) | qL (mg/g) | R2 |
| 22.2 | 0.185 | 24.6 | 0.9667 |
| Freundlich Isotherm | |||
| KF (mg/g) | 1/nF | R2 | |
| 6.16 | 0.318 | 0.8465 | |
| Sips Isotherm | |||
| KS | qS (mg/g) | 1/nS | R2 |
| 0.63 | 22.18 | 0.10 | 0.9884 |
| Sorbent | Sorption Capacities, mg Au(III)/g | Reference |
|---|---|---|
| 3-(8-Quinolinylazo)-4-hydroxybenzoic acid modified nanometer-sized alumina | 17.7 | [25] |
| Nanometer TiO2 immobilized on silica gel | 3.56 | [26] |
| Amberlite XAD-2000/DDTC | 12.3 | [27] |
| 2-Mercaptobenzothiazole-bonded silica gel | 4.5 | [28] |
| coP-TEDMA/EGDMA | 22.2 | Present study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negrea, A.; Ronka, S.; Ciopec, M.; Duteanu, N.; Negrea, P.; Mihailescu, M. Kinetics, Thermodynamics and Equilibrium Studies for Gold Recovery from Diluted Waste Solution. Materials 2021, 14, 5325. https://doi.org/10.3390/ma14185325
Negrea A, Ronka S, Ciopec M, Duteanu N, Negrea P, Mihailescu M. Kinetics, Thermodynamics and Equilibrium Studies for Gold Recovery from Diluted Waste Solution. Materials. 2021; 14(18):5325. https://doi.org/10.3390/ma14185325
Chicago/Turabian StyleNegrea, Adina, Sylwia Ronka, Mihaela Ciopec, Narcis Duteanu, Petru Negrea, and Maria Mihailescu. 2021. "Kinetics, Thermodynamics and Equilibrium Studies for Gold Recovery from Diluted Waste Solution" Materials 14, no. 18: 5325. https://doi.org/10.3390/ma14185325
APA StyleNegrea, A., Ronka, S., Ciopec, M., Duteanu, N., Negrea, P., & Mihailescu, M. (2021). Kinetics, Thermodynamics and Equilibrium Studies for Gold Recovery from Diluted Waste Solution. Materials, 14(18), 5325. https://doi.org/10.3390/ma14185325








