Synthesis and Characterization of a Trifunctional Photoinitiator Based on Two Commercial Photoinitiators with α-Hydroxyl Ketone Structure
Abstract
:1. Introduction
2. Experimental
2.1. Materials
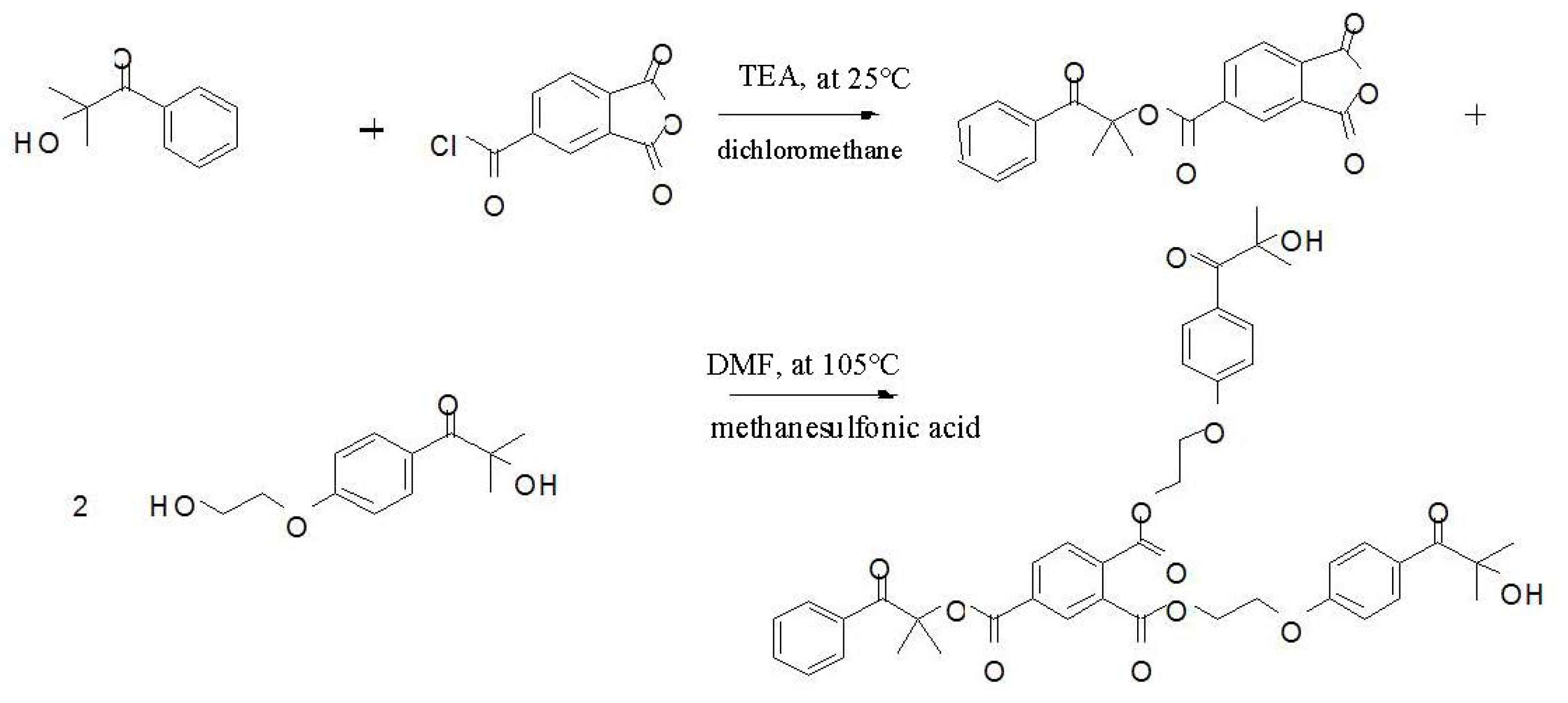
2.2. Preparation of the Trifunctional Photoinitiator (HTH)
2.3. Preparation of UV Curable Films
2.4. Characterization of the Products
2.5. Migration of the Photoinitiators
2.6. Photoinitiator Activity
3. Results and Discussion
3.1. FTIR
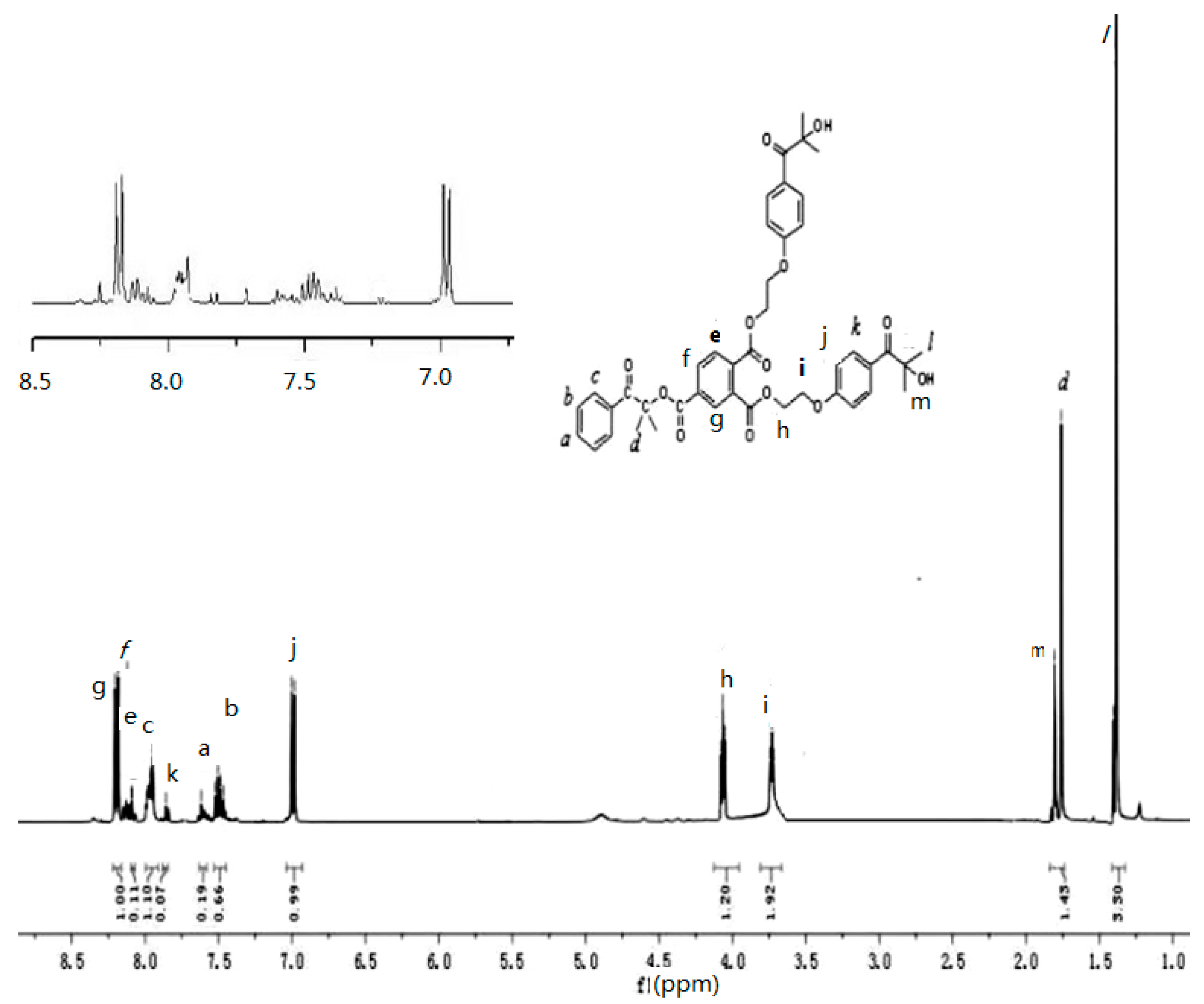
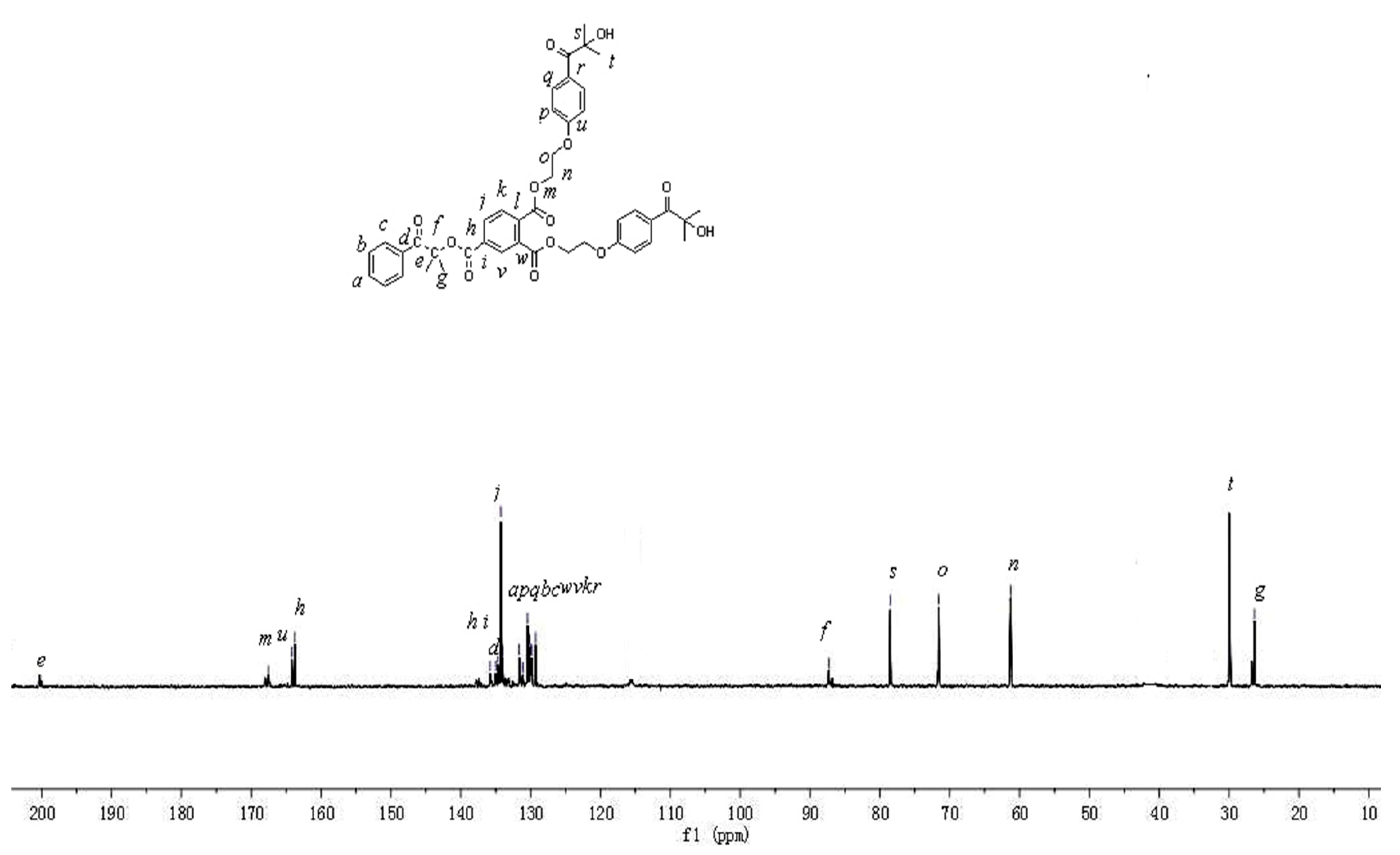
3.2. NMR
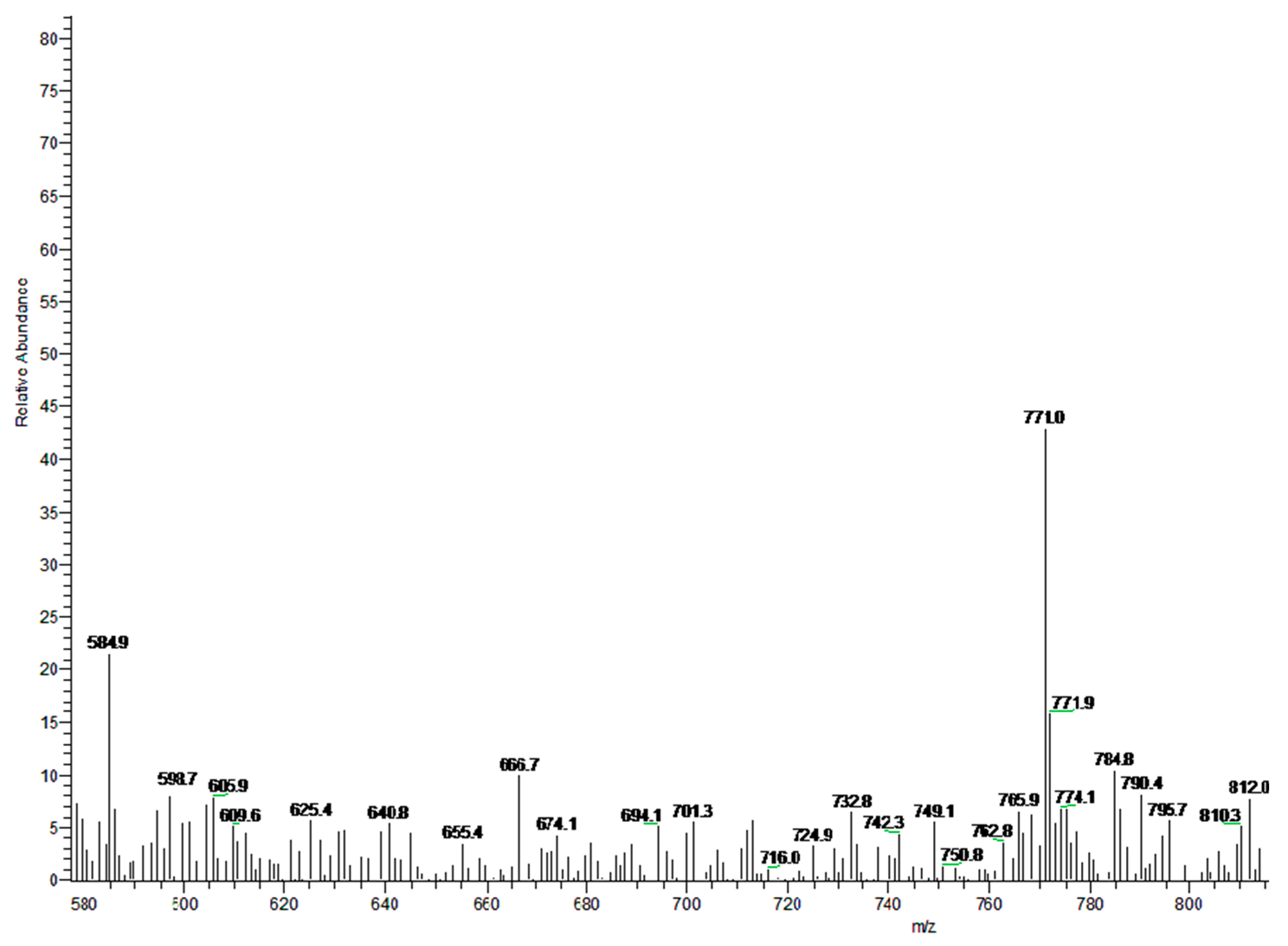
3.3. MS
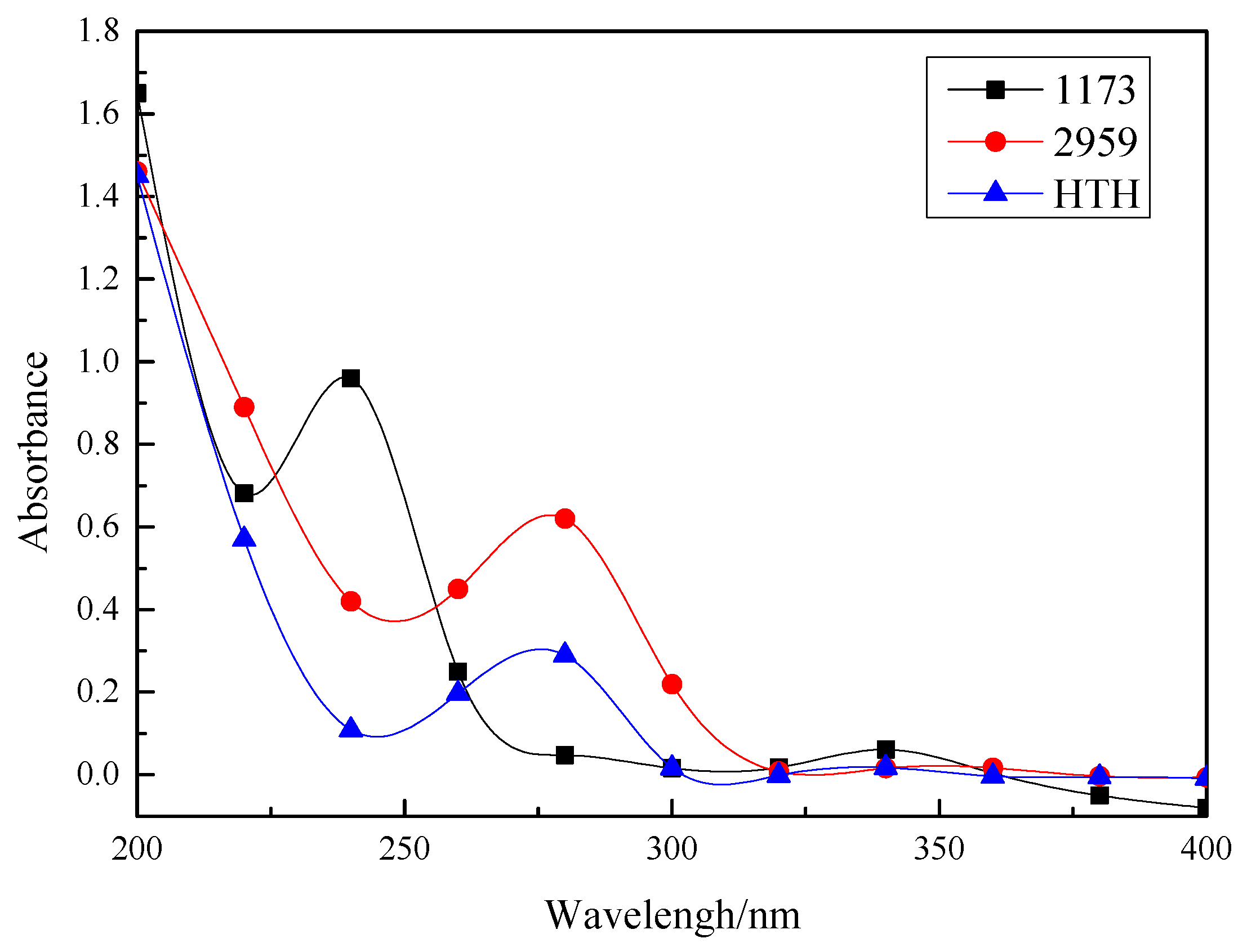
3.4. UV Absorption Spectra
3.5. Thermogravimetric Analysis (TGA)
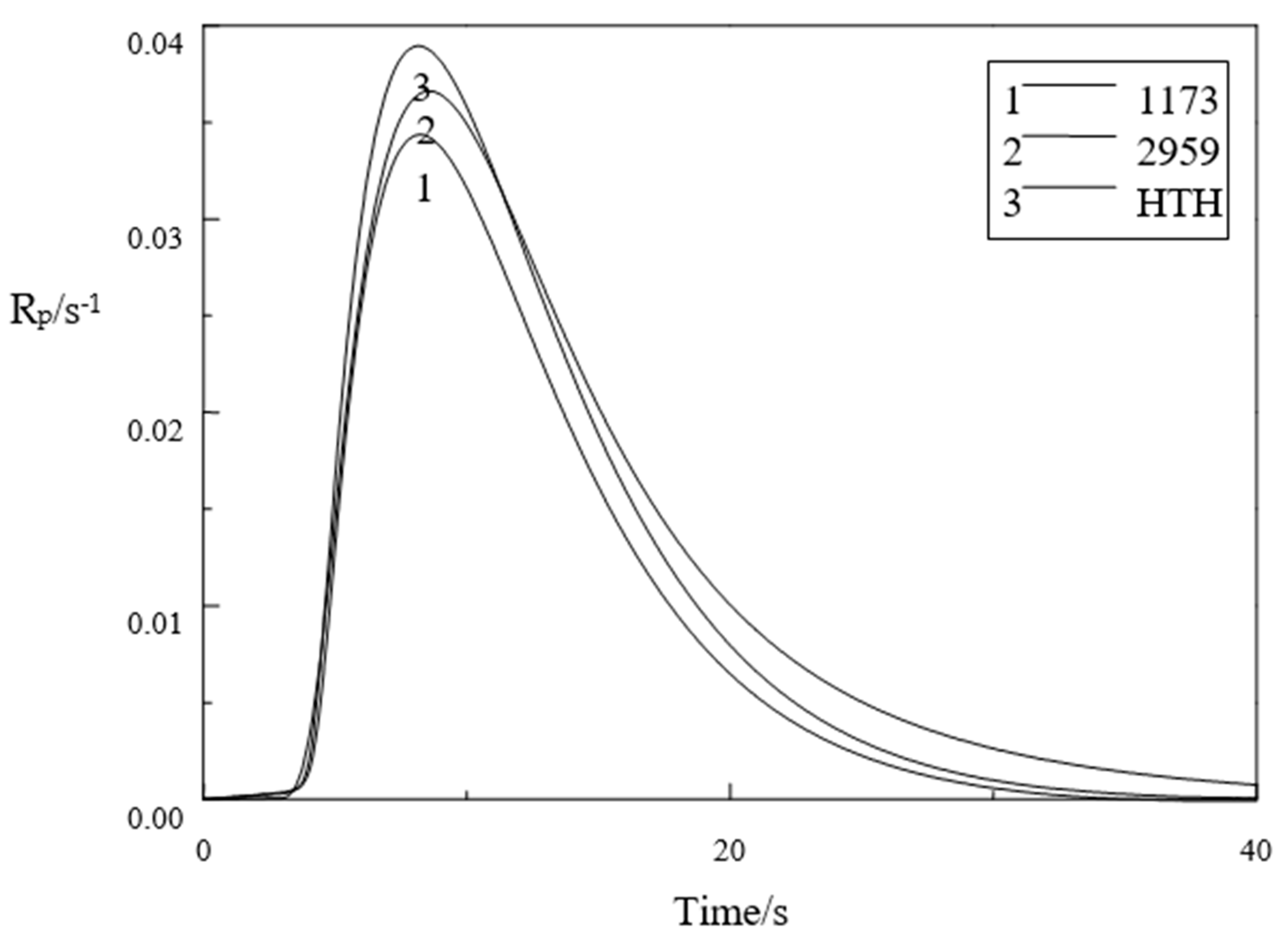
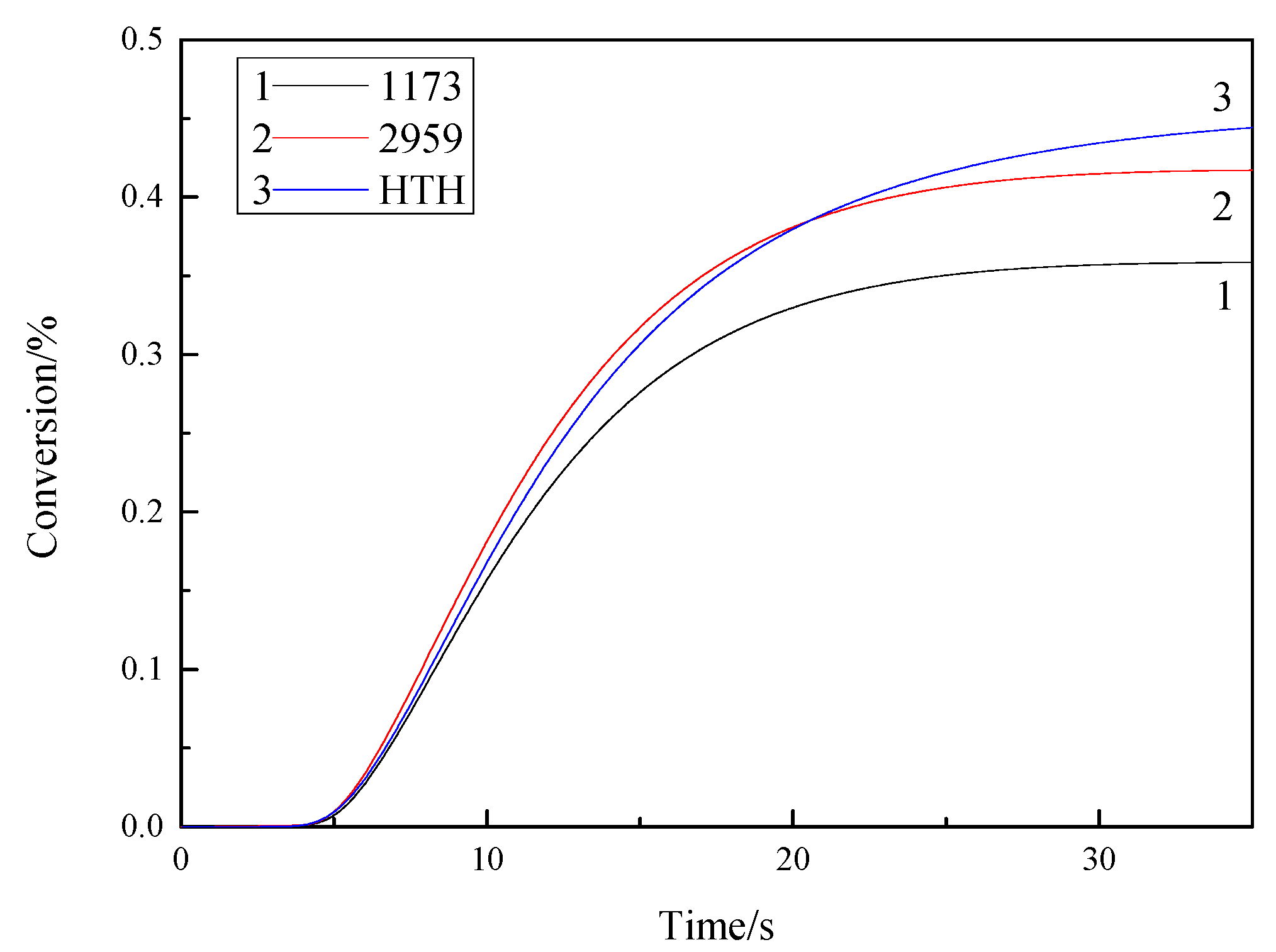
3.6. Photopolymerization Kinetics Analysis
3.7. Properties of the Cured Films
3.8. Migration of Residual Photoinitiator in the Cured Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhattcacharia, S.K.; Khan, M.A. Application of photocuring technique on wood surface and its prospects in Bangladesh. Nucl. Instrum. Methods Phys. Res. B 2005, 236, 359–365. [Google Scholar] [CrossRef]
- Decker, C. Photoinitiated crosslinking polymerization. Prog. Polym. Sci. 1996, 21, 593–650. [Google Scholar] [CrossRef]
- Lauppi, U.V. Radiation curing—An overview. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1990, 35, 30–35. [Google Scholar] [CrossRef]
- Liu, F.; Miao, L.; Wang, Y.; Xue, X.; Yang, H. Green fabrication of ultraviolet curable epoxy acrylate-silica hybrid coatings. Prog. Org. Coat. 2017, 109, 38–44. [Google Scholar] [CrossRef]
- Angiolini, L.; Carett, D.; Carlini, C.; Corelli, E.; Salatelli, E. Polymeric photoinitiators having benzoin methylether moieties connected to the main chain through the benzyl aromatic ring and their activity for ultra-violetcurable coatings. Polymer 1999, 40, 7197–7207. [Google Scholar] [CrossRef]
- Czech, Z. New copolymerizable photoinitiators for radiation curing of acrylic PSA. J. Adhes. Adhes. 2007, 27, 195–199. [Google Scholar] [CrossRef]
- Suyama, K.; Shirai, M. Photobase generators: Recent progress and application trend in polymer systems. Prog. Polym. Sci. 2009, 34, 194–209. [Google Scholar] [CrossRef]
- Mejiritski, A.; Sarker, A.M.; Wheaton, B.; Neckers, D.C. Novel method of thermal epoxy curing based photogeneration of polymeric amines and negative-tone image formation. Chem. Mater. 1997, 9, 1488–1494. [Google Scholar] [CrossRef]
- Kundu, P.P.; Larock, R.C. Novel conjugated linseed oil–styrene–divinylbenzene copolymers prepared by thermal polymerization 1: Effect of monomer concentration on the structure and properties. Biomacromolecules 2005, 6, 797–806. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, J.W.; Park, C.H.; Lim, D.H.; Kim, H.J.; Song, J.Y.; Lee, J.H. UV-curing and thermal stability of dual curable urethane epoxy adhesives for temporary bonding in 3D. Int. J. Adhes. Adhes. 2013, 44, 138–143. [Google Scholar] [CrossRef]
- Isobe, Y.; Yamada, K.; Nakano, T.; Okamoto, Y. Stereocontrol in the free-radical polymerization of methacrylates with fluoroalcohols. J. Polym. Sci. Part A Polym. Chem. 2000, 38, 4693–4703. [Google Scholar] [CrossRef]
- Nakano, T.; Okamoto, Y. Helix-Sense-Selective Free-Radical Polymerization of l-Phenyldibenzosuberyl Methacrylate Using a Cobalt(Ⅱ) Complex. Macromolecules 1999, 32, 2391–2393. [Google Scholar] [CrossRef]
- Liu, F.; Liu, A.; Tao, W.; Yang, Y. Preparation of UV curable organic/inorganic hybrid coatings-a review. Prog. Org. Coat. 2020, 145, 105685. [Google Scholar] [CrossRef]
- Zhou, W.; Kuebler, S.M.; Braun, K.L.; Yu, T.; Cammack, J.K.; Ober, C.K.; Perry, J.W.; Marder, S.R. An Efficient Two-Photon-Generated Photoacid Applied to Positive-Tone 3D Microfabrication. Science 2002, 296, 1106–1109. [Google Scholar] [CrossRef]
- Crivello, J.V.; Lee, J.L. Alkoxy-substituted diaryliodonium salt cationic photoinitiators. J. Polym. Sci. Part A Polym. Chem. 1989, 27, 3951–3968. [Google Scholar] [CrossRef]
- Ananda, M.S.; Lungu, A.; Mejiritski, A.; Kaneko, Y.; Neckers, D.C. Tetraorganylborate salts as convenient precursors for photogeneration of tertiary amines. J. Chem. Soc. Perkin Trans. 1998, 2, 2315–2321. [Google Scholar]
- Sarker, A.M.; Lungu, A.; Neckers, D.C. Synthesis and Characterization of a Novel Poly- meric System Bearing a Benzophenone Borate Salt as a New Photoinitiator for UV Curing. Macromolecules 1996, 29, 8047–8052. [Google Scholar] [CrossRef]
- Yagci, Y.; Jockusch, S.; Turro, N.J. Photoinitiated polymerization: Advances, challenges, and opportunities. Macromolecules 2010, 43, 6245–6260. [Google Scholar] [CrossRef]
- Oesterreicher, A.; Roth, M.; Hennen, D.; Mostegel, F.H.; Edler, M.; Kappaun, S.; Griesser, T. Low migration type I photoinitiators for biocompatiblethiol-ene formulations. Eur. Polym. J. 2017, 88, 393–402. [Google Scholar] [CrossRef]
- Dietliker, K.; Jung, T.; Benkhoff, J.; Kura, H.; Matsumoto, A.; Oka, H.; Hristova, D.; Gescheidt, G.; Rist, G. New developments in photoinitiators. MacromolSymp 2004, 217, 77–97. [Google Scholar] [CrossRef]
- Karahan, Ö.; Balta, D.K.; Arsu, N.; Avci, D. Synthesis and evaluations of novel photoinitiators with side-chain benzophenone, derived from alkyl α-hydroxymethacrylates. J. Photochem. Photobiol. A Chem. 2014, 274, 43–49. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Y.; Zhang, X.; Nie, J. A-hydroxyalkyl ketones derivatives used as photoinitiators for photografting field. J. Photochem. Photobiol. A Chem. 2017, 349, 193–196. [Google Scholar] [CrossRef]
- Kandirmaz, E.A.; Apohan, N.K.; Gencoglu, E.N. Preparation of novel thioxanthone based polymeric photoinitiator for flexographic varnish and determination of their migration behaviour. Prog. Org. Coat. 2018, 119, 36–43. [Google Scholar] [CrossRef]
- Chen, W.; Wang, L.; Liu, X.; Chen, B.; Zhao, G. Synthesis and preliminary photopolymerization evluation of novel photoinitiators containing phototrigger to overcome oxygen inhibition in the UV-curing system. J. Photochem. Photobiol. A Chem. 2020, 388, 112187. [Google Scholar] [CrossRef]
- Huo, S.; Zhou, H.Y.; Wang, J.X. Preparation and photochemical properties of PEG based alpha-hydroxy alkyl phenone photoinitiator. React. Funct. Polym. 2021, 163, 104892. [Google Scholar] [CrossRef]
- Zhou, J.; Allonas, X.; Ibrahim, A.; Liu, X. Progress in the Development of Polymeric and Multifunctional Photoinitiators. Prog. Polym. Sci. 2019, 99, 101165. [Google Scholar] [CrossRef]
- Douaihy, R.Z.; Telegeiev, I.; Nasrallah, H.; Lebedev, O.; Bazin, P.; Vimont, A.; Chailan, J.F.; Fahs, A.; Mohamad, E.R. Synthesis of silica-polymer core-shell hybrid materials with enhanced mechanical properties using a new bifuntional silane-based photointiator as coupling agent. Mater. Today Commun. 2021, 27, 102248. [Google Scholar] [CrossRef]
- Aparicio, J.L.; Elizalde, M. Migration of photoinitiators in food packaging: A review. Packag. Technol. Sci. 2015, 28, 181–203. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, T.; Xie, C.; Tian, X.; Song, L.; Liu, L.; Wang, Z.; Yu, Q. Naphthyl-based acylphosphine oxide photoinitiators with high efficiency and low migration. Prog. Org. Coat. 2020, 142, 105603. [Google Scholar] [CrossRef]
- Zubiaguirre, L.; Zabaleta, I.; Prieto, A.; Olivares, M.; Zuloaga, O.; Elizalde, M.P. Migration of photoinitiators, phthalates and plasticizers from paper and cardboard materials into different simulants and foodstuffs. Food Chem. 2020. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Hong, P.; Li, X.; Li, Z.; Chen, L.; Liu, X. Synthesis of organic and inorganic hybrid nanoparticles as multifunctional photonintiator and its application in UV-curable epoxyacrylate based coating systems. Prog. Org. Coat. 2020, 141, 105565. [Google Scholar] [CrossRef]
- Guo, X.D.; Zhou, H.Y.; Wang, J.X. A novel thioxanthone-hydroxyalkylphenone bifunctional photoinitiator: Synthesis, characterization and mechanism of Photopoymerization. Prog. Org. Coat. 2021, 154, 106214. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, Z.; Xie, G.; Jiao, C.; Zhou, Y.; Zhong, R. Synthesis and photoinitiation properties of pentaerythritol tetrabenzoyl formate. Chin. J. Appl. Chem. 2018, 35, 652–658. [Google Scholar]



| Photoinitiators | 1173 | 2959 | HTH |
|---|---|---|---|
| λmax/nm | 243 | 274 | 274.5 |
| εmax/(×104 L·mol−1·cm−1) | 9.2 | 5.7 | 7.8 |
| Photoinitiator | 1173 | 2959 | HTH |
|---|---|---|---|
| hardness/H | 3 | 4 | 4 |
| adhesion | 2 | 2 | 3 |
| ductility | good | good | good |
| Acid resistance * | No crack | No crack | No crack |
| Base resistance ** | No crack | No crack | No crack |
| Photoinitiator | HTH | 2959 | 1173 |
|---|---|---|---|
| A(λmax) | 0.32 | 0.605 | 0.96 |
| c/(mol·L−1) | 3.47 × 10−6 | 8.3 × 10−5 | 1.68 × 10−5 |
| R/% | 20.65 | 49.40 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, R.; Hu, H.; Zhou, Y. Synthesis and Characterization of a Trifunctional Photoinitiator Based on Two Commercial Photoinitiators with α-Hydroxyl Ketone Structure. Materials 2021, 14, 5272. https://doi.org/10.3390/ma14185272
Zhong R, Hu H, Zhou Y. Synthesis and Characterization of a Trifunctional Photoinitiator Based on Two Commercial Photoinitiators with α-Hydroxyl Ketone Structure. Materials. 2021; 14(18):5272. https://doi.org/10.3390/ma14185272
Chicago/Turabian StyleZhong, Rong, Hui Hu, and Yanfang Zhou. 2021. "Synthesis and Characterization of a Trifunctional Photoinitiator Based on Two Commercial Photoinitiators with α-Hydroxyl Ketone Structure" Materials 14, no. 18: 5272. https://doi.org/10.3390/ma14185272
APA StyleZhong, R., Hu, H., & Zhou, Y. (2021). Synthesis and Characterization of a Trifunctional Photoinitiator Based on Two Commercial Photoinitiators with α-Hydroxyl Ketone Structure. Materials, 14(18), 5272. https://doi.org/10.3390/ma14185272






