A Diamond-Based Dose-per-Pulse X-ray Detector for Radiation Therapy
Abstract
1. Introduction
2. Materials and Methods
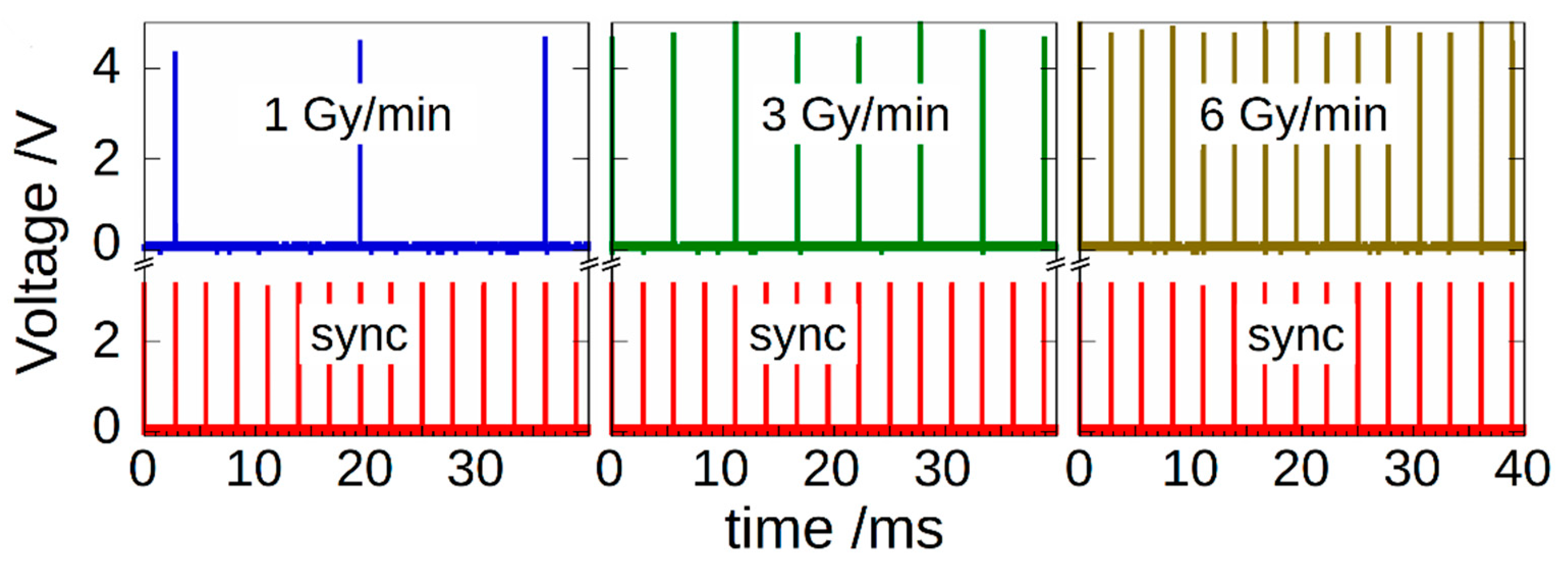
2.1. Pulsed X-ray Source
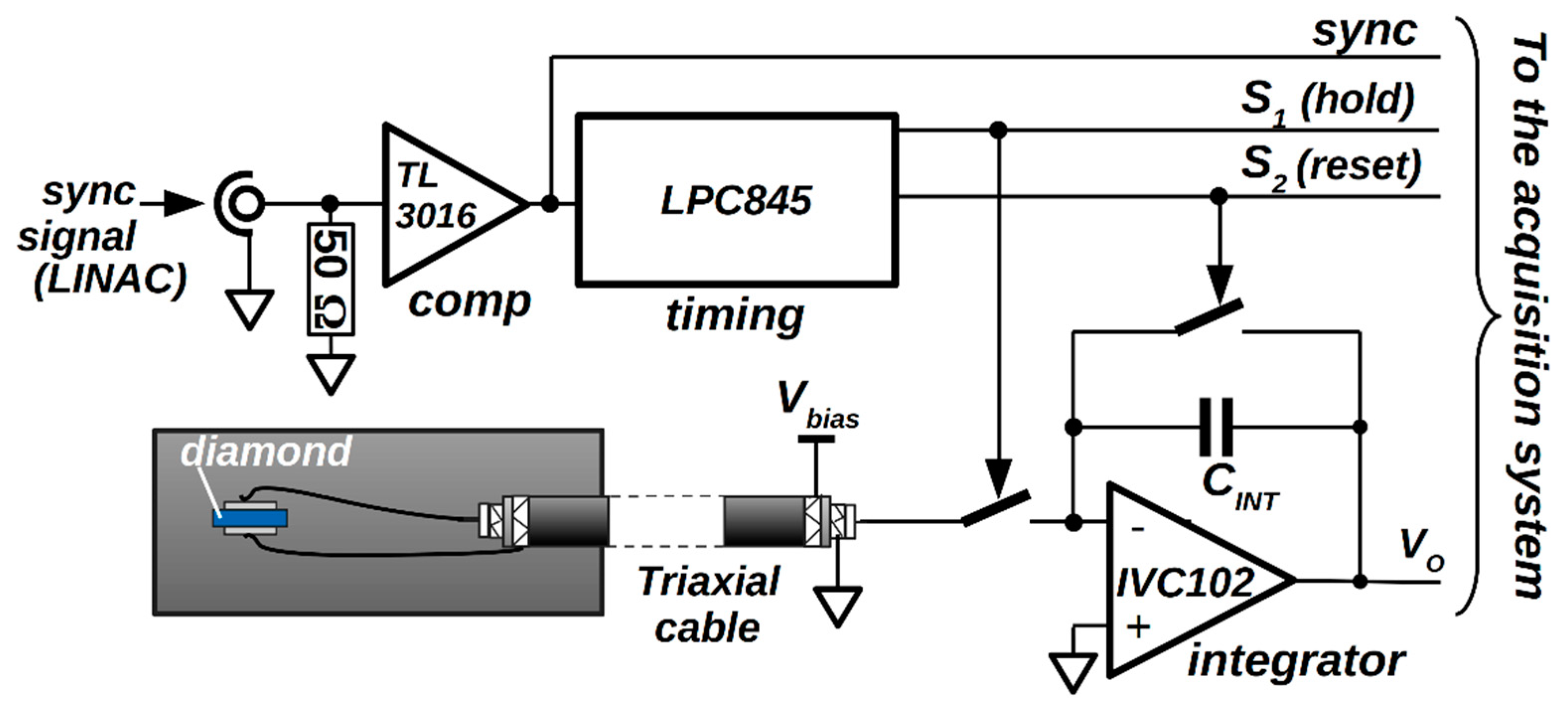
2.2. Diamond Detector
2.3. Gated Integration Readout Electronics
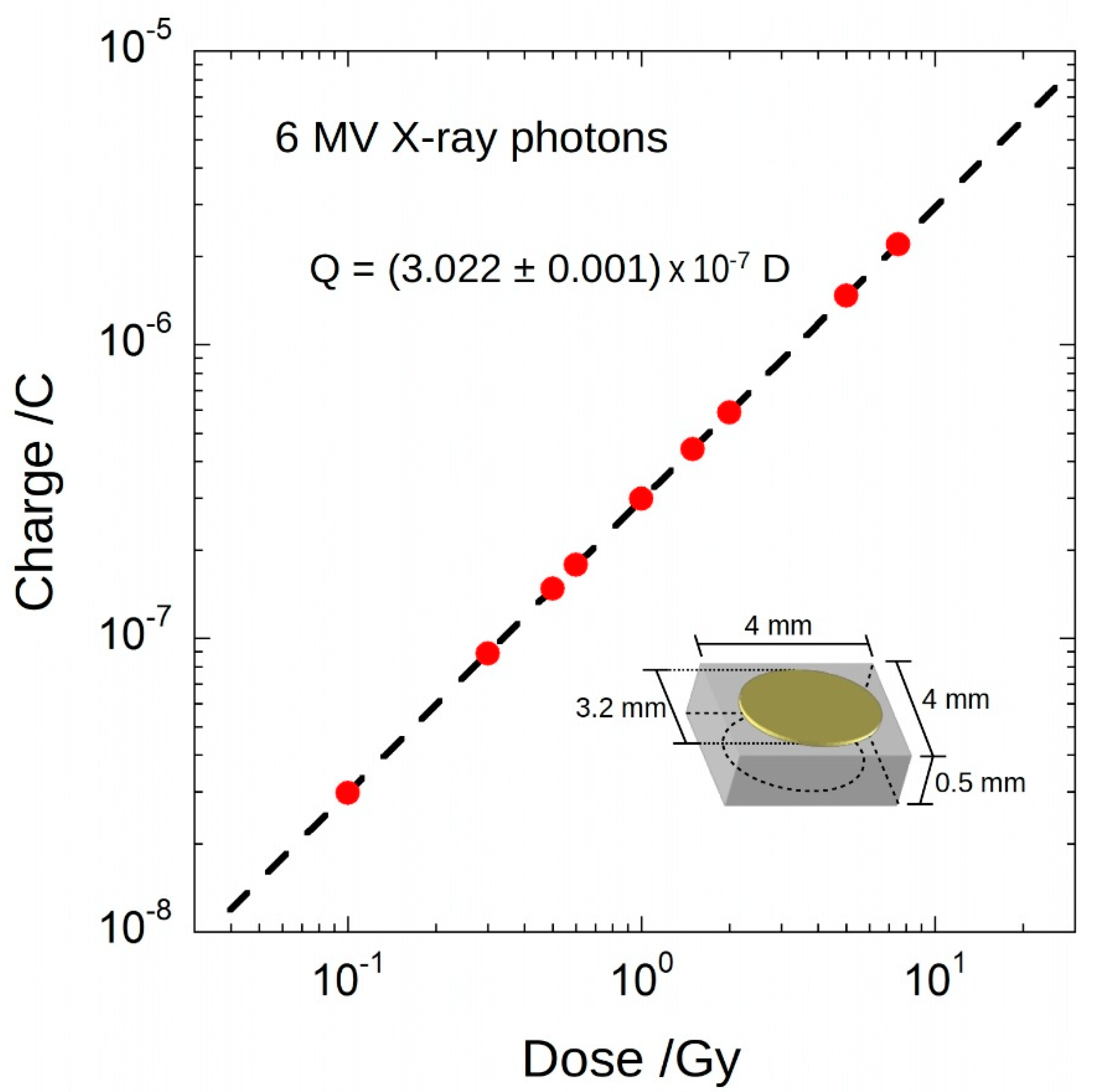
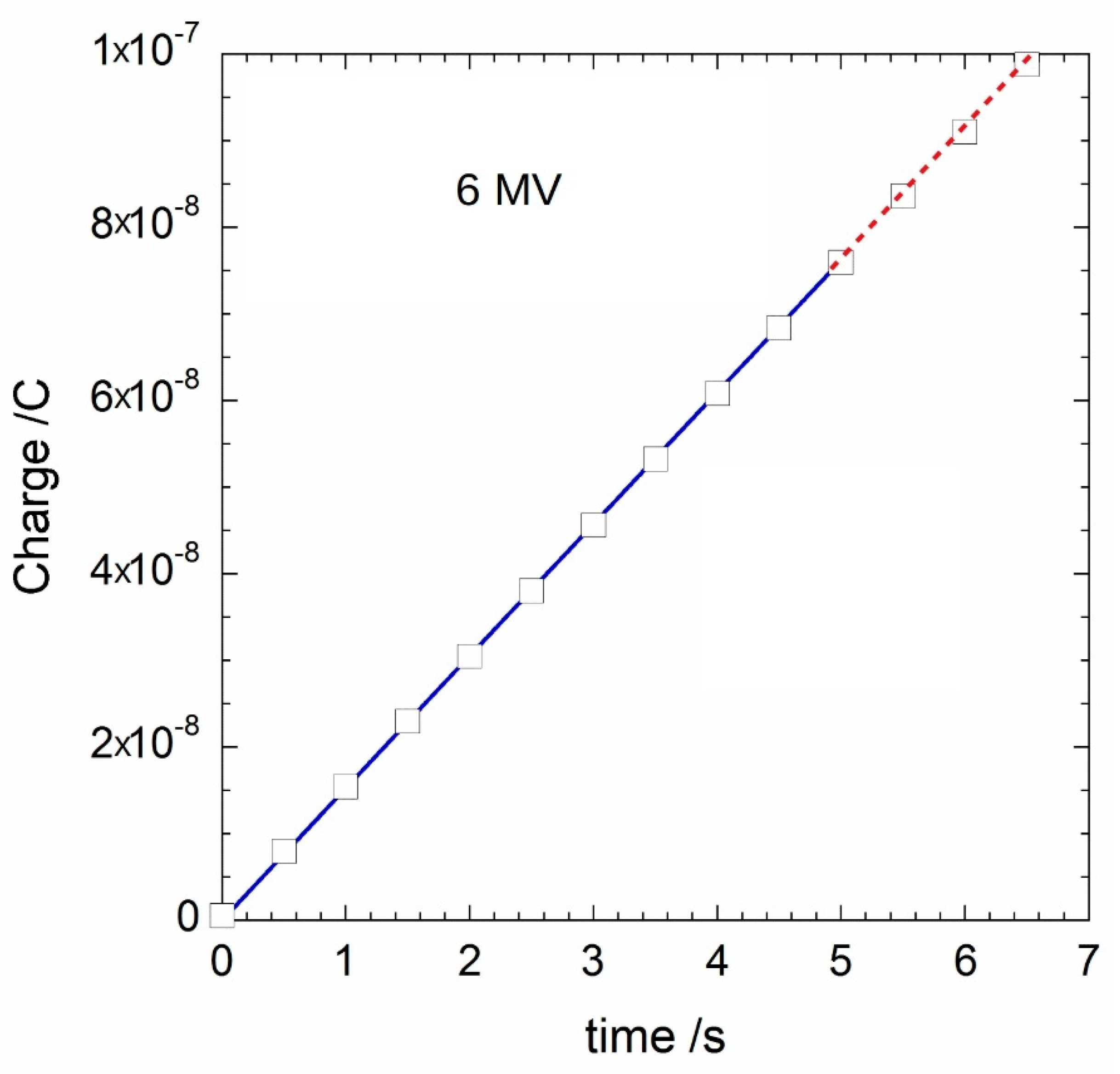
3. Experimental Results
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bewes, J.M.; Suchowerska, N.; Jackson, M.; Zhang, M.; McKenzie, D.R. The radiobiological effect of intra-fraction dose-rate modulation in intensity modulated radiation therapy (IMRT). Phys. Med. Biol. 2008, 53, 3567–3578. [Google Scholar] [CrossRef]
- Ashour, M.G.; Shouman, T.H.; Hassouna, A.H.; El Din, R.M.E.; Mokhtar, M.H.; Khalil, M.A. Measuring radiotherapy setup errors in IMRT treated head and neck cancer patients requiring bilateral neck irradiation, NCI-egypt experience. J. Cancer Ther. 2017, 8, 1160–1168. [Google Scholar] [CrossRef][Green Version]
- Dobler, B.; Groeger, C.; Treutwein, M.; Alvarez-Moret, M.; Goetzfried, T.; Weidner, K.; Haertl, P.; Koelbl, O. Commissioning of volumetric modulated arc therapy (VMAT) in a dual-vendor environment. Radiother. Oncol. 2011, 99, 86–89. [Google Scholar] [CrossRef] [PubMed]
- Matuszak, M.M.; Yan, D.; Grills, I.; Martinez, A. Clinical applications of volumetric modulated arc therapy. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Rapole, P.S.; Karunanithi, G.; Saravanan Kandasamy, S.P.; Kumar, R.; Vivekanandam, S. Dosimetric comparison and feasibility of Simultaneous Integrated Boost (SIB) in treatment of malignant gliomas using Intensity Modulated Radiotherapy (IMRT) or Volumetric Modulated Arc Therapy (VMAT). Asian Pac. J. Cancer Prev. APJCP 2018, 19, 2499. [Google Scholar] [CrossRef][Green Version]
- Jeong, Y.; Lee, S.W.; Kwak, J.; Cho, I.; Yoon, S.M.; Kim, J.H.; Park, J.H.; Choi, E.K.; Song, S.Y.; Kim, Y.S.; et al. A dosimetric comparison of Volumetric Modulated Arc Therapy (VMAT) and non-coplanar Intensity Modulated Radiotherapy (IMRT) for nasal cavity and paranasal sinus cancer. Radiat Oncol. 2014, 30, 193. [Google Scholar] [CrossRef]
- Teoh, M.; Clark, C.H.; Wood, K.; Whitaker, S.; Nisbet, A. Volumetric modulated arc therapy: A review of current literature and clinical use in practice. Br. J. Radiol. 2011, 84, 967–996. [Google Scholar] [CrossRef] [PubMed]
- Freiburg, P.T.W. Ionizing Radiation Detectors: Including Codes of Practice. 2013, p. 100. Available online: https://www.ptwdosimetry.com/fileadmin/user_upload/DETECTORS_Cat_en_16522900_12/blaetterkatalog/blaetterkatalog/pdf/complete.pdf (accessed on 3 September 2021).
- Electrometer, R.C. High-Performance Dual-Channel Reference Class Electrometer 2. Available online: https://www.iba-dosimetry.com/fileadmin/user_upload/products/02_radiation_therapy/Dose2/Absolute-Dosimetry_DOSE2-Electrometer-Rev.1.pdf (accessed on 3 September 2021).
- Element Six, Diamond Handbook. Available online: https://e6-prd-cdn-01.azureedge.net/mediacontainer/medialibraries/element6/documents/brochures/element_six_diamond_handbook_august_2020.pdf?ext=.pdf (accessed on 3 September 2021).
- Almaviva, S.; Ciancaglioni, I.; Consorti, R.; De Notaristefani, F.; Manfredotti, C.; Marinelli, M.; Milani, E.; Petrucci, A.; Prestopino, G.; Verona, C.; et al. Synthetic single crystal diamond dosimeters for Intensity Modulated Radiation Therapy applications. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2009, 608, 191–194. [Google Scholar] [CrossRef]
- Betzel, G.T.; Lansley, S.P.; Baluti, F.; Reinisch, L.; Meyer, J. Clinical investigations of a CVD diamond detector for radiotherapy dosimetry. Phys. Med. 2012, 28, 144–152, 2012. [Google Scholar] [CrossRef] [PubMed]
- Marsolat, F.; Tromson, D.; Tranchant, N.; Pomorski, M.; Le Roy, M.; Donois, M.; Moignau, F.; Ostrowsky, A.; De Carlan, L.; Bassinet, C.; et al. A new single crystal diamond dosimeter for small beam: Comparison with different commercial active detectors. Phys. Med. Biol. 2013, 58, 7647–7660. [Google Scholar] [CrossRef]
- Ravichandran, R.; Binukumar, J.P.; Al Amri, I.; Davis, C.A. Diamond detector in absorbed dose measurements in high-energy linear accelerator photon and electron beams. J. Appl. Clin. Med. Phys. 2016, 17, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Conte, G.; Girolami, M.; Salvatori, S.; Ralchenko, V. X-ray diamond detectors with energy resolution. Appl. Phys. Lett. 2007, 91, 6–9. [Google Scholar] [CrossRef]
- Girolami, M.; Allegrini, P.; Conte, G.; Trucchi, D.M.; Ralchenko, V.; Salvatori, S. Diamond detectors for UV and X-ray source imaging. IEEE Electron Device Lett. 2011, 33, 224–226. [Google Scholar] [CrossRef]
- Girolami, M.; Conte, G.; Salvatori, S.; Allegrini, P.; Bellucci, A.; Trucchi, D.M.; Ralchenko, V. Optimization of X-ray beam profilers based on CVD diamond detectors. J. Instrum. 2012, 7. [Google Scholar] [CrossRef]
- Mazzeo, G.; Salvatori, S.; Conte, G.; Ralchenko, V.; Konov, V. Electronic performance of 2D-UV detectors. Diam. Relat. Mater. 2007, 16, 1053–1057. [Google Scholar] [CrossRef]
- Salvatori, S.; Girolami, M.; Oliva, P.; Conte, G.; Bolshakov, A.; Ralchenko, V.; Konov, V. Diamond device architectures for UV laser monitoring. Laser Phys. 2016, 26, 84005. [Google Scholar] [CrossRef]
- Salvatori, S.; Oliva, P.; Pacilli, M.; Allegrini, P.; Conte, G.; Komlenok, K.; Khomich, A.A.; Bolshakov, A.; Ralchenko, V.; Konov, V. Nano-carbon pixels array for ionizing particles monitoring. Diam. Relat. Mater. 2017, 73, 132–136. [Google Scholar] [CrossRef]
- Girolami, M.; Conte, G.; Trucchi, D.M.; Bellucci, A.; Oliva, P.; Kononenko, T.; Khomich, A.; Bolshakov, A.; Ralchenko, V.; Konov, V.; et al. Investigation with β-particles and protons of buried graphite pillars in single-crystal CVD diamond. Diam. Relat. Mater. 2018, 84, 1–10. [Google Scholar] [CrossRef]
- Salvatori, S.; Rossi, M.C.; Conte, G.; Kononenko, T.V.; Komlenok, M.S.; Khomich, A.A.; Ralchenko, V.G.; Konov, V.I.; Provatas, G.; Jaksic, M. Diamond detector with laser-formed buried graphitic electrodes: Micron-scale mapping of stress and charge collection efficiency. IEEE Sens. J. 2019, 19, 11908–11917. [Google Scholar] [CrossRef]
- Khomich, A.; Ashikkalieva, K.; Bolshakova, A.; Kononenko, T.; Ralchenko, V.; Konova, V.; Oliva, P.; Conte, G.; Salvatori, S. Very long laser-induced graphitic pillars buried in single-crystal CVD-diamond for 3D detectors realization. Diam. Relat. Mater. 2018, 90, 84–92. [Google Scholar] [CrossRef]
- Girolami, M.; Bellucci, A.; Calvani, P.; Cazzaniga, C.; Rebai, M.; Rigamonti, D.; Tardocchi, M.; Pillon, M.; Trucchi, D.M. Mosaic diamond detectors for fast neutrons and large ionizing radiation fields. Phys. Status Solidi Appl. Mater. Sci. 2015, 212, 2424–2430. [Google Scholar] [CrossRef]
- Červ, M. The ATLAS diamond beam monitor. J. Instrum. 2014, 9, C02026. [Google Scholar] [CrossRef]
- Girolami, M.; Allegrini, P.; Conte, G.; Salvatori, S. CVD-diamond detectors for real-time beam profile measurements. Proc. IEEE Sensors 2008, 270–273. [Google Scholar] [CrossRef]
- Keithley Model 6517A Electrometer User’s Manual. 2014. Available online: https://download.tek.com/manual/6517_900_01D.pdf (accessed on 3 September 2021).
- Ashraf, M.R.; Rahman, M.; Zhang, R.; Cao, X.; Williams, B.B.; Hoopes, P.J.; Gladstone, D.J.; Pogue, B.W.; Bruza, P. Technical note: Single-pulse beam characterization for FLASH-RT using optical imaging in a water tank. Med. Phys. 2021, 48, 2673–2681. [Google Scholar] [CrossRef]
- Bradley, D.A.; Zubair, H.T.; Oresegun, A.; Louay, G.T.; Zin, H.M.; Ung, N.M.; Abdul-Rashid, H.A. Time-resolved dose measurements of linear accelerator pulses using a fibre optic sensor: Applications and challenges. Radiat. Phys. Chem. 2019, 167, 108212. [Google Scholar] [CrossRef]
- Velthuis, J.J.; Page, R.F.; Purves, T.M.; Beck, L.; Hanifa, M.A.M.; Hugtenburg, R.P. Toward pulse by pulse dosimetry using an SC CVD diamond detector. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 527–533. [Google Scholar] [CrossRef]
- Pettinato, S.; Orsini, A.; Girolami, M.; Trucchi, D.M.; Rossi, M.C.; Salvatori, S. A high-precision gated integrator for repetitive pulsed signals acquisition. Electronics 2019, 8, 1231. [Google Scholar] [CrossRef]
- Pettinato, S.; Orsini, A.; Rossi, M.C.; Tagnani, D.; Girolami, M.; Salvatori, S. A compact gated integrator for conditioning pulsed analog signals. In Applications in Electronics Pervading Industry, Environment and Society; Saponara, S., De Gloria, A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 33–39. [Google Scholar] [CrossRef]


| Dose Rate (Gy/min) | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| <Q> (pC) | 84.60 | 84.63 | 84.67 | 84.60 | 84.46 | 84.78 |
| Qmax (pC) | 88.83 | 82.01 | 82.64 | 81.13 | 81.97 | 82.48 |
| Qmin (pC) | 86.99 | 86.68 | 86.93 | 86.76 | 87.01 | 87.00 |
| t (s) | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Q(t) (nC) | 15.28 | 30.54 | 45.75 | 60.98 | 76.10 |
| Q6517(t) (nC) | 15.34 | 30.51 | 45.70 | 60.90 | 76.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pettinato, S.; Girolami, M.; Olivieri, R.; Stravato, A.; Caruso, C.; Salvatori, S. A Diamond-Based Dose-per-Pulse X-ray Detector for Radiation Therapy. Materials 2021, 14, 5203. https://doi.org/10.3390/ma14185203
Pettinato S, Girolami M, Olivieri R, Stravato A, Caruso C, Salvatori S. A Diamond-Based Dose-per-Pulse X-ray Detector for Radiation Therapy. Materials. 2021; 14(18):5203. https://doi.org/10.3390/ma14185203
Chicago/Turabian StylePettinato, Sara, Marco Girolami, Riccardo Olivieri, Antonella Stravato, Cristina Caruso, and Stefano Salvatori. 2021. "A Diamond-Based Dose-per-Pulse X-ray Detector for Radiation Therapy" Materials 14, no. 18: 5203. https://doi.org/10.3390/ma14185203
APA StylePettinato, S., Girolami, M., Olivieri, R., Stravato, A., Caruso, C., & Salvatori, S. (2021). A Diamond-Based Dose-per-Pulse X-ray Detector for Radiation Therapy. Materials, 14(18), 5203. https://doi.org/10.3390/ma14185203








