Chitin Cryogels Prepared by Regeneration from Phosphoric Acid Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
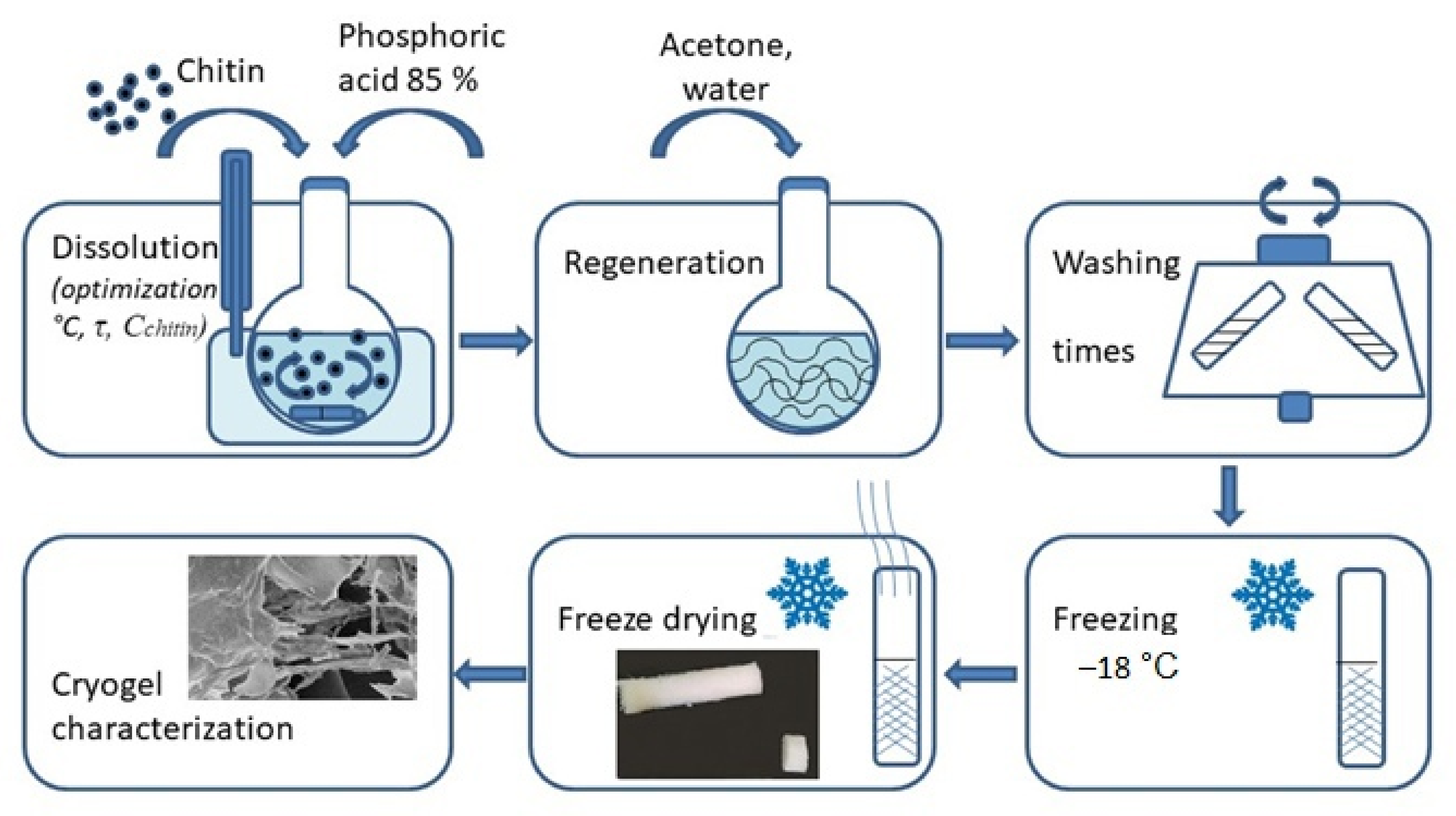
2.2. Preparation of Chitin Cryogels
2.3. General Methods
3. Results and Discussion
3.1. Chitin Dissolution in 85% Phosphoric Acid and Preparation of Chitin Cryogels
3.2. Swelling in Water of Chitin Cryogels
3.3. Specific Surface Area of Chitin Cryogels
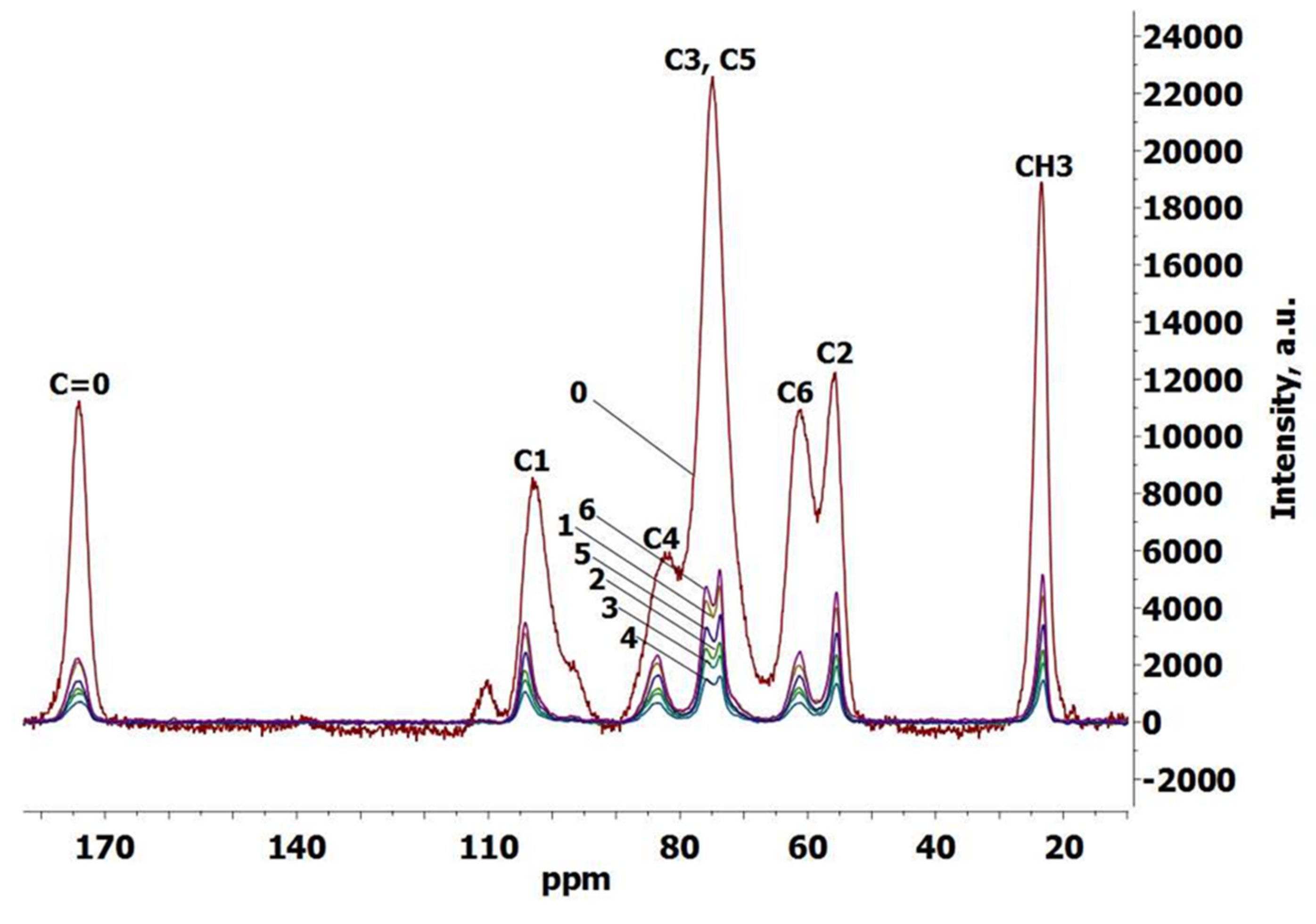
3.4. Crystallinity of Chitin and Chitin Cryogels
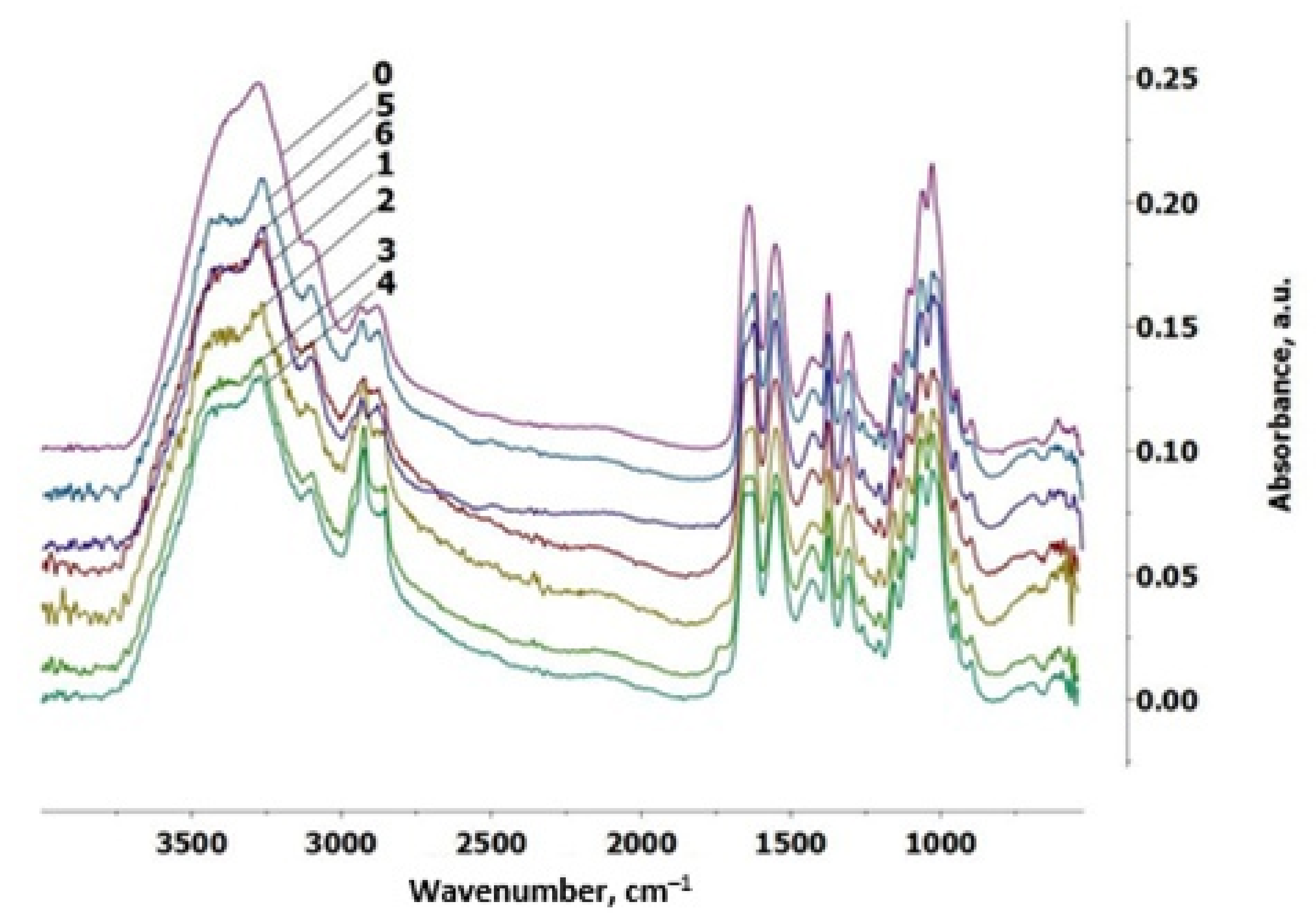
3.5. FTIR Spectroscopy
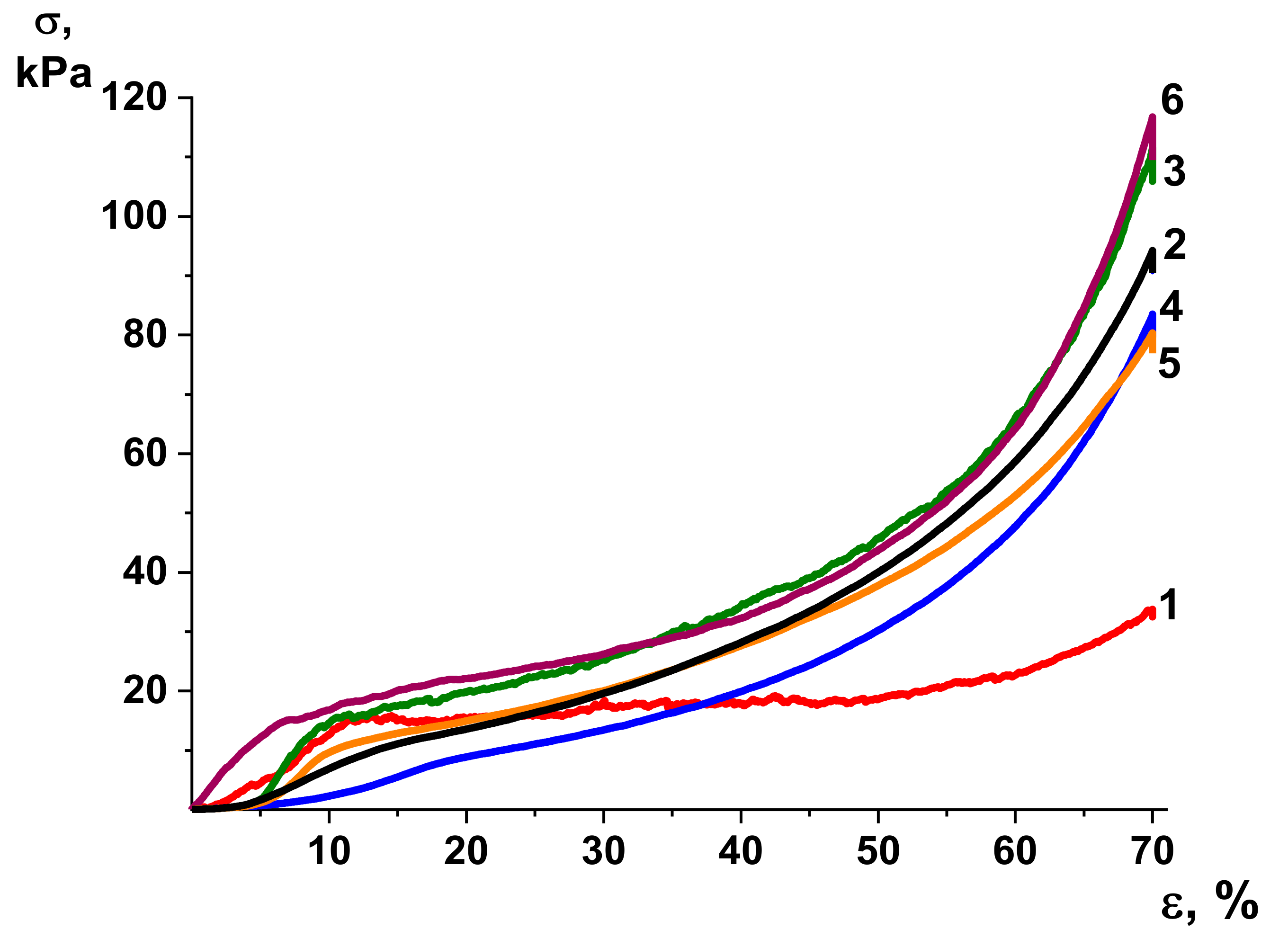
3.6. Mechanical Properties
3.7. SEM Morphology of Chitin Cryogels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Azuma, K.; Ifuku, S.; Osaki, T.; Okamoto, Y.; Minami, S. Preparation and biomedical applications of chitin and chitosan nanofibers. J. Biomed. Nanotechnol. 2014, 10, 2891–2920. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Kumar, P.S.; Nair, S.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Satitsri, S.; Muanprasat, C. Chitin and chitosan derivatives as biomaterial resources for biological and biomedical applications. Molecules 2020, 25, 5961. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.-L. Chitin-based materials in tissue engineering: Applications in soft tissue and epithelial organ. Int. J. Mol. Sci. 2011, 12, 1936–1963. [Google Scholar] [CrossRef] [Green Version]
- Park, B.K.; Kim, M.-M. Applications of chitin and its derivatives in biological medicine. Int. J. Mol. Sci. 2010, 11, 5152–5164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakhshpour, M.; Idil, N.; Perçin, I.; Denizli, A. Biomedical applications of polymeric cryogels. App. Sci. 2019, 9, 553. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Potential applications of natural origin polymer-based systems in soft tissue regeneration. Crit. Rev. Biotechnol. 2010, 30, 200–221. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.S.; Mano, J.F.; Reis, R.L. Ionic liquids in the processing and chemical modification of chitin and chitosan for biomedical applications. Green Chem. 2017, 19, 1208–1220. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, S.; Li, S. Chitin and chitosan dissolved in ionic liquids as reversible sorbents of CO2. Green Chem. 2006, 8, 630–633. [Google Scholar] [CrossRef]
- Takegawa, A.; Murakami, M.-A.; Kaneko, Y.; Kadokawa, J.-I. Preparation of chitin/cellulose composite gels and films with ionic liquids. Carbohydr. Polym. 2010, 79, 85–90. [Google Scholar] [CrossRef]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Tsioptsias, C.; Michailof, C.; Stauropoulos, G.; Panayiotou, C. Chitin and carbon aerogels from chitin alcogels. Carbohydr. Polym. 2009, 76, 535–540. [Google Scholar] [CrossRef]
- Abe, K.; Ifuku, S.; Kawata, M.; Yano, H. Preparation of tough hydrogels based on β-chitin nanofibers via naoh treatment. Cellulose 2014, 21, 535–540. [Google Scholar] [CrossRef]
- Chang, C.; Chen, S.; Zhang, L. Novel hydrogels prepared via direct dissolution of chitin at low temperature: Structure and biocompatibility. J. Mat Chem. 2011, 21, 3865–3871. [Google Scholar] [CrossRef]
- Hu, X.; Du, Y.; Tang, Y.; Wang, Q.; Feng, T.; Yang, J.; Kennedy, J.F. Solubility and property of chitin in naoh/urea aqueous solution. Carbohydr. Polym. 2007, 70, 451–458. [Google Scholar] [CrossRef]
- Tamura, H.; Nagahama, H.; Tokura, S. Preparation of chitin hydrogel under mild conditions. Cellulose 2006, 13, 357–364. [Google Scholar] [CrossRef]
- Tamura, H.; Furuike, T.; Nair, S.; Jayakumar, R. Biomedical applications of chitin hydrogel membranes and scaffolds. Carbohydr. Polym. 2011, 84, 820–824. [Google Scholar] [CrossRef]
- Silva, S.S.; Duarte, A.R.C.; Carvalho, A.P.; Mano, J.F.; Reis, R.L. Green processing of porous chitin structures for biomedical applications combining ionic liquids and supercritical fluid technology. Acta Biomater. 2011, 7, 1166–1172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.; Xu, D.; Zhao, Y.; Gao, H.; Shi, X.; Cai, J.; Deng, H.; Chen, Y.; Du, Y. Electroassembly of chitin nanoparticles to construct freestanding hydrogels and high porous aerogels for wound healing. ACS Appl. Mater. Interfaces 2019, 11, 34766–34776. [Google Scholar] [CrossRef]
- Heath, L.; Zhu, L.; Thielemans, W. Chitin nanowhisker aerogels. ChemSusChem 2013, 6, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Bai, L.; Tripathi, A.; Yu, J.; Wang, Z.; Borghei, M.; Fan, Y.; Rojas, O.J. High axial ratio nanochitins for ultrastrong and shape-recoverable hydrogels and cryogels via ice templating. ACS Nano 2019, 13, 2927–2935. [Google Scholar] [CrossRef] [Green Version]
- Peter, M.; Kumar, P.T.S.; Binulal, N.S.; Nair, S.V.; Tamura, H.; Jayakumar, R. Development of novel α-chitin/nanobioactive glass ceramic composite scaffolds for tissue engineering applications. Carbohydr. Polym. 2009, 78, 926–931. [Google Scholar] [CrossRef]
- Ingavle, G.C.; Baillie, L.W.; Zheng, Y.; Lis, E.K.; Savina, I.N.; Howell, C.A.; Mikhalovsky, S.V.; Sandeman, S.R. Affinity binding of antibodies to supermacroporous cryogel adsorbents with immobilized protein a for removal of anthrax toxin protective antigen. Biomaterials 2015, 50, 140–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, T.; Wang, G.; Gao, C.; Chen, Z.; Feng, L.; Wang, P.; Zeng, X.; Wu, Z. Phosphoric acid-based preparing of chitin nanofibers and nanospheres. Cellulose 2016, 23, 477–491. [Google Scholar] [CrossRef]
- Vincendon, M. Regenerated chitin from phosphoric acid solutions. Carbohydr. Polym. 1997, 32, 233–237. [Google Scholar] [CrossRef]
- Assuncao, M.C.; Cote, G.; Andre, M.; Halleux, H.; Chagnes, A. Phosphoric acid recovery from concentrated aqueous feeds by a mixture of di-isopropyl ether (dipe) and tri-n-butylphosphate (tpb): Extraction data and modelling. RSC Adv. 2017, 7, 6922–6930. [Google Scholar] [CrossRef] [Green Version]
- Satish, N.; Patkar, P.D.P. Development and validation of method for molecular weight determination of cellulose using gpc column in hplc. Int. J. Adv. Res. 2016, 4, 516–530. [Google Scholar]
- Ding, B.; Cai, J.; Huang, J.; Zhang, L.; Chen, Y.; Shi, X.; Du, Y.; Kuga, S. Facile preparation of robust and biocompatible chitin aerogels. J. Mater. Chem. 2012, 22, 5801–5809. [Google Scholar] [CrossRef]
- Tyshkunova, I.V.; Chukhchin, D.G.; Gofman, I.V.; Poshina, D.N.; Skorik, Y.A. Cellulose cryogels prepared by regeneration from phosphoric acid solutions. Cellulose 2021, 28, 4975–4989. [Google Scholar] [CrossRef]
- Kameda, T.; Miyazawa, M.; Ono, H.; Yoshida, M. Hydrogen bonding structure and stability of α-chitin studied by 13c solid-state nmr. Macromol. Biosci. 2005, 5, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Ioelovich, M. Crystallinity and hydrophility of chitin and chitosan. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Buchtova, N.; Budtova, T. Cellulose aero-, cryo-and xerogels: Towards understanding of morphology control. Cellulose 2016, 23, 2585–2595. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Koga, H.; Qi, Z.-D.; Saito, T.; Isogai, A. Nanofibrillar chitin aerogels as renewable base catalysts. Biomacromolecules 2014, 15, 4314–4319. [Google Scholar] [CrossRef] [PubMed]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On chemistry of γ-chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Cuong, H.N.; Minh, N.C.; Van Hoa, N.; Trung, T.S. Preparation and characterization of high purity β-chitin from squid pens (loligo chenisis). Int. J. Biol. Macromol. 2016, 93, 442–447. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Tanner, S.F.; Chanzy, H.; Vincendon, M.; Roux, J.C.; Gaill, F. High-resolution solid-state carbon-13 nuclear magnetic resonance study of chitin. Macromolecules 1990, 23, 3576–3583. [Google Scholar] [CrossRef]
- Kumirska, J.; Czerwicka, M.; Kaczyński, Z.; Bychowska, A.; Brzozowski, K.; Thöming, J.; Stepnowski, P. Application of spectroscopic methods for structural analysis of chitin and chitosan. Mar. Drugs 2010, 8, 1567–1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukhchin, D.G.; Malkov, A.; Tyshkunova, I.V.; Mayer, L.V.; Novozhilov, E.V. Diffractometric method for determining the degree of crystallinity of materials. Crystallogr. Rep. 2016, 61, 371–375. [Google Scholar] [CrossRef]
- Cárdenas, G.; Cabrera, G.; Taboada, E.; Miranda, S.P. Chitin characterization by sem, ftir, xrd, and 13c cross polarization/mass angle spinning nmr. J. Appl. Polym. Sci. 2004, 93, 1876–1885. [Google Scholar] [CrossRef]
- Blackwell, J.; Parker, K.D.; Rudall, K.M. Chitin in pogonophore tubes. J. Mar. Biol. Assoc. U. K. 1965, 45, 659–661. [Google Scholar] [CrossRef]
- Kumar, K.V.; Rao, B.V.A.; Hebalkar, N.Y. Phosphorylated chitin as a chemically modified polymer for ecofriendly corrosion inhibition of copper in aqueous chloride environment. Res. Chem. Intermed. 2017, 43, 5811–5828. [Google Scholar] [CrossRef]
- Shih, T.-Y.; Blacklow, S.O.; Li, A.W.; Freedman, B.R.; Bencherif, S.; Koshy, S.T.; Darnell, M.C.; Mooney, D.J. Injectable, tough alginate cryogels as cancer vaccines. Adv. Healthc. Mater. 2018, 7, 1701469. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.T.; Zhang, D.K.; Grolman, J.M.; Stafford, A.G.; Mooney, D.J. Injectable nanocomposite cryogels for versatile protein drug delivery. Acta Biomater. 2018, 65, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Chau, T.T.L.; Le, D.Q.T.; Le, H.T.; Nguyen, C.D.; Nguyen, L.V.; Nguyen, T.-D. Chitin liquid-crystal-templated oxide semiconductor aerogels. ACS Appl. Mater. Interfaces 2017, 9, 30812–30820. [Google Scholar] [CrossRef]


| Sample | Chitin Concentration, % | Dissolution Time, h | Sample Shape | Yield, % | Cryogel Volume, cm3 | Volume Shrinkage, % | ∆V, % | ρ, g/cm3 | Porosity, % | % | Mw (Ð) * | SR, g/g | Specific Surface Area, m2/g |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 24 | Intact cylindrical | 60.7 | 3.3 | 33.5 | 20 | 0.027 | 98.1 | 6388 | 41,000 (4.6) | Sample fall apart | 0.334 |
| 2 | 5 | 24 | 77.6 | 3.7 | 26.0 | 74 | 0.050 | 96.3 | 4416 | 73,000 (7.3) | 7.2 | 0.292 | |
| 3 | 10 | 24 | 77.4 | 7.8 | −56.0 | 148 | 0.049 | 96.5 | 3091 | 80,450 (7.8) | 7.8 | 0.660 | |
| 4 | 15 | 25 | 76.4 | 9.7 | −93.3 | 170 | 0.059 | 95.8 | 2282 | 63,830 (7.4) | 7.2 | 0.330 | |
| 5 | 5 | 6 | 66.8 | 6.8 | −35.6 | 90 | 0.025 | 98.3 | 5737 | 274,600 (15.3) | 16.4 | - | |
| 6 | 5 | 16 | 81.2 | 4.8 | 3.9 | 90 | 0.042 | 97.0 | 4610 | 78,980 (8.2) | 10.0 | - | |
| 7 | 5 | 48 | Powder | 45.4 | - | - | - | - | - | - | 10,140 (2.0) | - | - |
| Sample | E, kPa | σy, kPa | σmax, kPa | εmax, % |
|---|---|---|---|---|
| 1 | 118 ± 6 | 11 ± 3 | 33 ± 4 | 70 |
| 2 | 170 ± 21 | 10 ± 1 | 87 ± 7 | 70 |
| 3 | 345 ± 23 | 15 ± 3 | 112 ± 22 | 70 |
| 4 | 154 ± 37 | 9 ± 2 | 83 ± 16 | 70 |
| 5 | 230 ± 7 | 11 ± 1 | 80 ± 12 | 70 |
| 6 | 214 ± 23 | 18 ± 7 | 116 ± 21 | 70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyshkunova, I.V.; Chukhchin, D.G.; Gofman, I.V.; Pavlova, E.N.; Ushakov, V.A.; Vlasova, E.N.; Poshina, D.N.; Skorik, Y.A. Chitin Cryogels Prepared by Regeneration from Phosphoric Acid Solutions. Materials 2021, 14, 5191. https://doi.org/10.3390/ma14185191
Tyshkunova IV, Chukhchin DG, Gofman IV, Pavlova EN, Ushakov VA, Vlasova EN, Poshina DN, Skorik YA. Chitin Cryogels Prepared by Regeneration from Phosphoric Acid Solutions. Materials. 2021; 14(18):5191. https://doi.org/10.3390/ma14185191
Chicago/Turabian StyleTyshkunova, Irina V., Dmitry G. Chukhchin, Iosif V. Gofman, Ekaterina N. Pavlova, Vadim A. Ushakov, Elena N. Vlasova, Daria N. Poshina, and Yury A. Skorik. 2021. "Chitin Cryogels Prepared by Regeneration from Phosphoric Acid Solutions" Materials 14, no. 18: 5191. https://doi.org/10.3390/ma14185191
APA StyleTyshkunova, I. V., Chukhchin, D. G., Gofman, I. V., Pavlova, E. N., Ushakov, V. A., Vlasova, E. N., Poshina, D. N., & Skorik, Y. A. (2021). Chitin Cryogels Prepared by Regeneration from Phosphoric Acid Solutions. Materials, 14(18), 5191. https://doi.org/10.3390/ma14185191






