3.1. Results of the Effect of REMs on the Formed Inclusions, Microstructural and Mechanical Properties of Low-Alloyed Structural Cr-Mo Steel Made from Heat A
The production of steel (heat A) was conducted by oxidative melting technology using the technological residue in EAF (Electric Arc Furnace) and subsequently processed on secondary metallurgy equipment LF + VD (Ladle Furnace and Vacuum Degassing). After the evacuation, steel samples, slags were taken, the hydrogen content was measured by HYDRIS
® [
35], and Ca-Si (0.2 kg/t) was added in the form of a profile. The melt temperature of the steel was 1596 °C; the hydrogen content was 0.000094 wt.%). The chemical composition of the steel after vacuum degassing (before the addition of REMs) and after the addition of REMs is given in
Table 1. The analysis of slag after vacuum degassing is given in
Table 2. After sampling for chemical analyses, the ladle was driven back into the VD, and after creating a vacuum, REMs in the form of mischmetal were added into the steel in the amount of 70 kg, which corresponded to the assumed final cerium content in the steel of 0.05–0.07 wt.%. The course of alloying with mischmetal pieces was calm; there was no visible reaction of the mischmetal with steel, which was also proved by a camera placed on the lid of the vacuum degassing chamber. Alloying with mischmetal lasted about 5 minutes, and after its completion, the steel was briefly bubbled with argon, taken out of the vacuum degassing chamber, and the steel temperature, oxygen activity, and hydrogen content were measured. CELOX
® [
36] and HYDRIS
® were used to measure the oxygen, respectively hydrogen activity. Subsequently, steel and slag samples were taken.
The temperature drop after alloying with mischmetal was 23 °C, i.e., about 2.3 °C/min. In the case of oxygen activity, its value decreased from the original 0.000385 wt.% to 0.000120 wt.%, i.e., a decrease by 0.000265 wt.%. The melting loss of cerium in the melt was 56.3%, which is also confirmed by the analysis of cerium from the laboratory (0.035 wt.%). Interestingly, there was a decrease in cerium content in steel during casting, with a total decrease of 0.009 wt.%, which corresponds to a melting loss of about 26% compared to that of the melting analysis, suggesting additional reoxidation of cerium during casting with atmospheric oxygen. The cerium melting losses ranged from 50–56% during alloying and VD, and it is also necessary to consider the decrease in the cerium content during casting. If it is required to alloy other similar melts with mischmetal targeting the cerium content in the melt of 0.05–0.07 wt.%, it will be necessary to increase the amount of mischmetal used, which will include higher losses due to melting loss and reoxidation. There was no significant decrease in the contents of Mn and Si; the content of Al decreased only by 0.004 wt.%. Thus, Ce had a favorable effect on the melting losses of these elements.
Slag sampling was performed in three stages of steel production. First, at the beginning of steel ladle processing (LF), then after vacuum degassing, and finally, after the addition of cerium in the form of mischmetal. No significant changes in the composition of slag (CaO, SiO
2, Al
2O
3, MgO) occurred, only the higher content of FeO after alloying with mischmetal was analyzed. This is a fundamental difference compared to that of the experiment in [
31], where differences in SiO
2 and MgO contents were noted, which were explained by the use of a filled profile with cerium containing 33% Si, the age of the ladle, and thus, its wear (MgO).
The cerium content of the slag was also analyzed. Prior to mischmetal alloying, the Ce content in the slag was 0.10 wt.%, after alloying 1.0 wt.%. It is not clear why this happened, and it is surprising; additional analysis would be very useful. According to the calculation, 80 kg of cerium was added to the melt (70 kg was a fact). Therefore, the cerium melting loss was 13.8 wt.%, and cerium utilization would be 86.1%. If this calculation were correct, the cerium content in the steel should be 0.078 wt.%, which, due to the melting analysis of 0.035 wt.%, was not proved. This fact, therefore, indicates a higher melting loss of cerium, namely 50–56%, as mentioned above.
From the experimental heat A, two pieces of forging ingots were cast, each weighing about 23 t. A teeming ladle with a larger diameter of ladle nozzle (90 mm) than in that of [
31] was chosen for casting to avoid clogging [
15,
16,
17]. To prevent reoxidation of the steel by atmospheric oxygen, the casting stream was protected with argon. Despite this type of protection, however, partial oxidation of cerium occurred. The casting process was standard, with no signs of problems. The ingot heads were treated with exothermic and insulating powder. The quality of the ingot heads was very good, without significant shrinkage. The ingot surfaces were free of obvious defects.
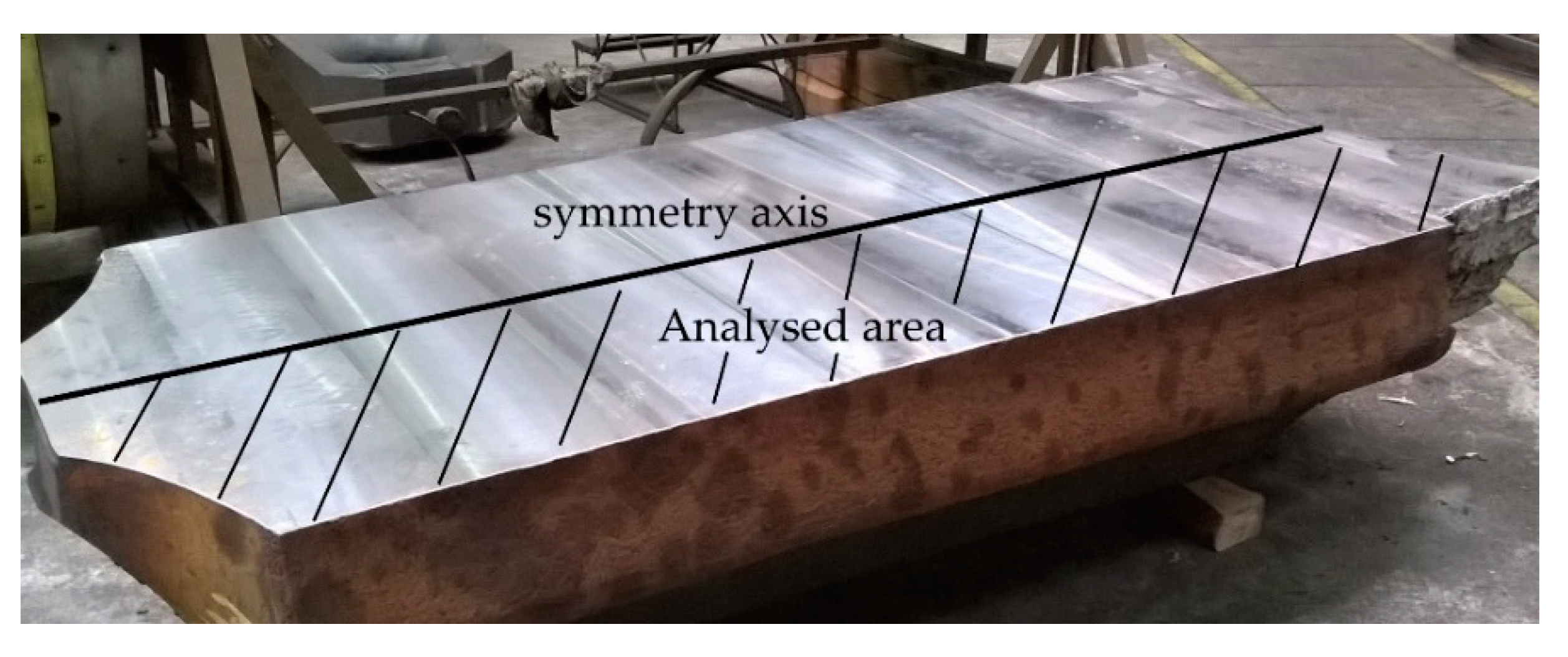
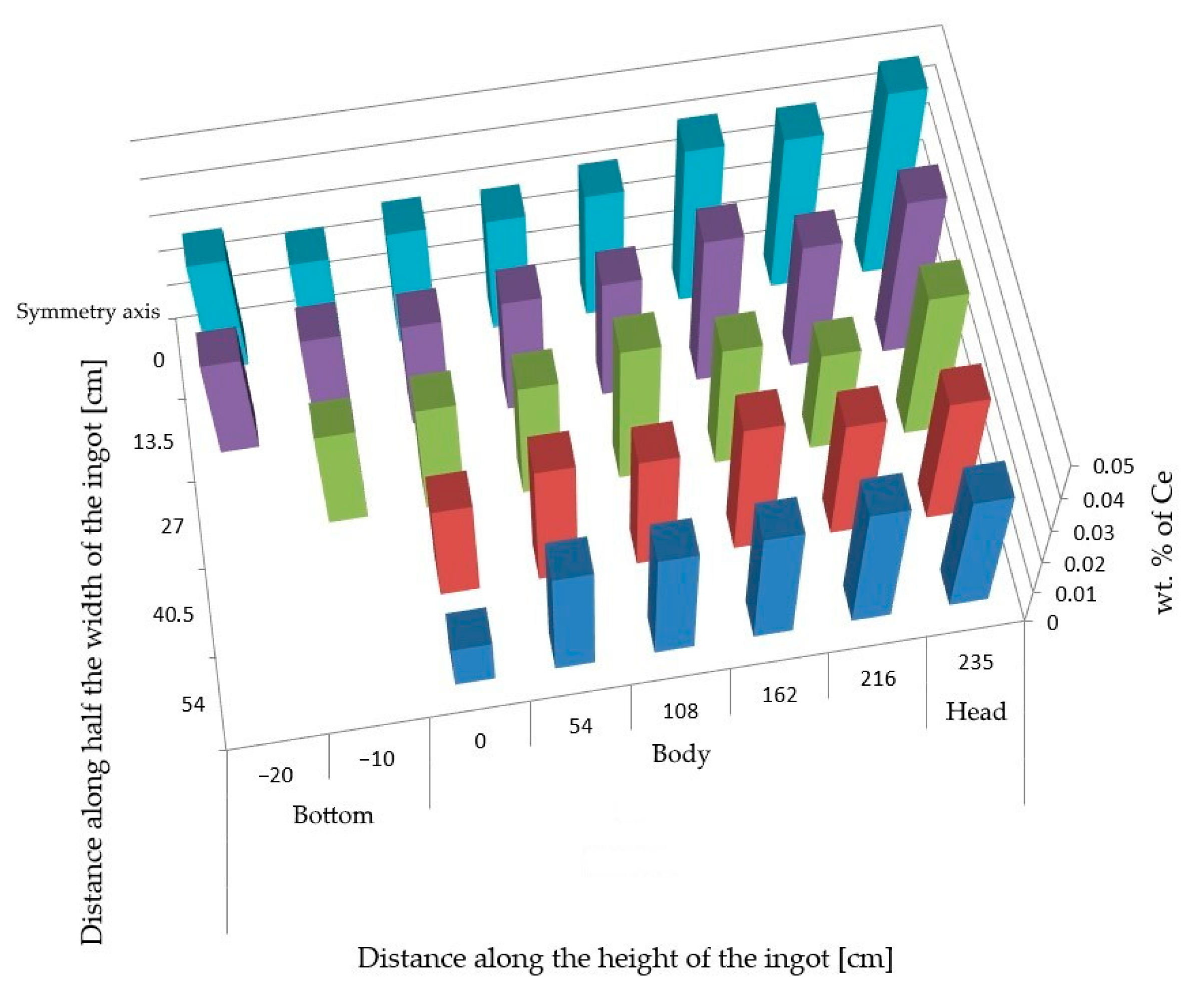
The performed analyses of the cerium content in the cross-section of the ingot from heat A (
Figure 1) show its uneven distribution (
Figure 2). The highest cerium content was found in the axial part of the head and in the subhead region of the ingot and was around 0.042 wt.%. Towards the bottom of the ingot, its content then gradually decreased. At the ingot wall, the cerium content was around 0.032 wt.%, and in the bottom area, it ranged from 0.027–0.031 wt.%
The carbon concentration was highest in the subhead area of the ingot in the axial sections. The cross-sectional distribution was more or less uniform, which was similar in the case of sulfur. The hydrogen content was 0.00010 wt.%, 0.000143 wt.%, 0.000127 wt.%, and 0.000132 wt.%.
On the forged bar from the second ingot of heat A, point indications with a size of max 3 mm, clusters of indications and elongated indications with a length of max 2 mm were detected by ultrasonic test.
This was followed by a control chemical analysis, the results of which are shown in
Table 3. The values given were always determined by the average of three measurements. The measured contents of the key elements for a given steel grade corresponded to the melting chemical composition; see
Table 1. The determined cerium content ranged from 0.023–0.028 wt.%, and thus, a further decrease was recorded compared to that of the melting analysis given in
Table 1.
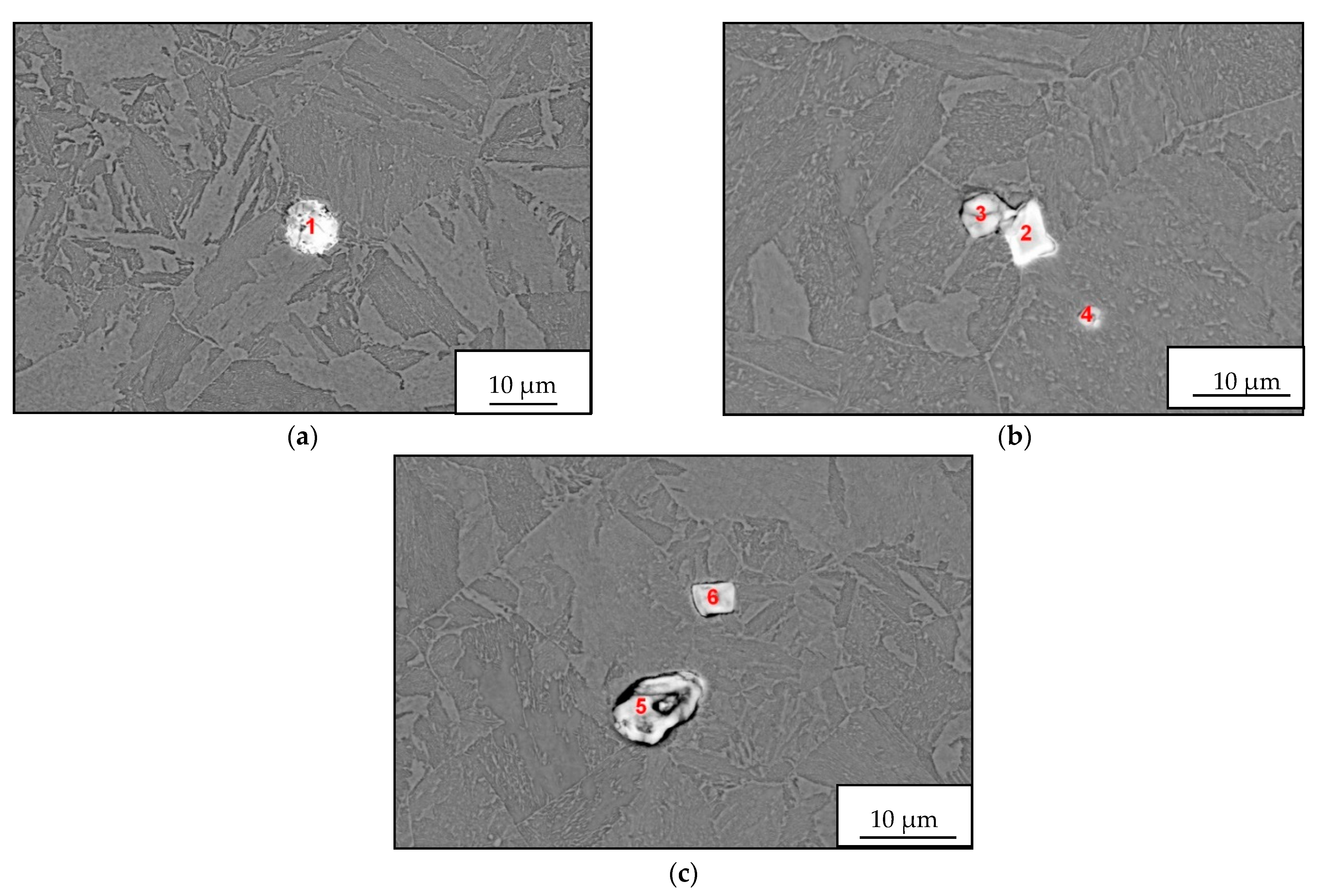
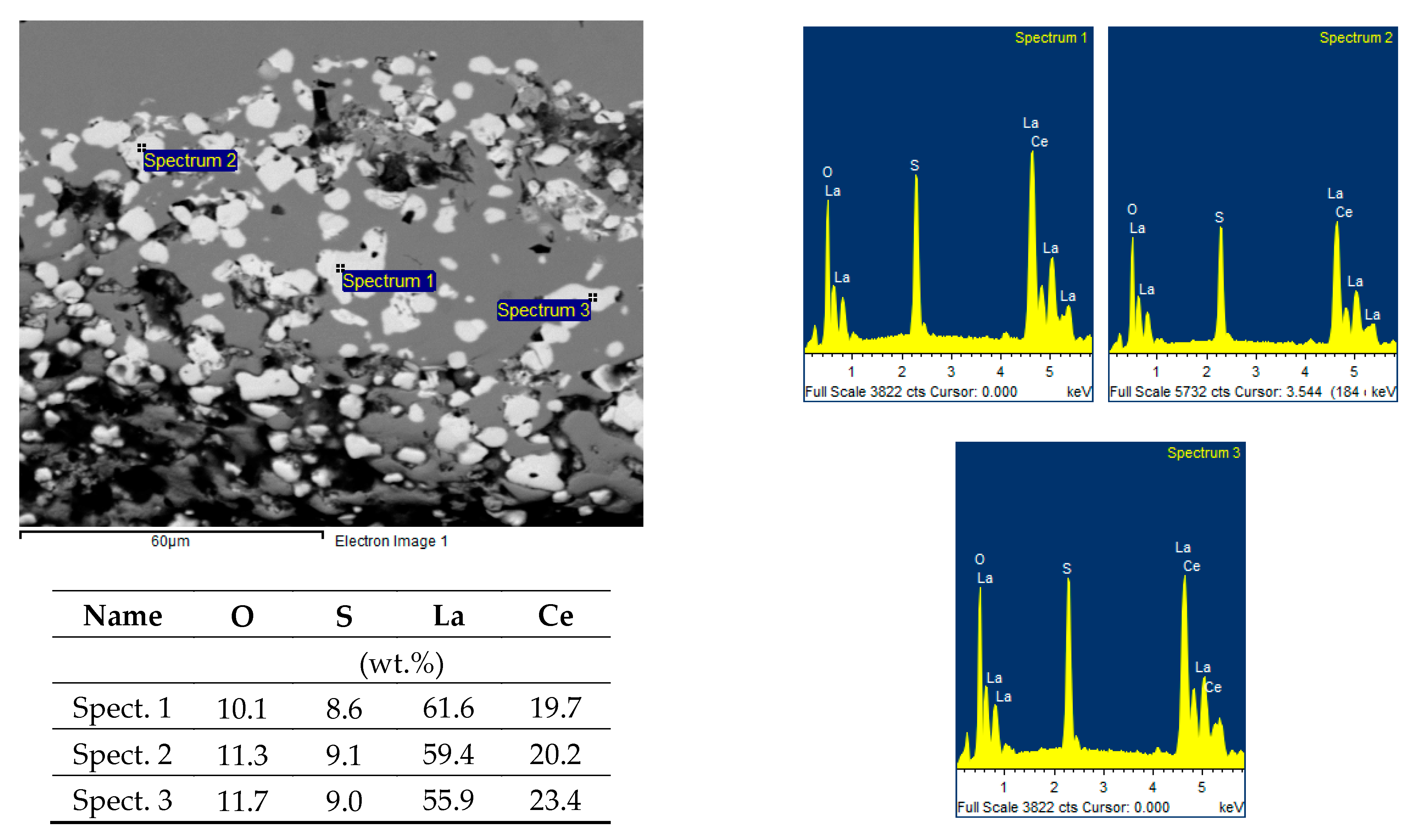
The microcleanliness of all studied samples was very good, with occasional inclusions of type B and D inclusions. In rare cases, type D inclusions with a diameter of about 20 µm were found. Examples of inclusions found are shown in
Figure 3a–c; EDS microanalysis of inclusions and area chemical analyses of the sample are in
Table 4.
The detected inclusions in the steel matrix were statistically analyzed. Based on the achieved results, four types of inclusions were found, see
Table 5. The first group (No. 1) consists of oxide-sulfide inclusions bound to lanthanum and cerium with stoichiometric composition (La-Ce)
2O
2S + (La-Ce)O
2 + SiO
2(minority).
The second group of inclusions (No. 2) is stoichiometrically complex. Oxygen, phosphorus, arsenic, and antimony bound to lanthanum and cerium were detected. There is probably a bond with iron (Fe content was about 20%).
The third group of inclusions (No. 3) are oxides La + Ce, MgO, Al2O3 a SiO2. The presence of sulfur or phosphorus was not detected.
The fourth group of inclusions (No. 4) is similar to the first group. Stoichiometrically, it corresponds to the complex of phases formed by the compounds (La-Ce)2O2S, FeO, SiO2 a CaO or CaS. Interestingly, neither chromium nor manganese was identified at any stage.
The microstructure was evaluated on transverse cuts of samples, and in all monitored areas (subsurface, ¼ of diameter, the center of forging), it was very similar; uneven, formed by blocks of bainite and ferrite grains and perlite nodules (see
Figure 4). The size of the original austenitic grain was the same in the whole cross-section of the forging, G = 9.
The results of tensile tests (average of three tests) and Charpy impact test are given in
Table 6, which shows relatively high values of the tensile strength for a given quality and type of the forging while maintaining excellent plastic properties. As expected, all stress characteristics decreased from the surface towards the center of the forging. The achieved values of impact energy seem to be relatively low, even though the microcleanliness of the steel was at an excellent level, as stated above.
3.2. Results of the Effect of REMs on the Formed of Inclusions and Microstructural Properties of Low-Alloyed Structural Cr-Mo Steel Made from Heat B
Based on the findings presented in chapter 3.1, experimental heat B was produced. The course of the production at the steel plant was similar to that of heat A. The main difference was the increase of alloying with mischmetal to 100 kg, which was to ensure the final cerium content in the steel in the range of 0.05–0.07 wt.%, as well as the use of higher quality casting ceramics and certain modifications concerning the Ar protection of the casting stream of the steel to minimize the secondary reoxidation of the steel by atmospheric oxygen. The chemical composition of heat B before and after the addition of mischmetal to VD is shown in
Table 7. Vacuum degassing of the steel lasted 33 min, bubbling the melt by argon 22 min. The reaction of REMs with Al
2O
3 formed clusters during casting [
14,
15], which resulted in a narrowing of the casting stream. The optimum casting speed was achieved only from the body/head interface of the ingot.
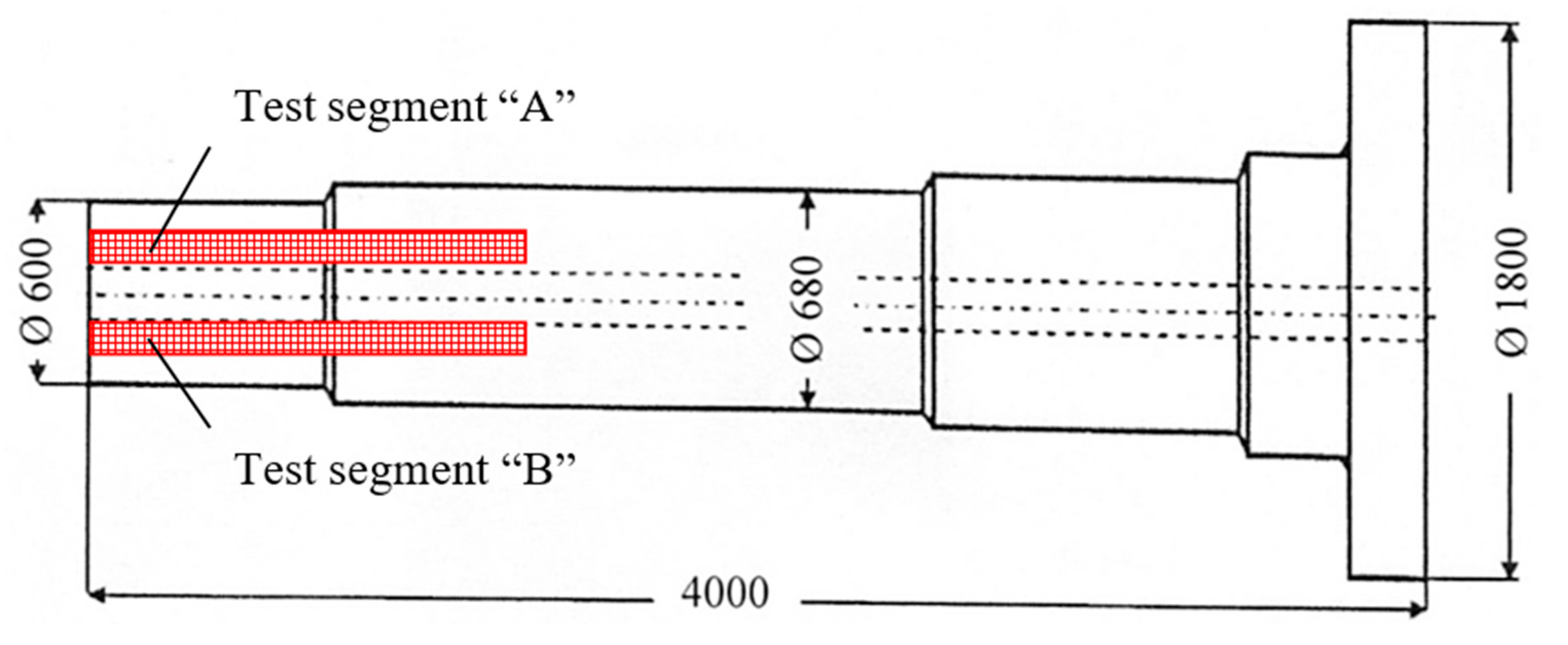
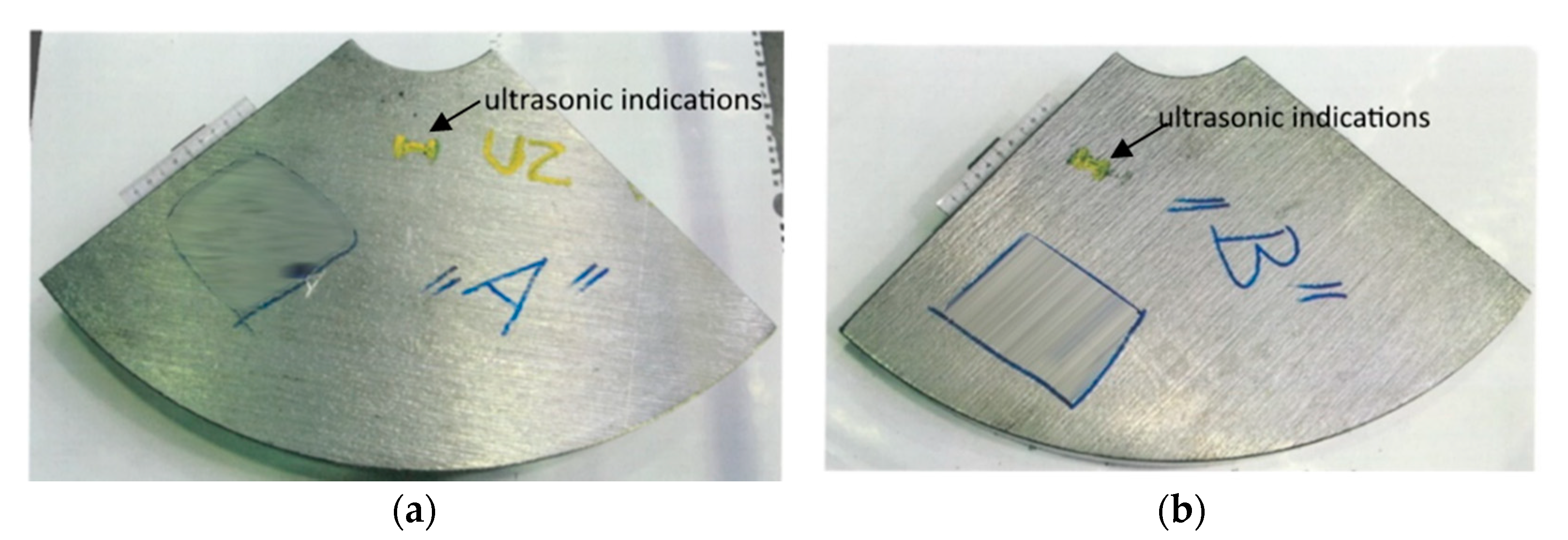
The wind power shaft was forged from a cast ingot weighing approximately 50 t. Unfortunately, during the ultrasonic inspection of the shaft, unacceptable indications were detected; the area of their occurrence is marked in red in
Figure 5. This was a frequent occurrence of point indications up to the equivalent diameter of approximately 5 mm, and three elongated unsatisfactory indications. To determine the cause of these unacceptable indications, test segments A, B (see
Figure 6) were taken from the “worst” areas of the shaft, from which samples 34/A, 34/B were prepared for chemical and structural analyses.
The control chemical analysis confirmed that the content of all elements mentioned above is in accordance with the melting chemical composition (
Table 7) except for the carbon, which showed a slightly higher content, see
Table 8.
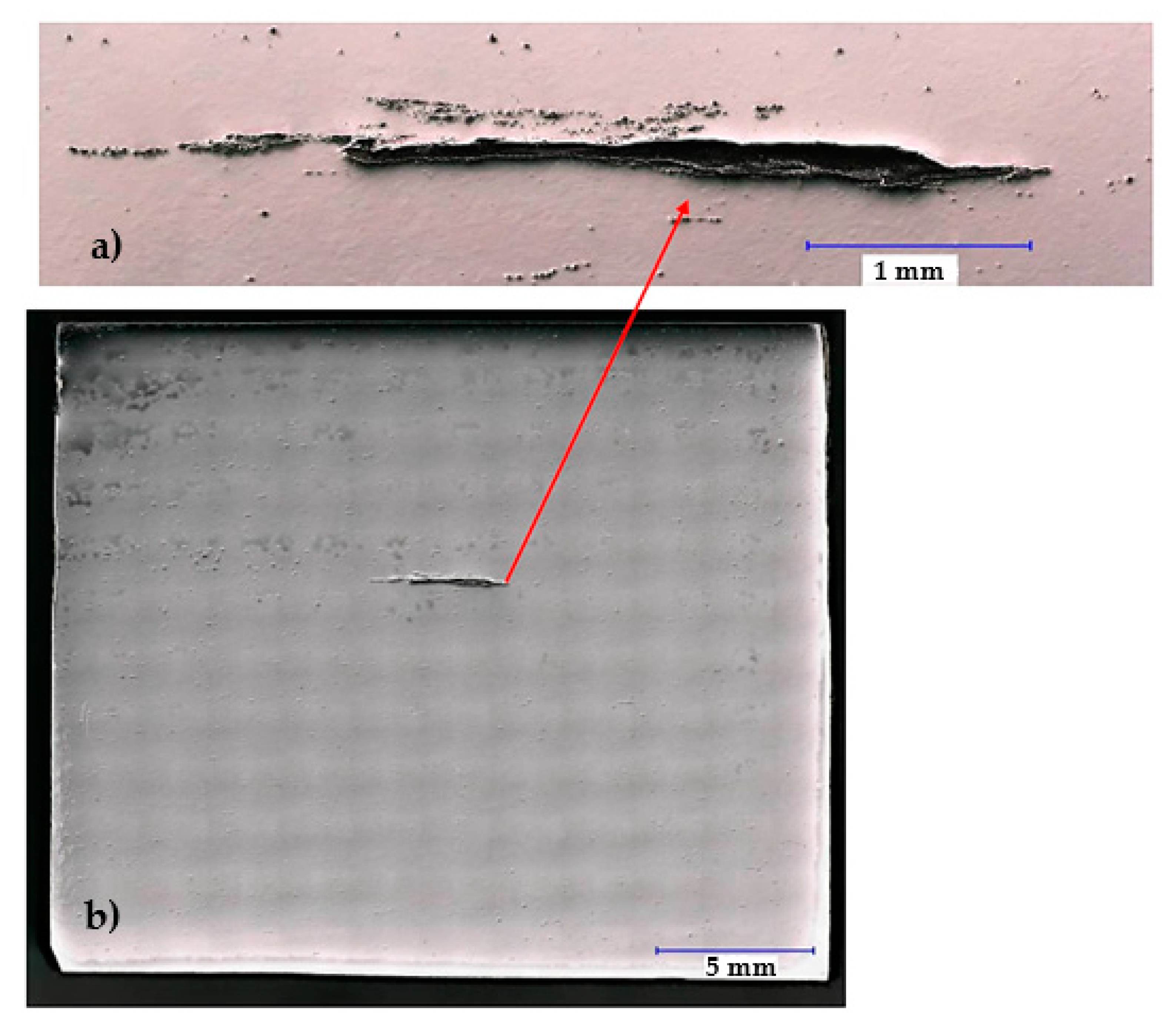
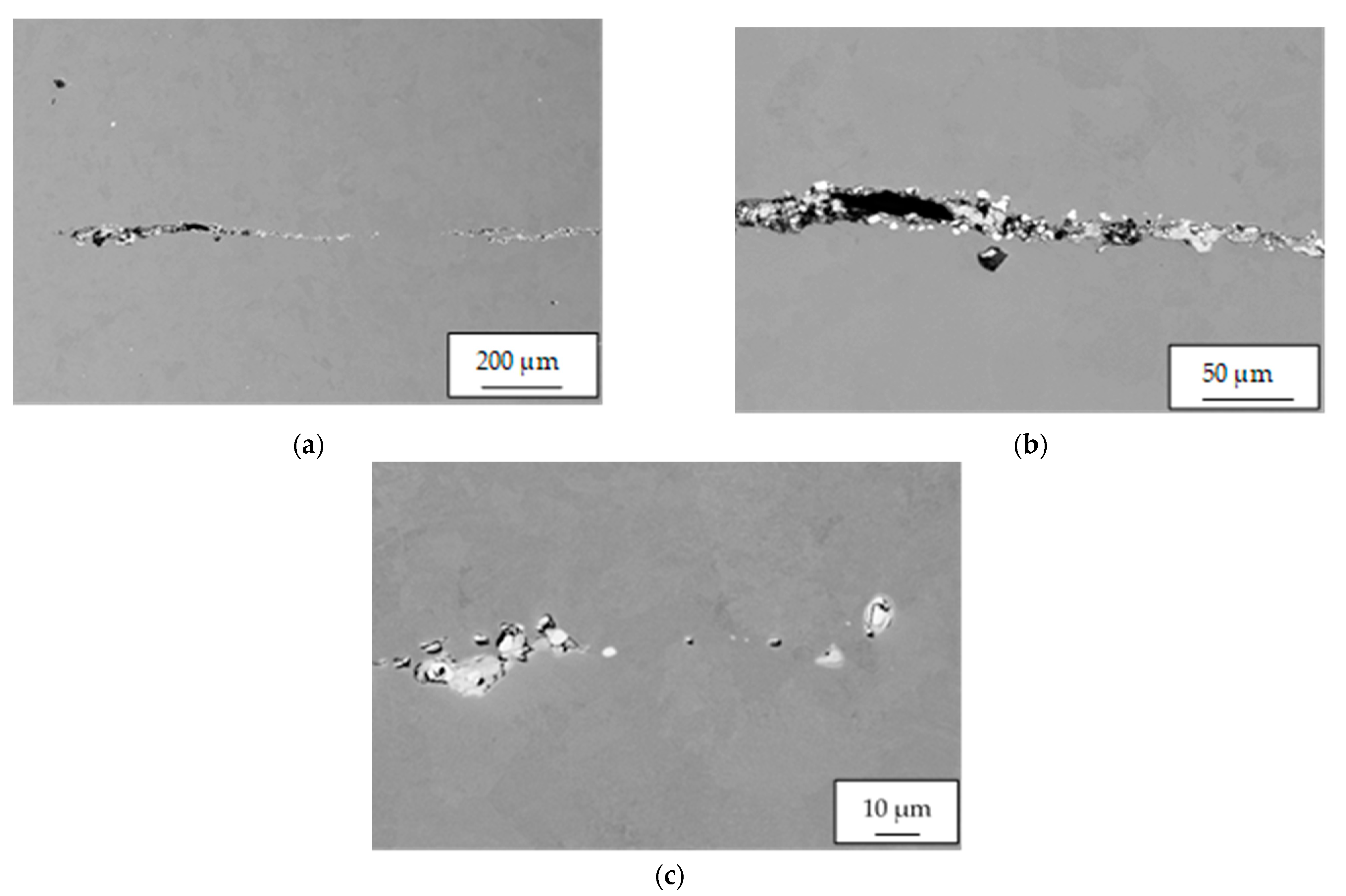
Based on the visual inspection of the products carried out in accordance with the guidelines of the quality management principles [
65], the crack about of 4.5 mm in length was observed in the metal matrix of sample 34/A (
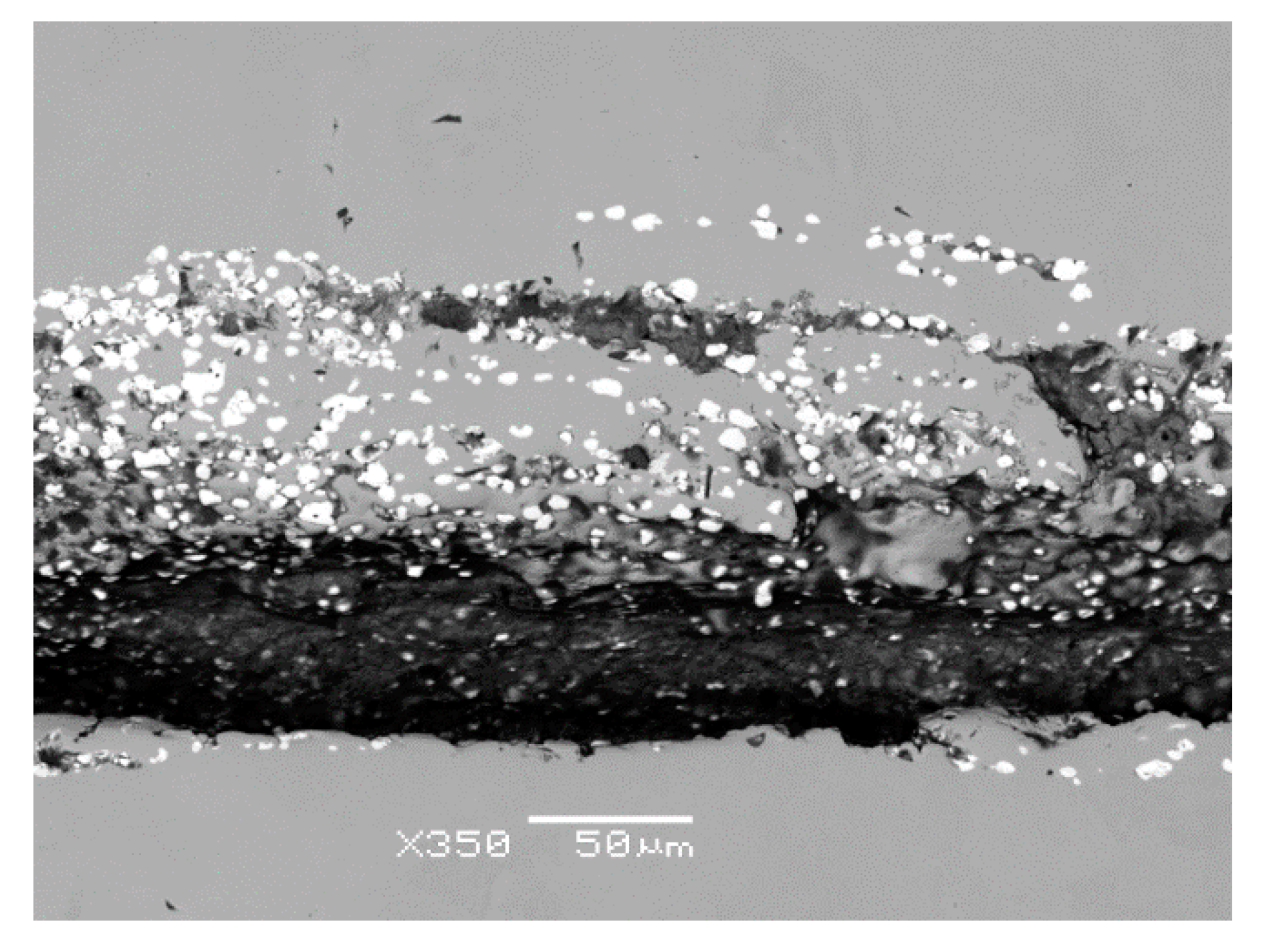
Figure 7). In the vicinity and in the filling of considerably coarse and open crack, mostly row-oriented, nonmetallic inclusions were observed, or clusters of nonmetallic inclusions with several contrasts (
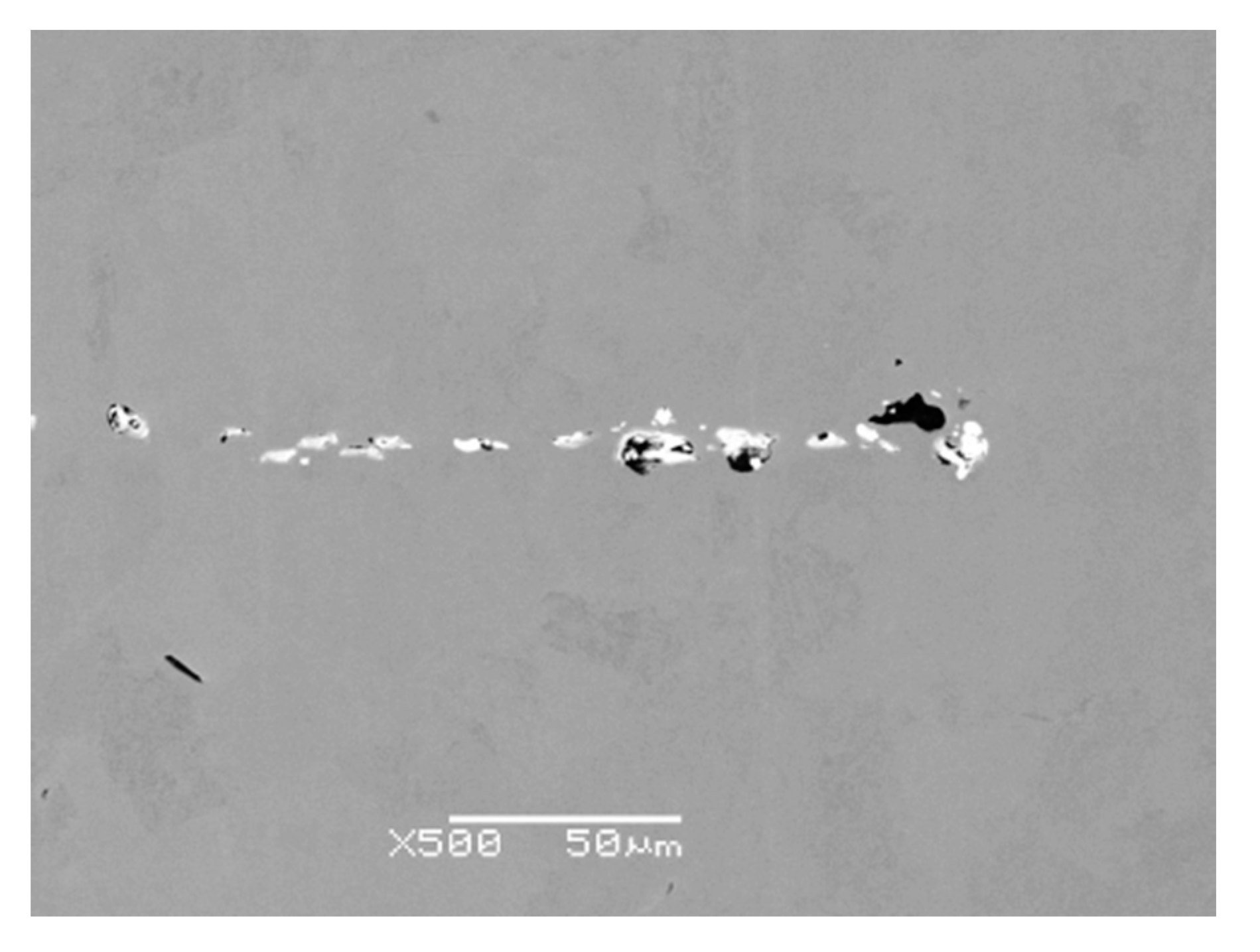
Figure 8). Point nonmetallic inclusions were present in the metal matrix, except for the crack (
Figure 9).
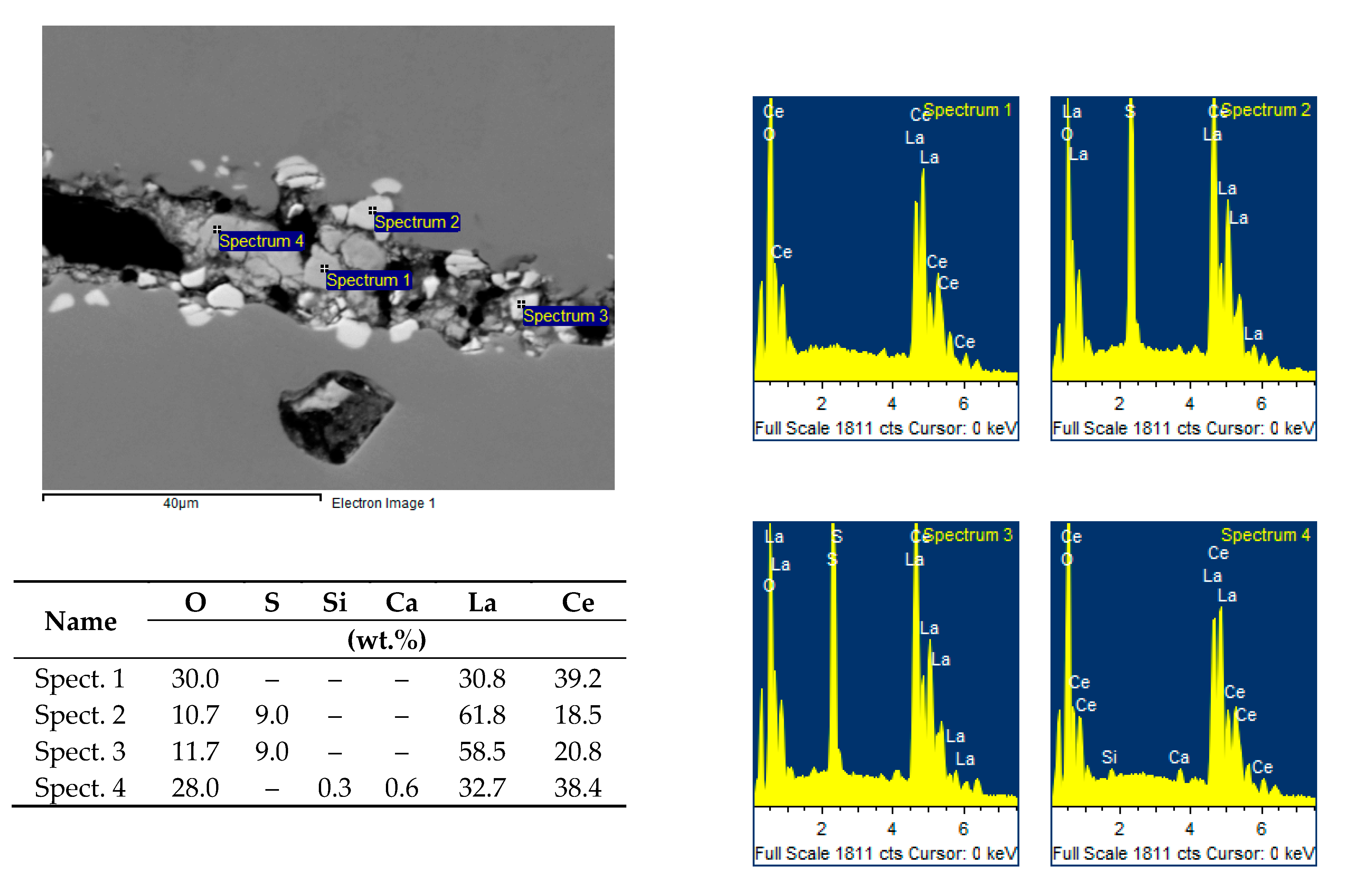
X-ray microanalysis showed that the chemical composition of various nonmetallic inclusions corresponds to complex oxides, or lanthanum and cerium oxysulphides (
Figure 10).
For sample 34/B, the observed crack was significantly smaller and finer (
Figure 11a) than for that of sample 34/A (
Figure 7) and was about 1.7 mm in length. Nonmetallic inclusions in sample 34/B were observed both in its filling (
Figure 11b) and in the matrix. Locally, these inclusions interconnected, and small chains were formed (
Figure 11c). The frequency of inclusions was also high in sample 34/B.
The identification of the chemical composition of both bright white and slightly grey inclusions proved that these are complex oxides based on lanthanum and cerium with variable amounts of calcium and silicon, or lanthanum and cerium oxysulphides, see
Figure 12.
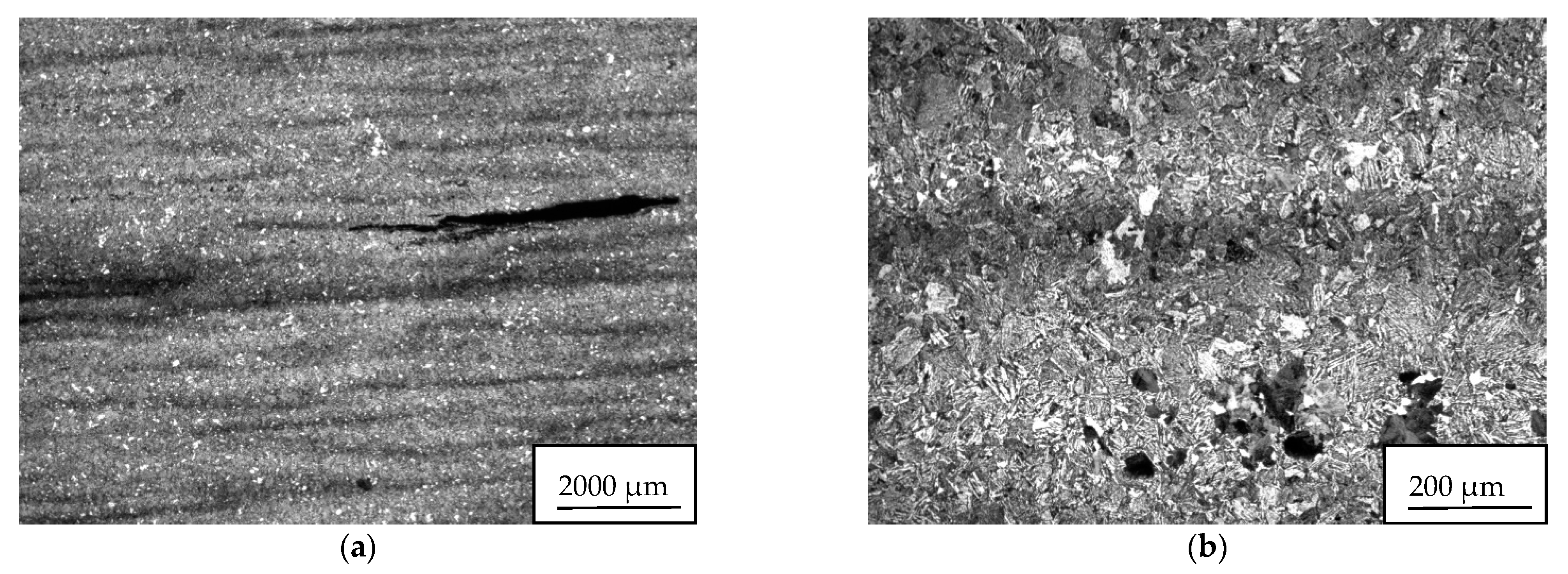
The microstructure of both studied samples 34/A, 34/B was identical, quenched and tempered with the occurrence of segregation bands of darker contrast, guided in the direction of material formation. Perlite, locally small islands of ferrite, were observed in smaller amounts in the metal matrix. A higher frequency of precipitate was found in the segregation. No decarburization was observed around the cracks. Clear photo documentation is shown in
Figure 13.