Determination of the Influence of Hydraulic Additives on the Foaming Process and Stability of the Produced Geopolymer Foams
Abstract
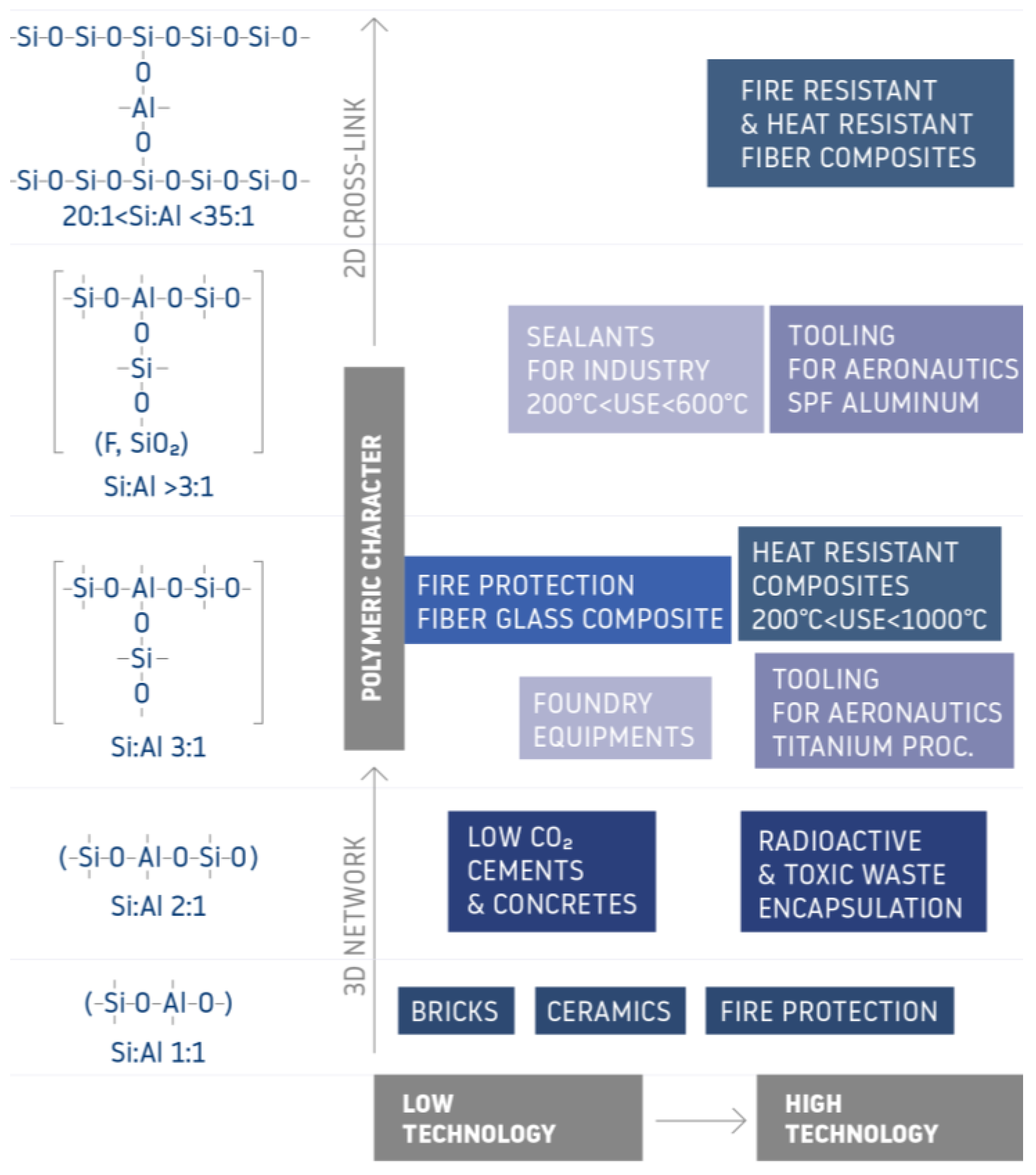
1. Introduction
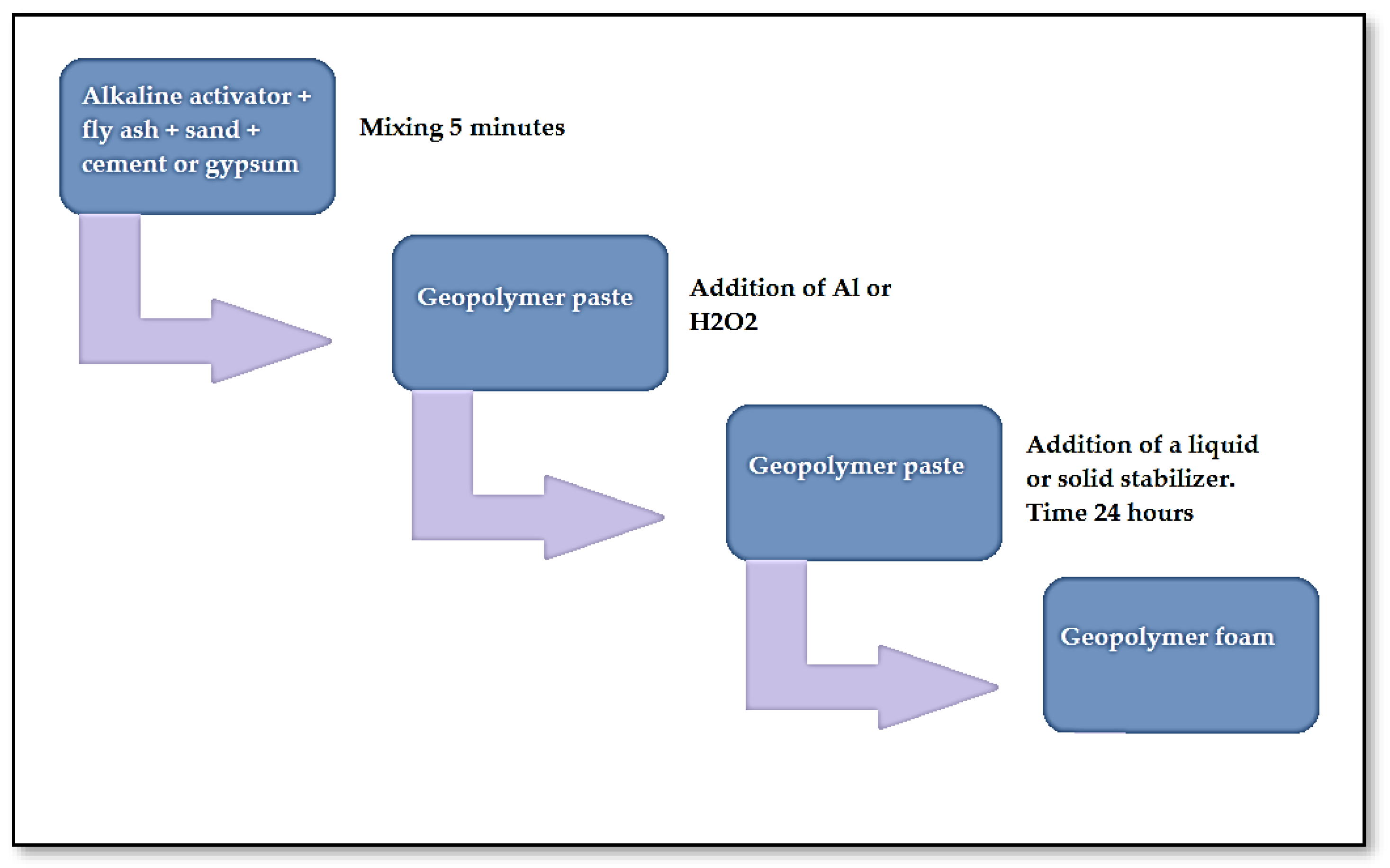
2. Materials and Methods
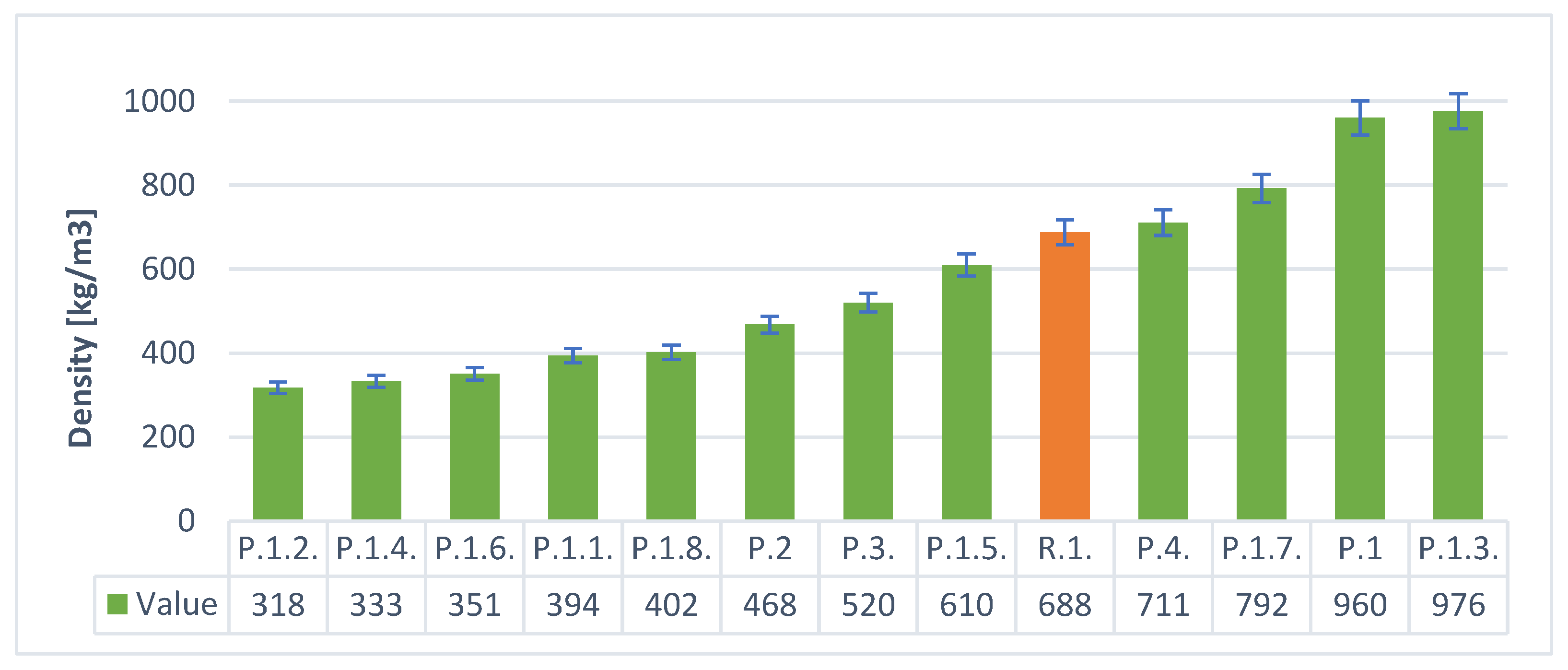
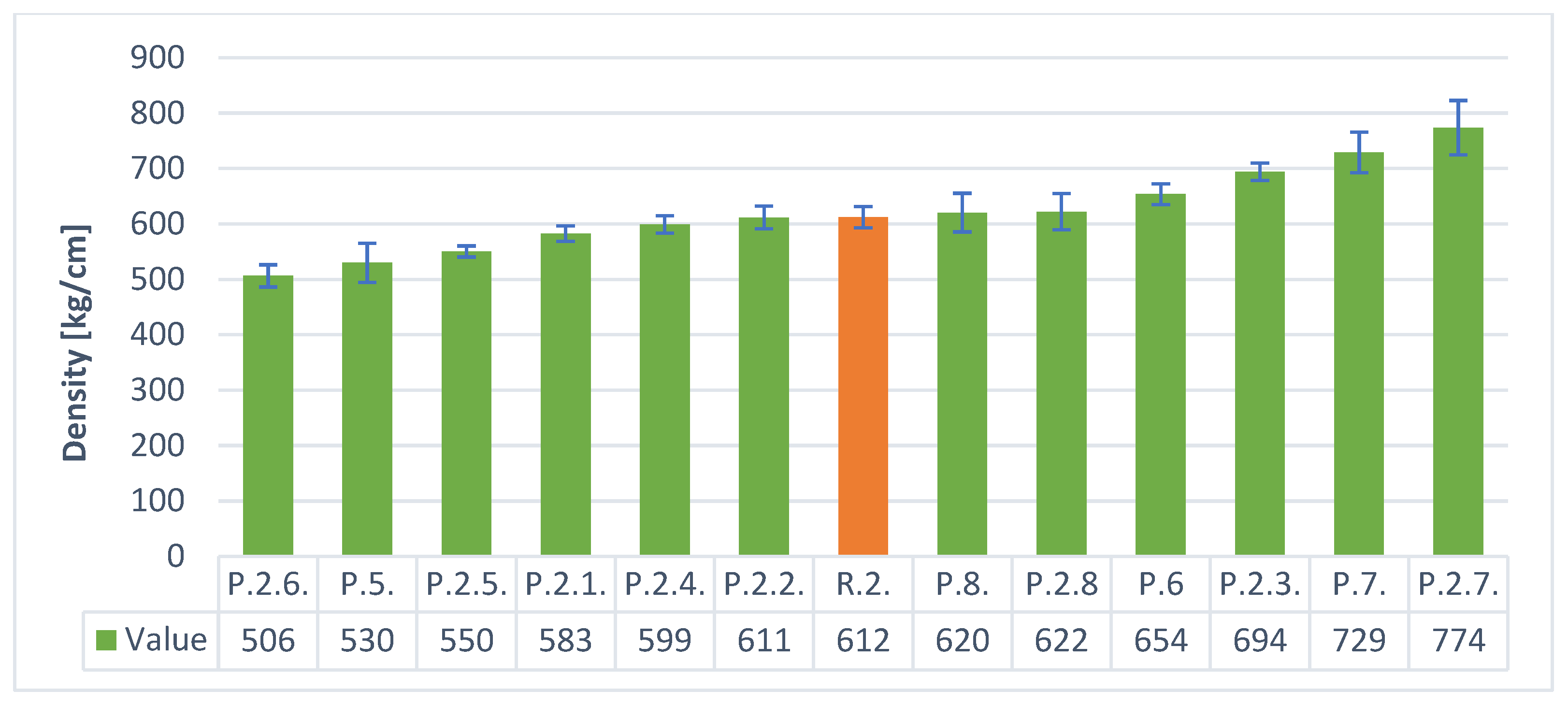
3. Results
4. Conclusions
- Comparing the foaming efficiency of geopolymers using hydrogen peroxide and aluminum powder as a foaming agent, it was found that better effects were obtained for H2O2—it is a better foaming agent for geopolymers than Al powder.
- By using the hydraulic additive—stabilizer in the form of cement—lower densities and better insulation parameters were obtained than when using gypsum. Portland cement is a better stabilizer than gypsum (calcium sulfates).
- The addition of surfactants had a beneficial effect on reducing density and improving thermal conductivity. It should be noted that both hydraulic additives such as cement or gypsum have a positive effect on the stability of the geopolymer foams produced, but also the addition of surfactants has a positive effect. The combination of these two additives gives the best results. The samples without the addition of surfactants, but only with hydraulic stabilizers, performed worse.
Author Contributions
Funding
Conflicts of Interest
References
- Chandra, S.; Berntsson, L. Lightweight Aggregate Concrete: Science, Technology and Applications; William Andrew Publishing: New York, NY, USA, 2002. [Google Scholar]
- Wang, S.; Li, V.C. Lightweight engineered cementitious composites (ECC). In Proceedings of 4th International RILEM Workshop on High Performance Fiber Reinforced Cement Composites (HPFRCC 4); RILEM Publications: Recteur Poincaré, Paris, France, 2003; Volume 1, pp. 379–390. [Google Scholar]
- Zhang, M.H.; Gjvorv, O.E. Mechanical properties of highstrength lightweight concrete. ACI Mater. J. 1991, 88, 240–247. [Google Scholar]
- Mikuła, J.; Łach, M. Eco-Friendly Solutions for Production. Modern Eco-Friendly Composite Materials; Wydawnictwo Politechniki Krakowskiej: Kraków, Poland, 2014; pp. 13–32. [Google Scholar]
- Davidovits, J. Geopolymer Chemistry and Applications, 5th ed.; Geopolymer Institute: Saint-Quentin, France, 2020. [Google Scholar]
- Mikuła, J.; Łach, M. Manufacturing method and properties of geopolymer products based on volcanic tuff. Mater. Eng. 2014, 35, 270–276. [Google Scholar]
- Fongang, R.T.T.; Pemndje, J.; Lemougna, P.N.; Melo, U.C.; Nanseu, C.P.; Nait-Ali, B.; Kamseu, E.; Leonelli, C. Cleaner production of the lightweight insulating composites: Microstructure, pore network and thermal conductivity. Energy Build. 2015, 107, 113–122. [Google Scholar] [CrossRef]
- Van Riessen, A. Thermo-mechanical and microstructural characterisation of sodium-poly(sialate-siloxo) (Na-PSS) geopolymers. J. Mater. Sci. 2007, 42, 3117–3123. [Google Scholar]
- Nguyen, T.T.; Bui, H.H.; Ngo, T.D.; Nguyen, G.D.; Kreher, M.U.; Darve, F. A micromechanical investigation for the effects of pore size and its distribution on geopolymer foam concrete under uniaxial compression. Eng. Fract. Mech. 2019, 209, 228–244. [Google Scholar] [CrossRef]
- Pantongsuk, T.; Kittisayarm, P.; Muenglue, N.; Benjawn, S.; Thavorniti, P.; Tippayasam, C.; Nilpairach, S.; Heness, G.; Chaysuwan, D. Effect of hydrogen peroxide and bagasse ash additions on thermal conductivity and thermal resistance of geopolymer foams. Mater. Today Commun. 2021, 26, 202149. [Google Scholar]
- Kaddami, A.; Pitois, O. A physical approach towards controlling the microstructure of metakaolin- based geopolymer foams. Cem. Concr. Res. 2019, 124, 1–9. [Google Scholar] [CrossRef]
- Singh, N. Foamed geopolymer concrete. Mater. Today Proc. 2018, 5, 15243–15252. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Mechanical, thermal insulation, thermal resistance and acoustic absorption properties of geopolymer foam concreto. Cem. Concr. Compos. 2015, 62, 97–105. [Google Scholar] [CrossRef]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Cem. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 4th ed.; Geopolymer Institute: Saint-Quentin, France, 2008. [Google Scholar]
- Lyon, R.E.; Foden, A.J.; Balaguru, P.N.; Davidovits, J.; Davidovics, M. Fire resistant aluminosilicate composite. Fire Mater. 1997, 21, 67–73. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P. Enhancing the strength of pre-made foams for foam concrete applications. Cem. Concr. Compos. 2018, 87, 164–171. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P.; Sanjayan, J. Regulating the chemical foaming reaction to control the porosity of geopolymer foams. Mater. Des. 2017, 120, 255–265. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Ngo, T.; Mendis, P. How does aluminium foaming agent impact the geopolymer formation mechanism? Cem. Concr. Compos. 2017, 80, 277–286. [Google Scholar] [CrossRef]
- Ducman, V.; Korat, L. Characterization of geopolymer fly-ash based foams obtained with the addition of Al powder or H2O2 as foaming agents. Mater. Charact. 2016, 113, 207–213. [Google Scholar] [CrossRef]
- Novais, R.M.; Ascensão, G.; Buruberri, L.H.; Senff, L.; Labrincha, J.A. Influence of blowing agent on the fresh- and hardened-state properties of lightweight geopolymers. Mater. Des. 2016, 108, 551–559. [Google Scholar] [CrossRef]
- Wang, M.R.; Jia, D.; He, P.G.; Zhou, Y. Microstructural and mechanical characterization of fly ash cenosphere/metakaolin-based geopolymeric composites. Ceram. Int. 2011, 37, 1661–1666. [Google Scholar] [CrossRef]
- Korniejenko, K.; Łach, M.; Chou, S.Y.; Gądek, S.; Mikuła, J. Mechanical properties of short fiber-reinforced geopolymers made by casted and 3D printing methods: A comparative study. Materials 2020, 13, 579. [Google Scholar] [CrossRef]
- Zhao, Y.; Jow, J.; Cai, X.; Lai, S.-Y. Fly Ash-Based Geopolymer Foam Technology for Thermal Insulation and Fire Protection Applications. In Proceedings of the 2015 World of Coal Ash (WOCA) Conference, Nashville, TN, USA, 5–7 May 2015. [Google Scholar]
- Cui, Y.; Wang, D.; Zhao, J.; Li, D.; Ng, S.; Rui, Y. Effect of calcium stearate based foam stabilizer on pore characteristics and thermal conductivity of geopolymer foam material. J. Build. Eng. 2018, 20, 21–29. [Google Scholar] [CrossRef]
- Lertcumfu, N.; Kaewapai, K.; Jaita, P.; Sanjoom, R.; Rujijanagul, G.; Tunkasiri, T. Synergistic effect of animal oil or butter and hydrogen peroxide on physical and mechanical properties of porous alumino-siliceous materials. Sci. Asia 2020, 46S, 58–65. [Google Scholar] [CrossRef]
- Aliabdo, A.A.; Elmoaty, A.; Salem, H.A. Effect of cement addition, solution resting time and curing characteristics on fly ash based geopolymer concrete performance. Constr. Build. Mater. 2016, 123, 581–593. [Google Scholar] [CrossRef]
- Kretzer, M.B.; Effting, C.; Schwaab, S.; Schackow, A. Hybrid geopolymer-cement coating mortar optimized based on metakaolin, fly ash, and granulated blast furnace slag. Clean. Eng. Technol. 2021, 4, 100153. [Google Scholar] [CrossRef]
- Syringaldehyde. Available online: https://www.sigmaaldrich.com/PL/pl/product/aldrich/w404926?context=product (accessed on 21 August 2021).
- Poly(Ethylene Glycol) Diacrylate. Available online: https://www.sigmaaldrich.com/PL/pl/product/aldrich/437441# (accessed on 21 August 2021).
- Mixer Type TM 100-700. Available online: https://www.l-m-b.eu/en/mixer.php (accessed on 5 September 2021).
- Colangelo, G.; Roviello, G.; Ricciotti, L. Mechanical and thermal properties of lightweight geopolymer composites. Cem. Concr. Compos. 2018, 86, 266–272. [Google Scholar] [CrossRef]
- Nematollahi, B.; Ranade, R.; Sanjayan, J.; Ramakrishnan, S. Thermal and mechanical properties of sustainable lightweight strain hardening geopolymer composites. Arch. Civ. Mech. Eng. 2017, 17, 55–64. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, R.; Gong, L.; Cao, W.; Cheng, X. Development of porous fly ash-based geopolymer with low thermal conductivity. Mater. Des. 2015, 65, 529–533. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, Z.; Zhang, Y.; Li, D. Preparation and characterization of ultra-lightweight foamed geopolymer (UFG) based on fly ash-metakaolin blends. Constr. Build. Mater. 2018, 168, 771–779. [Google Scholar] [CrossRef]
- Wongsa, A.; Sata, V.; Nauklong, P.; Chindaprasirt, P. Use of crushed clay brick and pumice aggregates in lightweight geopolymer concrete. Constr. Build. Mater. 2018, 188, 1025–1034. [Google Scholar] [CrossRef]
- Demirboğa, R.; Gül, R. The effects of expanded perlite aggregate, silica fume and fly ash on the thermal conductivity of lightweight concrete. Cem. Concr. Res. 2003, 33, 723–727. [Google Scholar] [CrossRef]
- Łach, M. Geopolymer foams—Will they ever become a viable alternative to popular insulation materials?—A critical opinion. Materials 2021, 14, 3568. [Google Scholar] [CrossRef] [PubMed]
- Łach, M.; Korniejenko, K.; Mikuła, J. Thermal insulation and thermally resistant materials made of geopolymer foams. Proc. Eng. 2016, 151, 410–416. [Google Scholar] [CrossRef]
- Łach, M.; Mierzwiński, D.; Korniejenko, K.; Mikuła, J. Geopolymer foam as a passive fire protection. MATEC Web Conf. 2018, 247, 1–6. [Google Scholar] [CrossRef][Green Version]







| Precursor | Oxide Composition (wt%) | |||||||
| SiO2 | TiO2 | Fe2O3 | Al2O3 | CaO | MgO | K2O | Na2O | |
| Fly ash | 55.9 | 1.09 | 5.92 | 23.49 | 2.72 | 2.61 | 3.55 | 0.59 |
| Name of Stablizer | Syringaldehyde | Poly(Ethylene Glycol) Diacrylate—Average Mn 575 |
|---|---|---|
| Brand | Sigma-Aldrich | Aldrich |
| Chemical formula | C9H10O4 | (C2H4O)nC6H6O3 |
| Molecular weight | 182.17 g/mol | - |
| Appearance (Form/color) | solid/beige | Liquid/colorless to faint yellow |
| Temperature melting point | 110–113 °C | 104 °C |
| Temperature range boiling | 192–193 °C (in 14 mmHg) | - |
| Density | 1.01 g/cm3 | 1.12 g/cm3 (in 25 °C) |
| Application | laboratory chemicals, production of substances | cross-linking reagent, polymerization reactions |
| Sample Name | Building Sand Mass | Fly Ash Mass | Hydraulic Additive | Stabilizer Weight | Alkaline Activator |
|---|---|---|---|---|---|
| R.1. | 100 g | 900 g | - | - | 350 mL |
| P.1. | 100 g | 850 g | 50 g gypsum | - | 350 mL |
| P.2. | 100 g | 850 g | 50 g cement | - | 350 mL |
| P.3. | 100 g | 800 g | 100 g gypsum | - | 400 mL |
| P.4. | 100 g | 800 g | 100 g cement | - | 400 mL |
| P.1.1. | 100 g | 845 g | 50 g gypsum | 5 g syringaldehyde | 360 mL |
| P.1.2. | 100 g | 845 g | 50 g cement | 5 g syringaldehyde | 350 mL |
| P.1.3. | 100 g | 795 g | 100 g gypsum | 5 g syringaldehyde | 400 mL |
| P.1.4. | 100 g | 795 g | 100 g cement | 5 g syringaldehyde | 400 mL |
| P.1.5. | 100 g | 850 g | 50 g gypsum | 20 mL average Mn 575 | 360 mL |
| P.1.6. | 100 g | 850 g | 50 g cement | 20 mL average Mn 575 | 360 mL |
| P.1.7. | 100 g | 800 g | 100 g gypsum | 20 mL average Mn 575 | 400 mL |
| P.1.8. | 100 g | 800 g | 100 g cement | 20 mL average Mn 575 | 400 mL |
| Sample Name | Building Sand Mass | Fly Ash Mass | Hydraulic Additive | Stabilizer Weight | Alkaline Activator |
| R.2. | 100 g | 900 g | - | - | 360 mL |
| P.5. | 100 g | 850 g | 50 g gypsum | - | 360 mL |
| P.6. | 100 g | 850 g | 50 g cement | - | 360 mL |
| P.7. | 100 g | 800 g | 100 g gypsum | - | 400 mL |
| P.8. | 100 g | 800 g | 100 g cement | - | 400 mL |
| P.2.1. | 100 g | 845 g | 50 g gypsum | 5 g syringaldehyde | 365 mL |
| P.2.2. | 100 g | 845 g | 50 g cement | 5 g syringaldehyde | 370 mL |
| P.2.3. | 100 g | 795 g | 100 g gypsum | 5 g syringaldehyde | 400 mL |
| P.2.4. | 100 g | 795 g | 100 g cement | 5 g syringaldehyde | 380 mL |
| P.2.5. | 100 g | 850 g | 50 g gypsum | 20 mL average Mn 575 | 360 mL |
| P.2.6. | 100 g | 850 g | 50 g cement | 20 mL average Mn 575 | 360 mL |
| P.2.7. | 100 g | 800 g | 100 g gypsum | 20 mL average Mn 575 | 380 mL |
| P.2.8. | 100 g | 800 g | 100 g cement | 20 mL average Mn 575 | 380 mL |
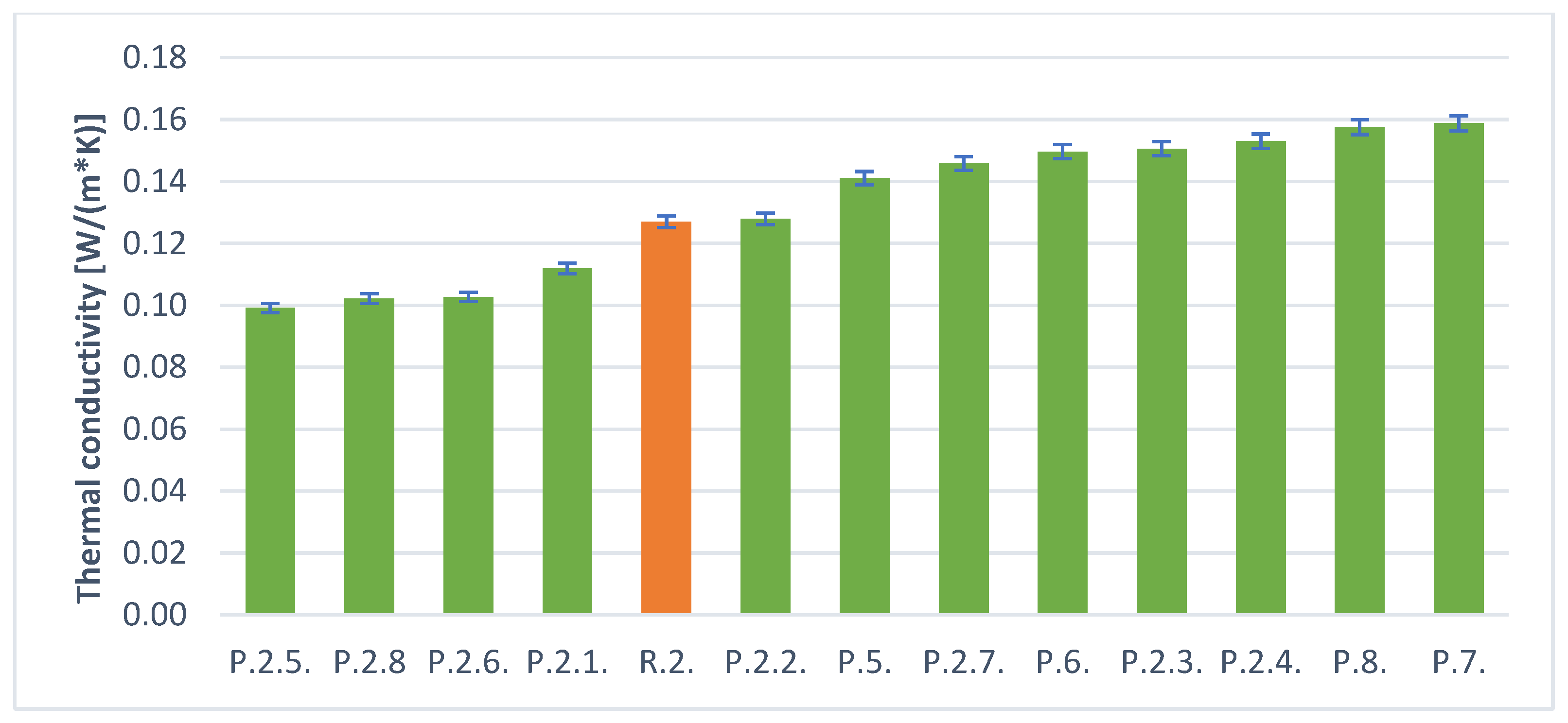
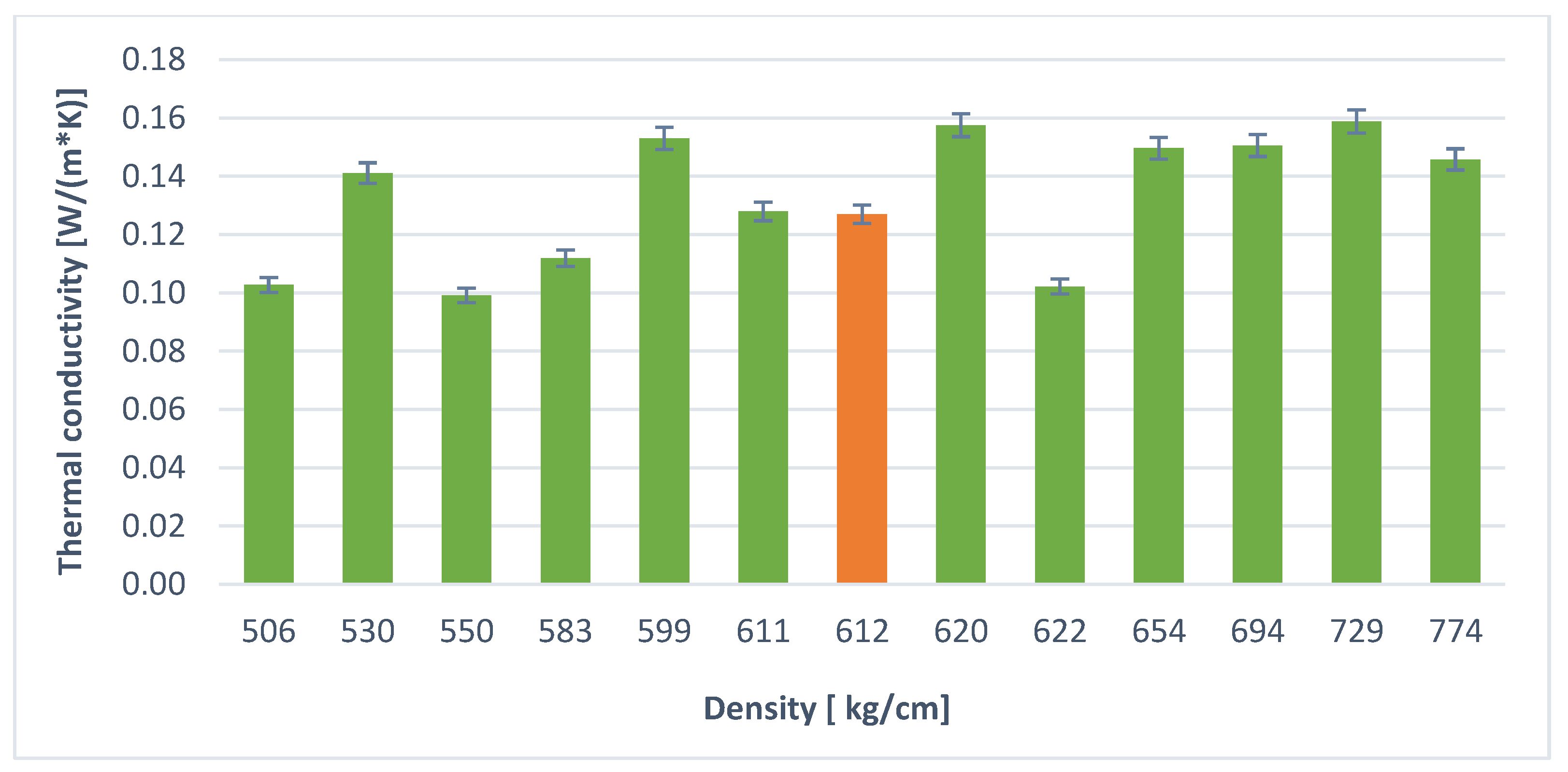
| Foaming Agent | Sample Determination | Results [W/(m × K)] | Foaming Agent | Sample Determination | Results [W/(m × K)] |
|---|---|---|---|---|---|
| H2O2 (30 mL) | R.1. | 0.12596 | Al powder (1.5 g) | R.2. | 0.12698 |
| P.1. | 0.17359 | P.5. | 0.14107 | ||
| P.2. | 0.10913 | P.6. | 0.14961 | ||
| P.3. | 0.13936 | P.7. | 0.15875 | ||
| P.4. | 0.16986 | P.8. | 0.15750 | ||
| P.1.1. | 0.09564 | P.2.1. | 0.11186 | ||
| P.1.2. | 0.08696 | P.2.2. | 0.12789 | ||
| P.1.3. | 0.16108 | P.2.3. | 0.15054 | ||
| P.1.4. | 0.07985 | P.2.4. | 0.15299 | ||
| P.1.5. | 0.12154 | P.2.5. | 0.09914 | ||
| P.1.6. | 0.08251 | P.2.6. | 0.10271 | ||
| P.1.7. | 0.11655 | P.2.7. | 0.14576 | ||
| P.1.8. | 0.09647 | P.2.8. | 0.10214 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łach, M.; Pławecka, K.; Bąk, A.; Lichocka, K.; Korniejenko, K.; Cheng, A.; Lin, W.-T. Determination of the Influence of Hydraulic Additives on the Foaming Process and Stability of the Produced Geopolymer Foams. Materials 2021, 14, 5090. https://doi.org/10.3390/ma14175090
Łach M, Pławecka K, Bąk A, Lichocka K, Korniejenko K, Cheng A, Lin W-T. Determination of the Influence of Hydraulic Additives on the Foaming Process and Stability of the Produced Geopolymer Foams. Materials. 2021; 14(17):5090. https://doi.org/10.3390/ma14175090
Chicago/Turabian StyleŁach, Michał, Kinga Pławecka, Agnieszka Bąk, Katarzyna Lichocka, Kinga Korniejenko, An Cheng, and Wei-Ting Lin. 2021. "Determination of the Influence of Hydraulic Additives on the Foaming Process and Stability of the Produced Geopolymer Foams" Materials 14, no. 17: 5090. https://doi.org/10.3390/ma14175090
APA StyleŁach, M., Pławecka, K., Bąk, A., Lichocka, K., Korniejenko, K., Cheng, A., & Lin, W.-T. (2021). Determination of the Influence of Hydraulic Additives on the Foaming Process and Stability of the Produced Geopolymer Foams. Materials, 14(17), 5090. https://doi.org/10.3390/ma14175090









