Corrosion Inhibition of Mild Steel and 304 Stainless Steel in 1 M Hydrochloric Acid Solution by Tea Tree Extract and Its Main Constituents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Weight Loss Measurement
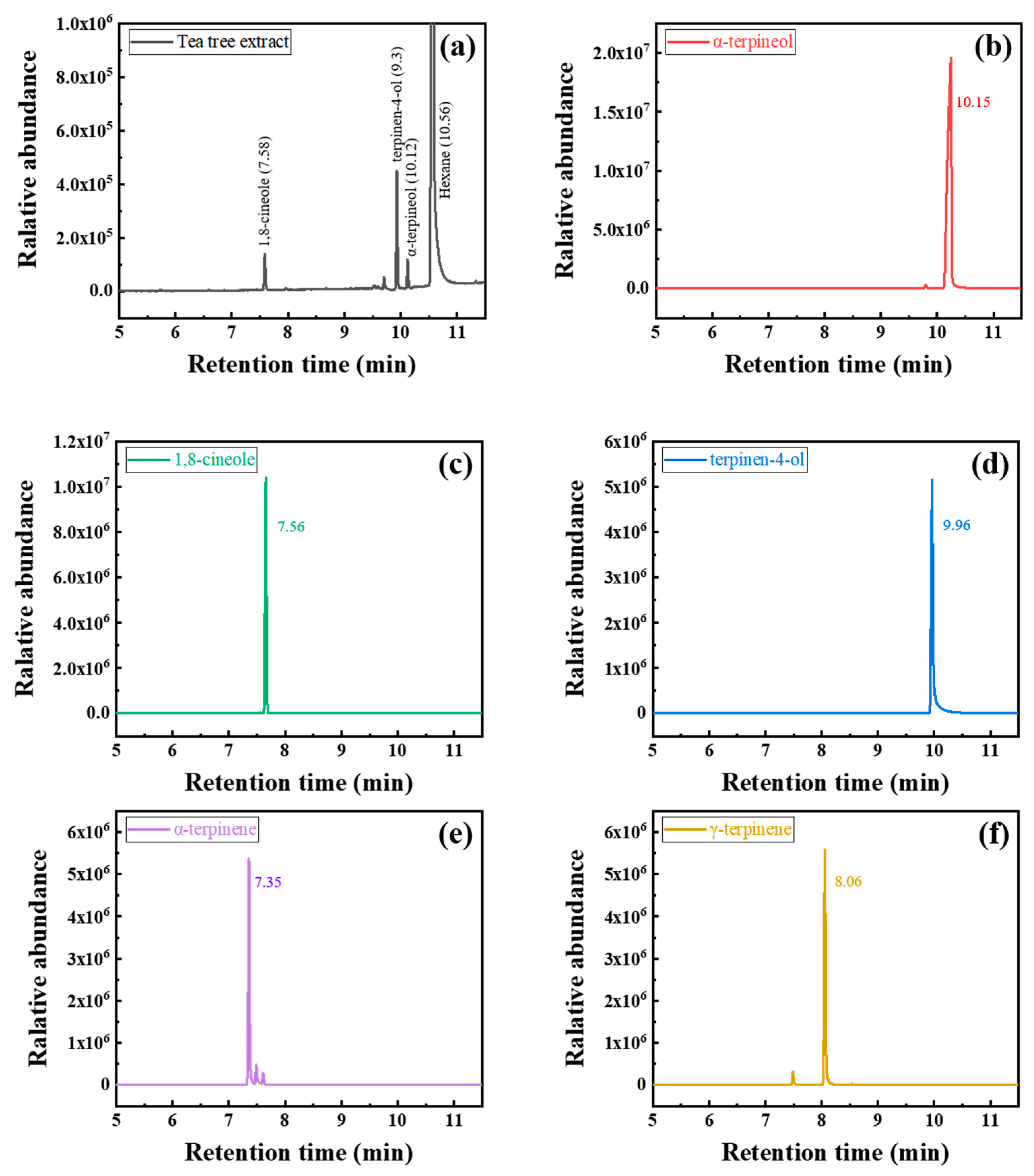
2.2. Gas Chromatography–Mass Spectrometry
2.3. Electrochemical Measurements
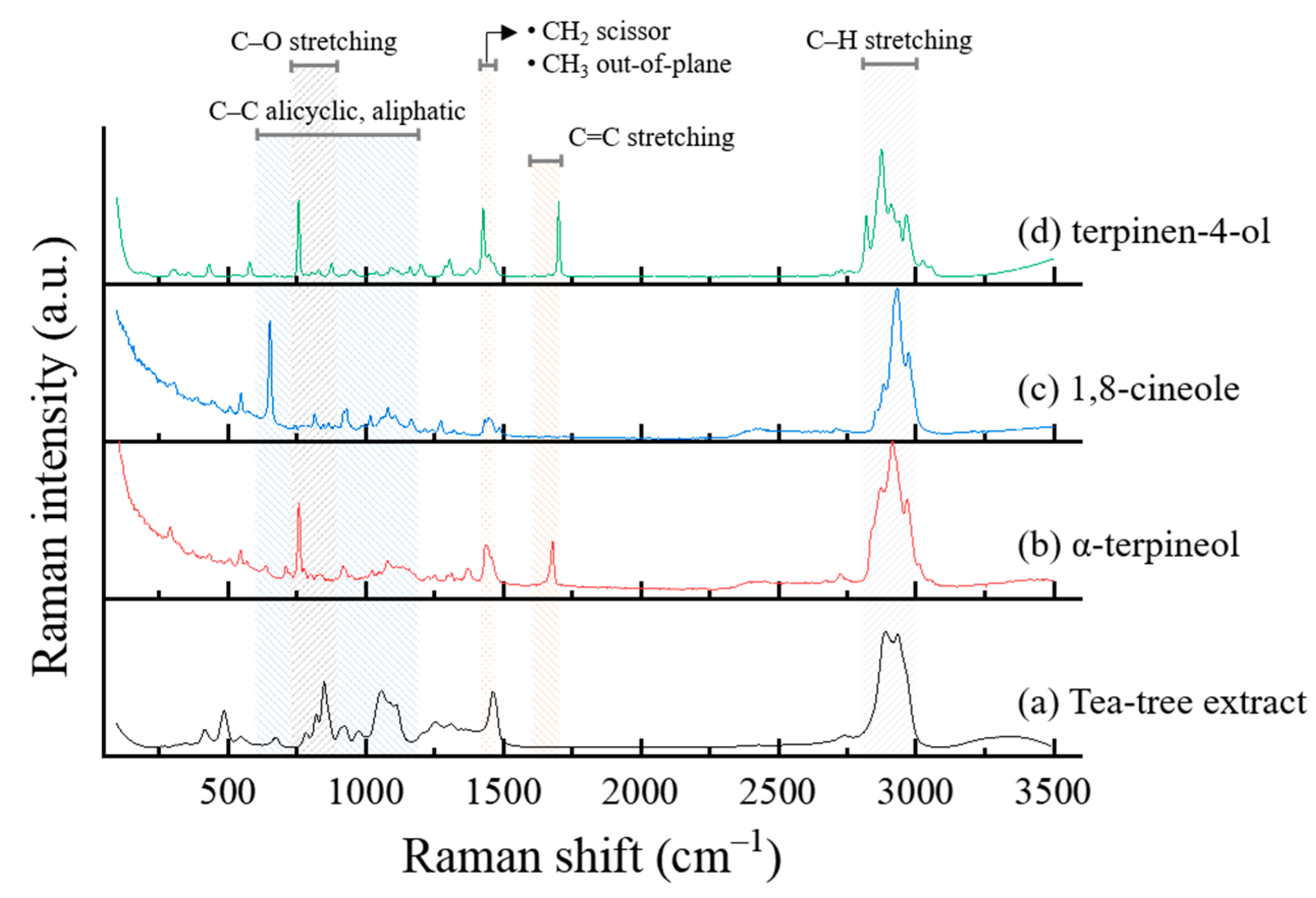
2.4. FTIR and Raman Spectroscopy
3. Results and Discussion
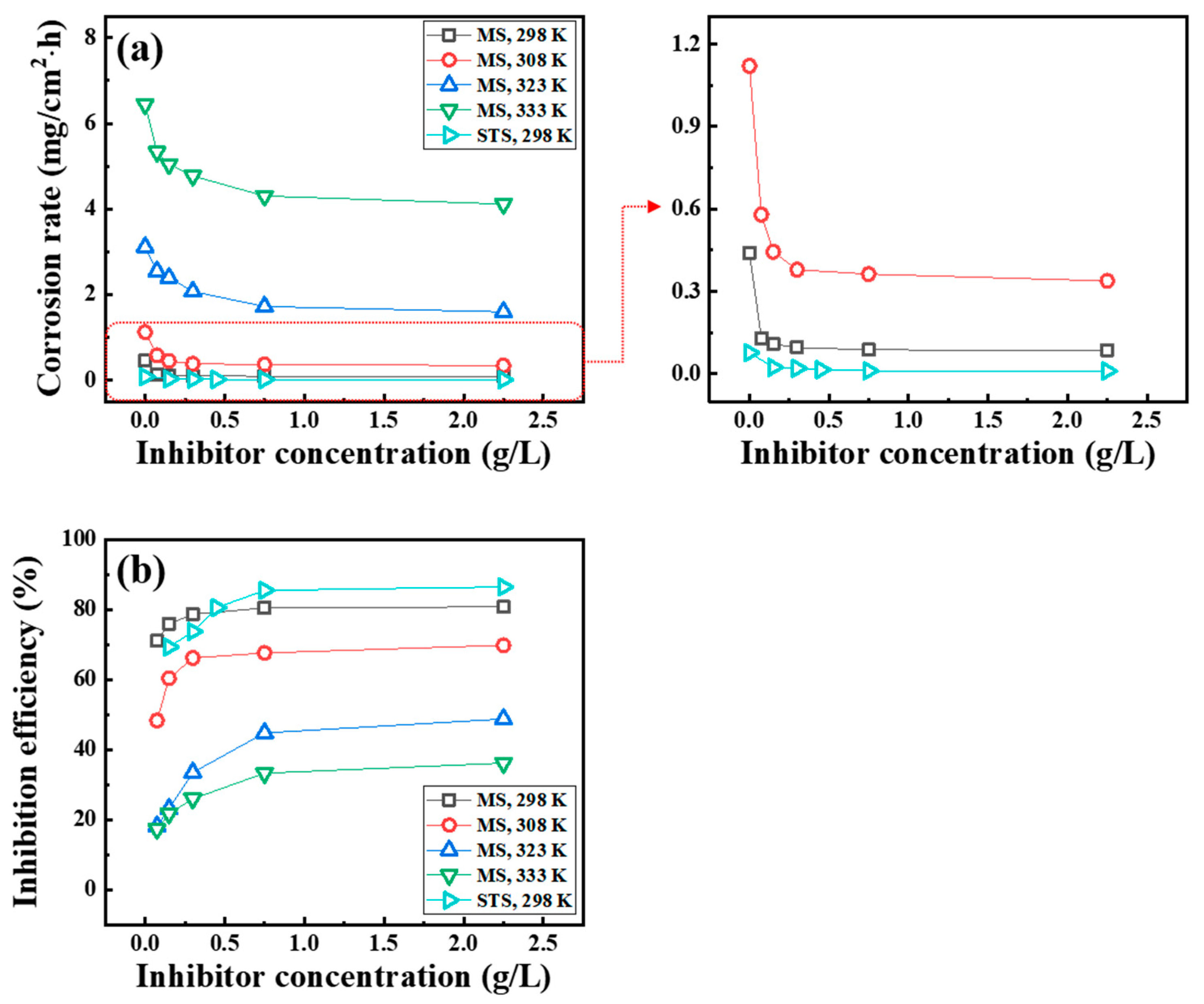
3.1. Weight Loss Measurements
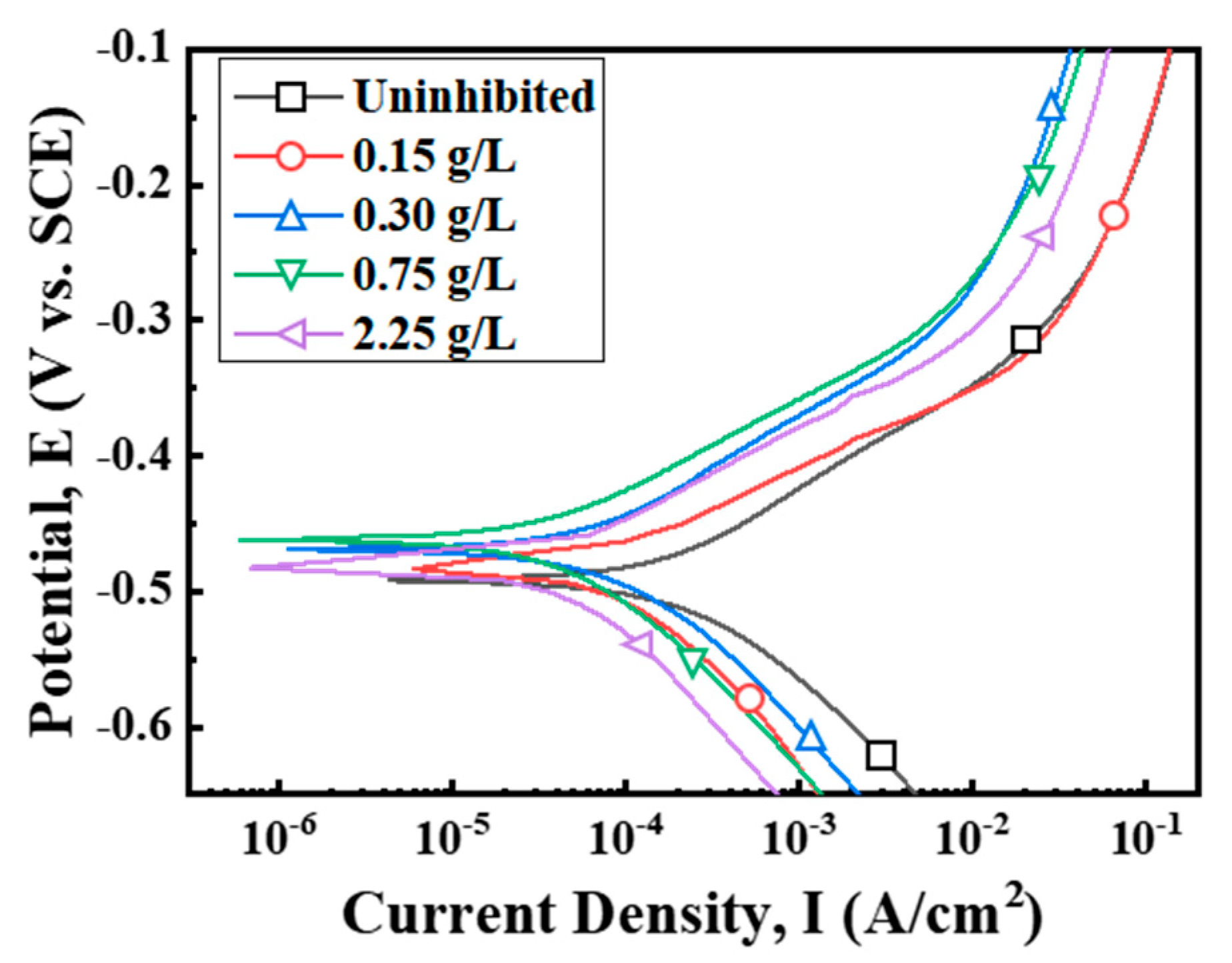
3.2. Polarization Measurements
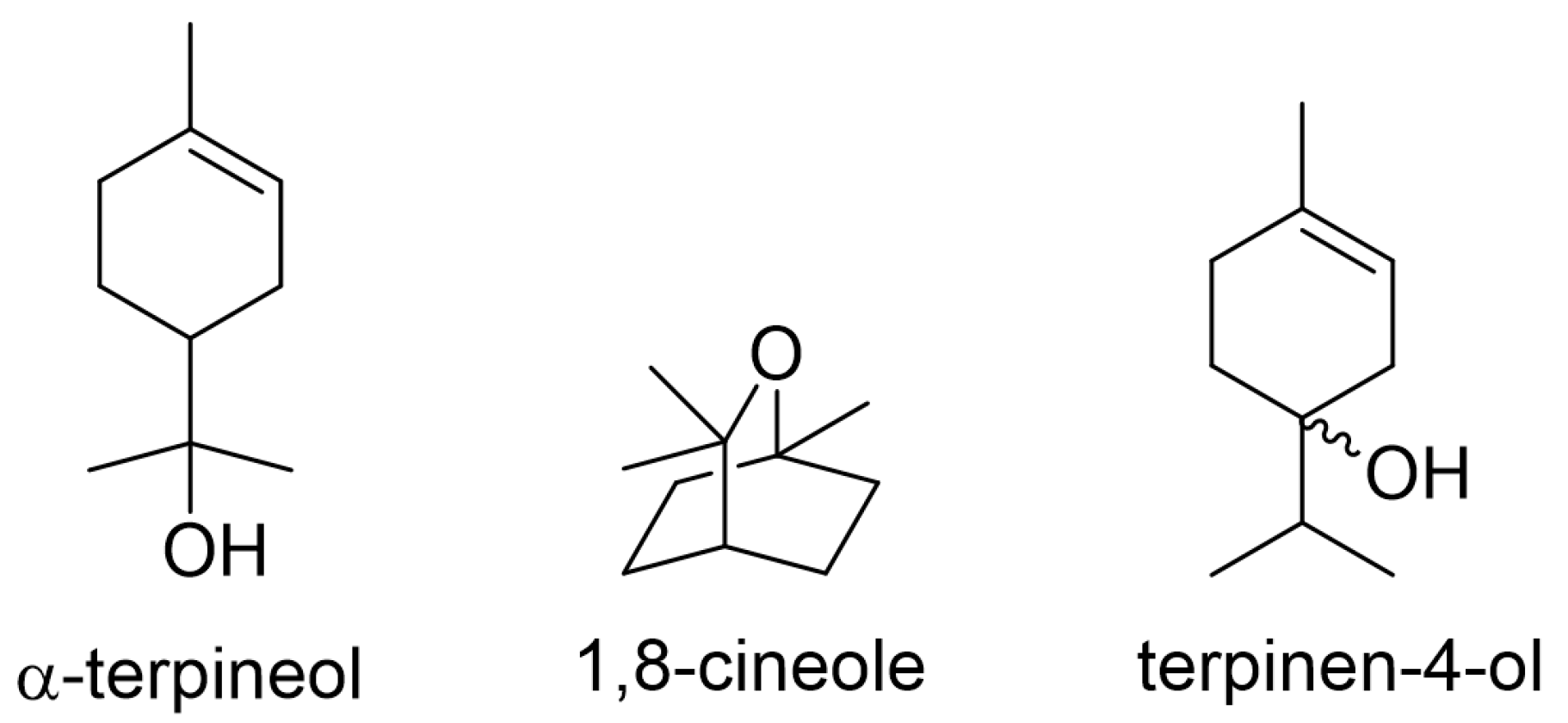
3.3. Corrosion Inhibition Effect of Constituents
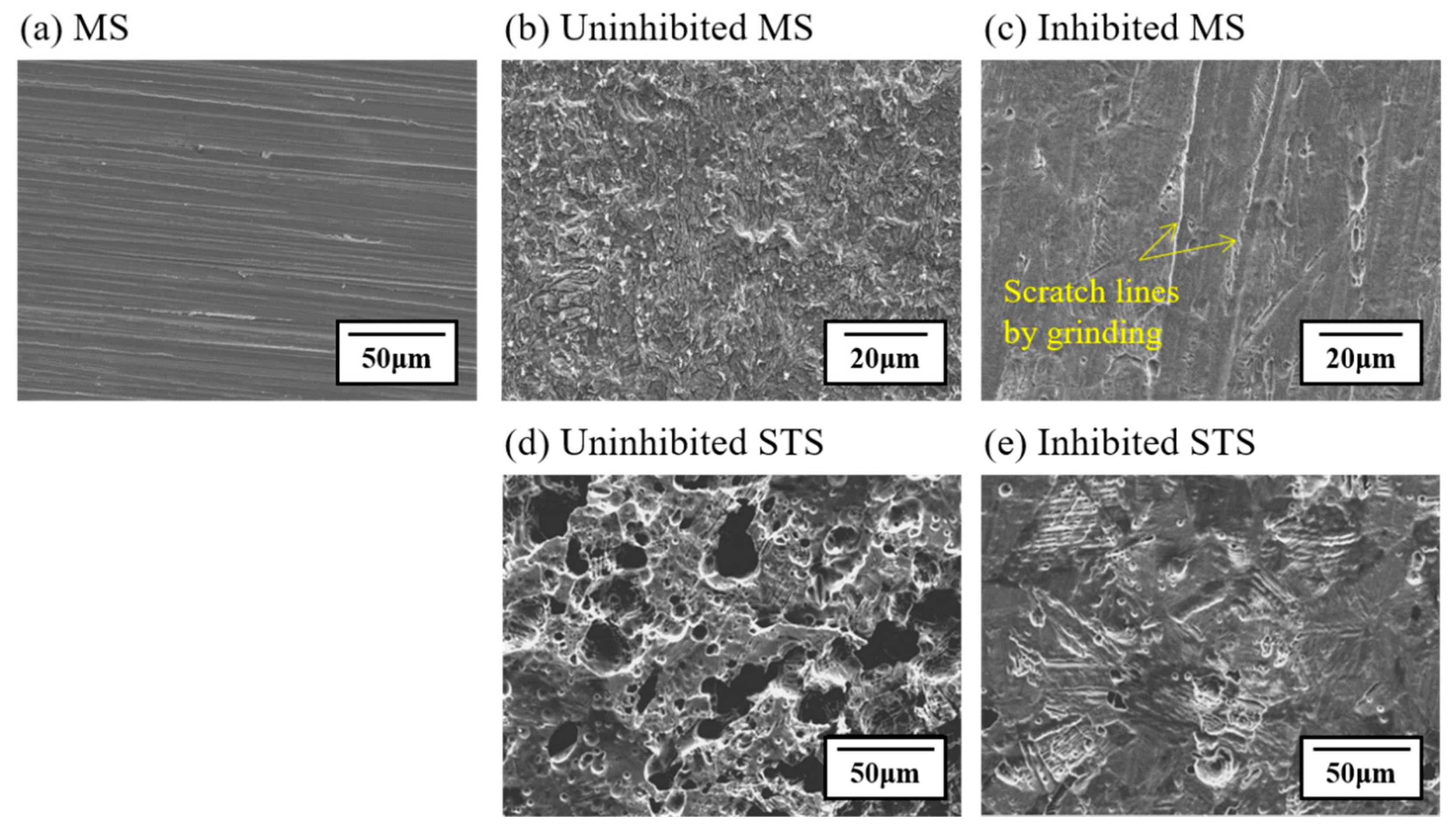
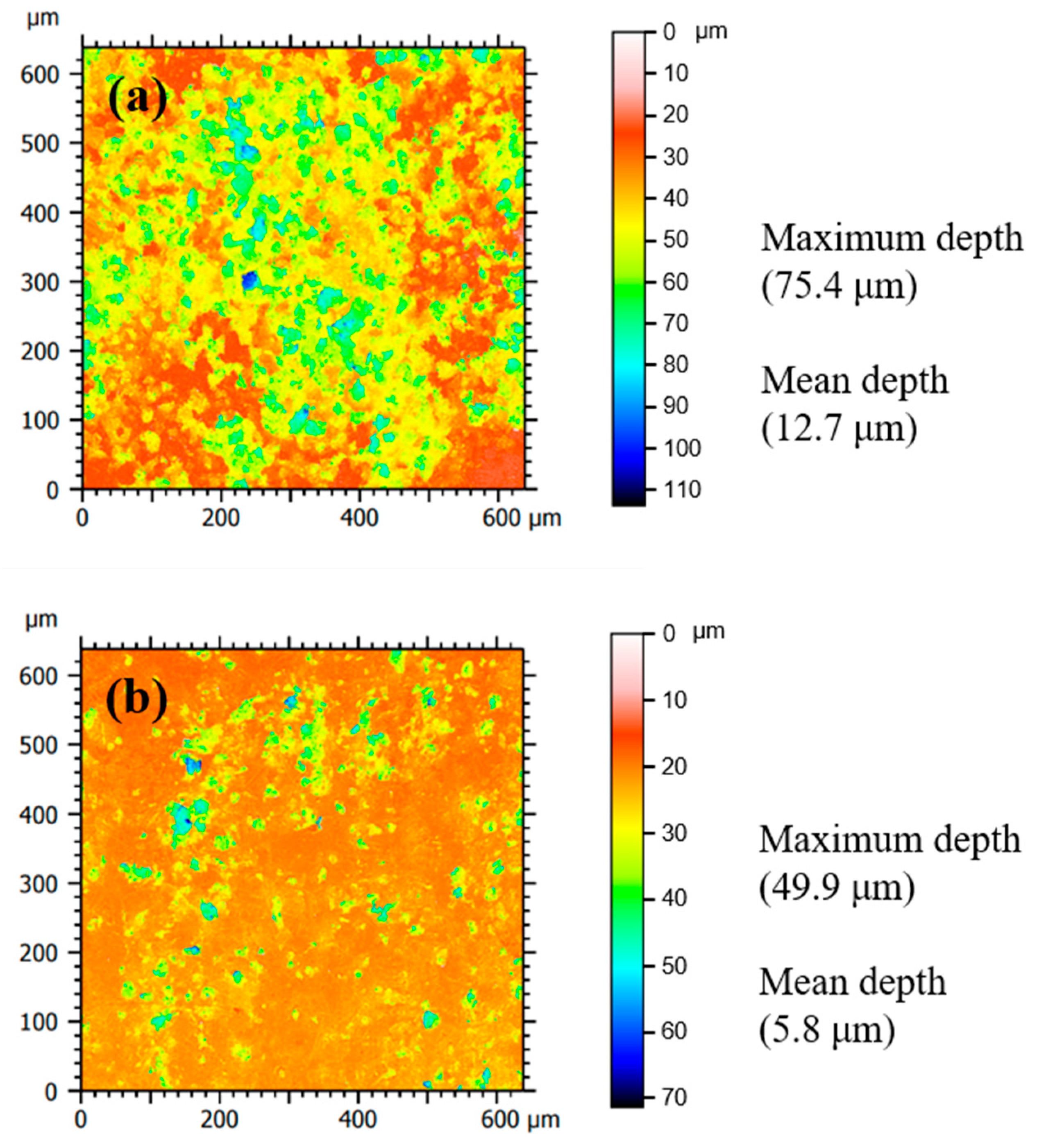
3.4. Surface Characterization
3.4.1. FTIR
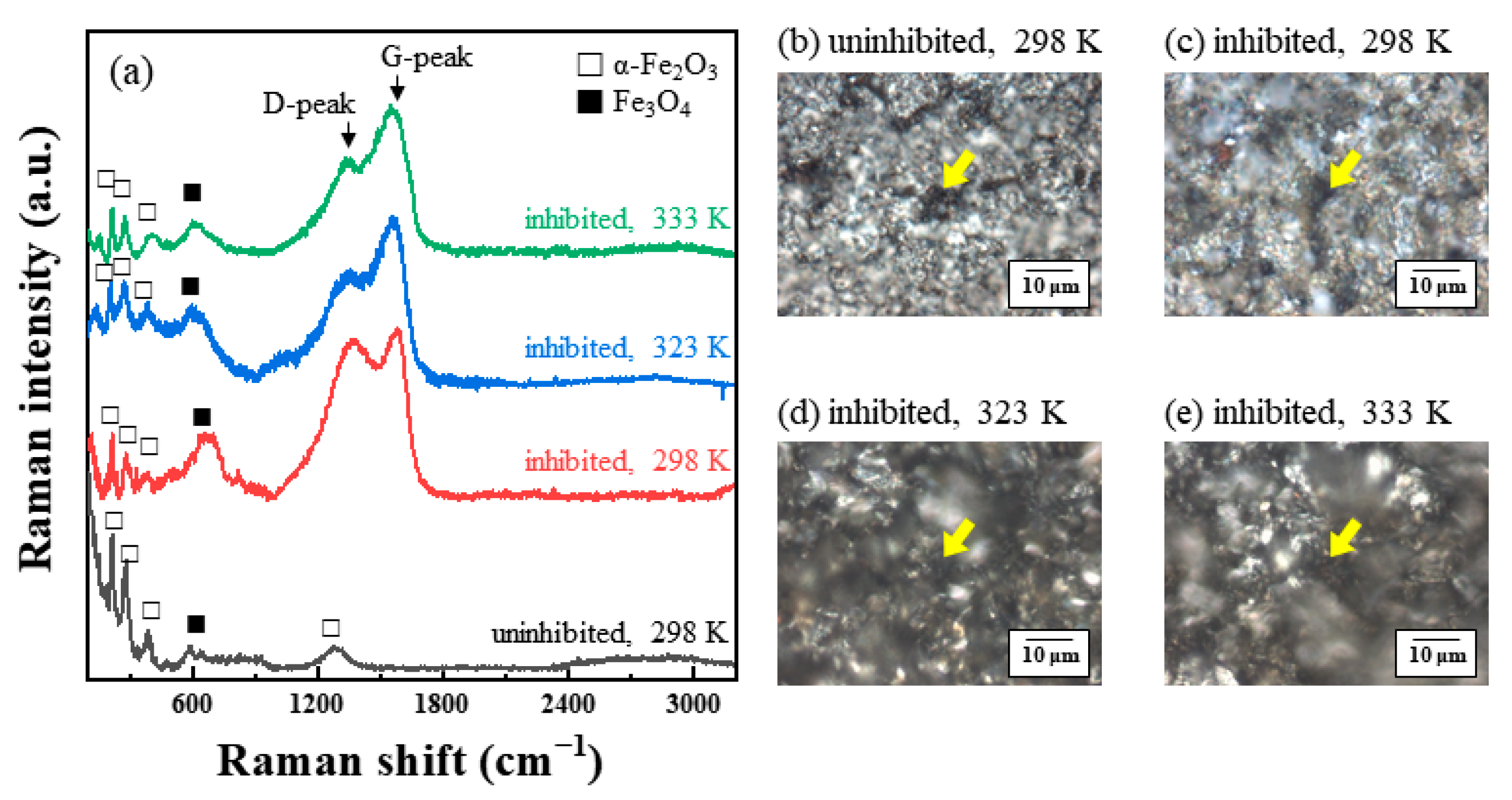
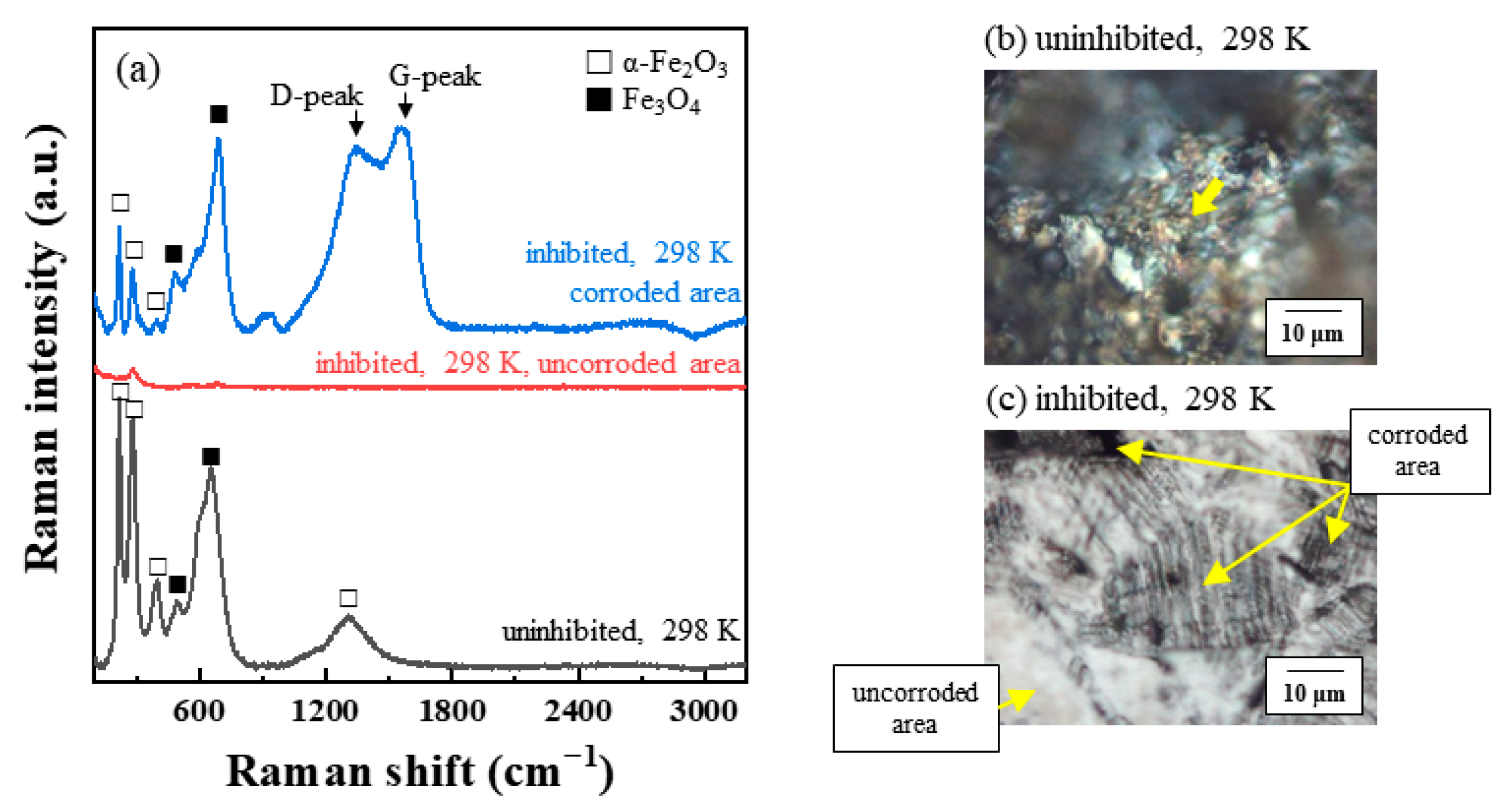
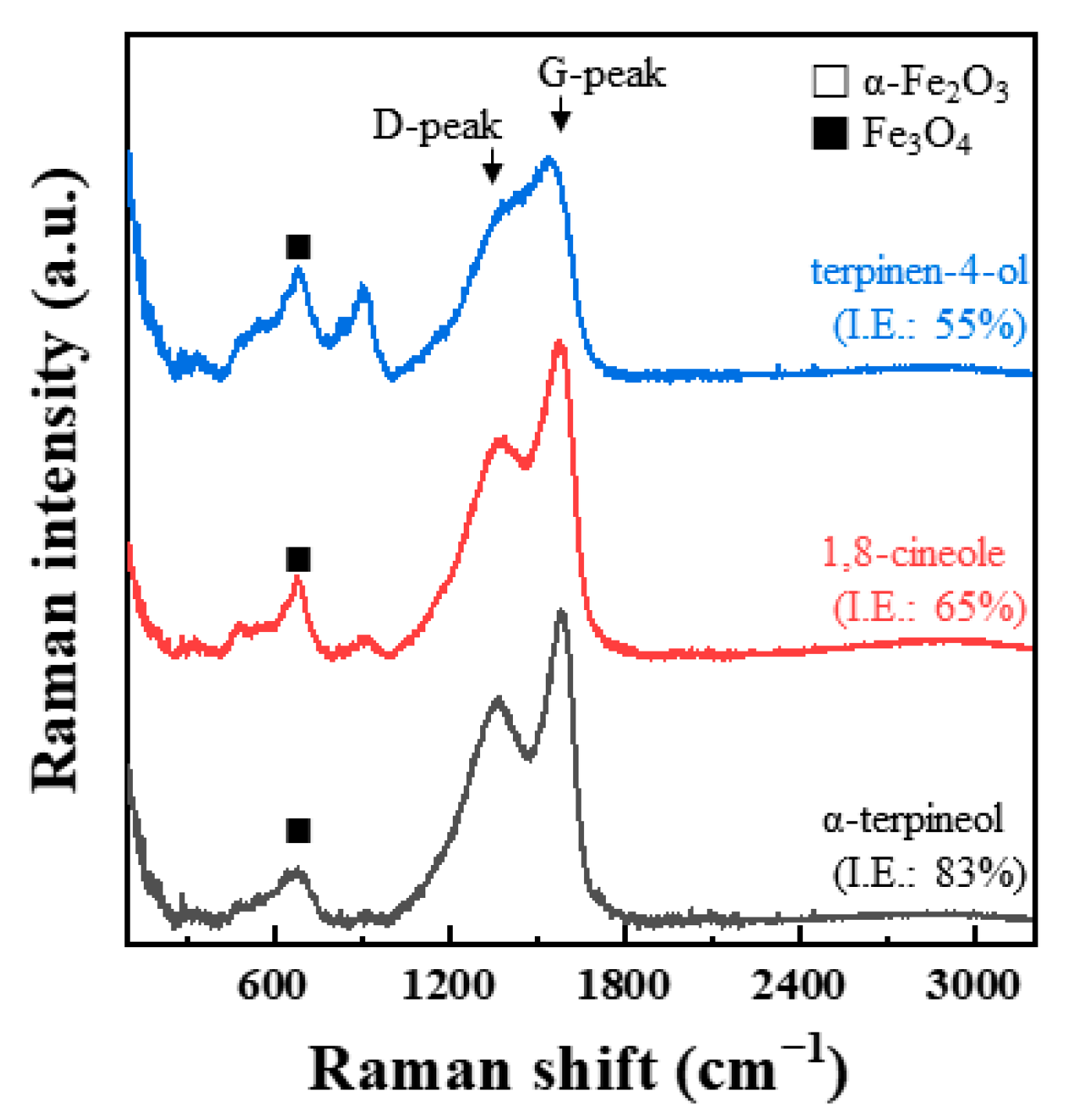
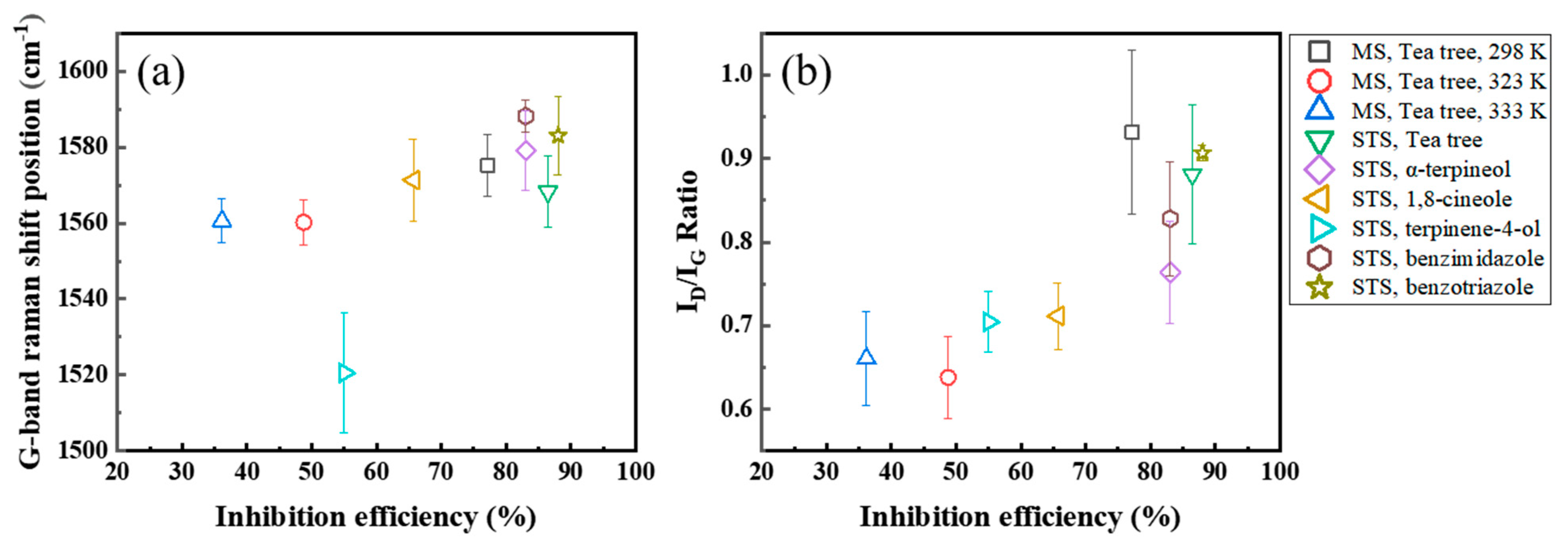
3.4.2. Raman Spectroscopy
3.4.3. Electrochemical Impedance Spectroscopy (EIS)
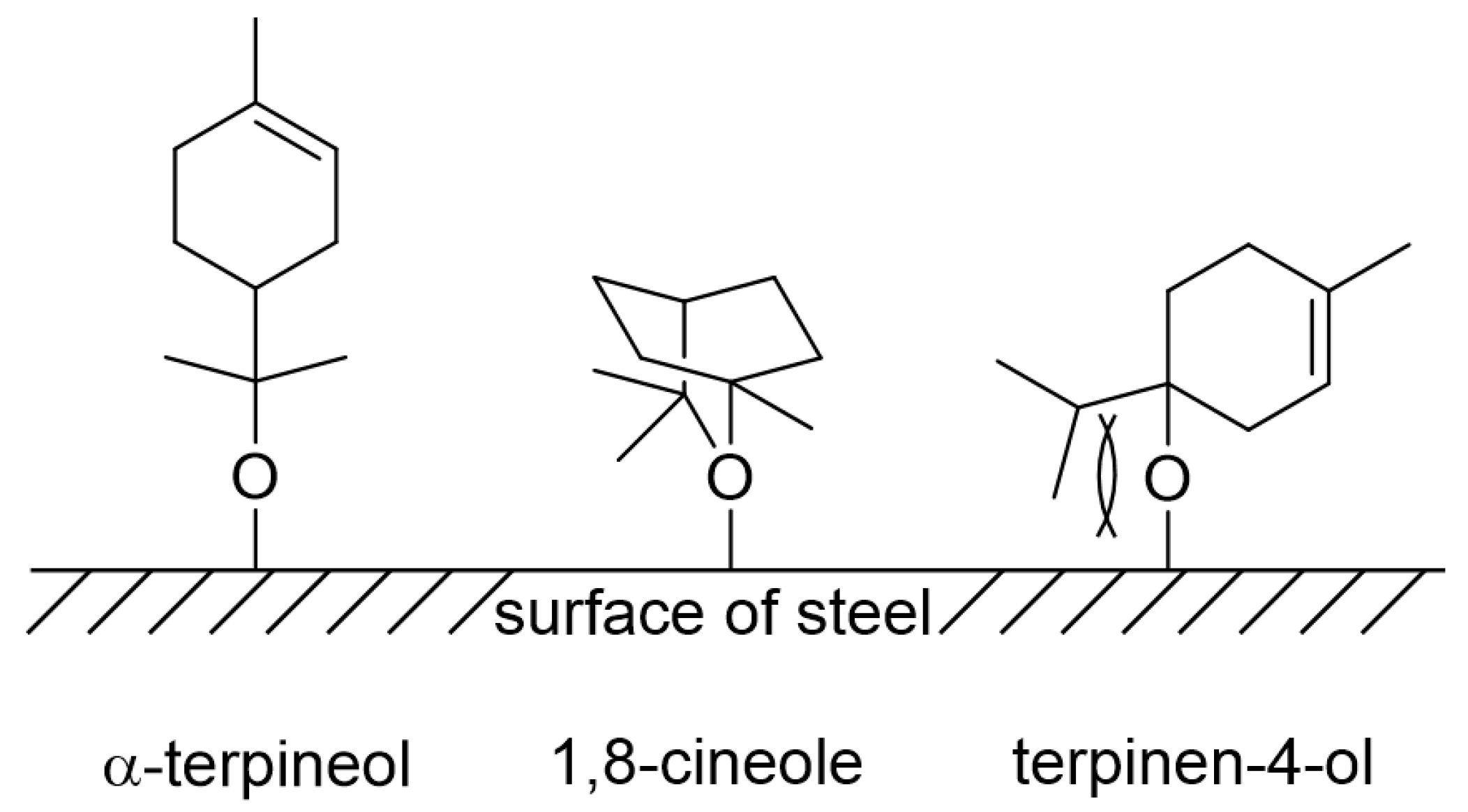
3.5. Corrosion Inhibition Mechanism
- In the case of passivated STS, the adsorbed chloride anions cause local breakdown of the protective passive layers on the steel–solution interface, resulting in pitting corrosion.
- In the presence of tea tree extract, organic inhibitors containing oxygen adsorb to the corrosive areas, i.e., all surface areas for MS of uniform corrosion morphology (Figure 9a) and the local pits for STS of pitting corrosion morphology (Figure 10a). The pre-adsorbed chloride anions are replaced by organic inhibitors on the surface of the steel [50,51], and the organic-Fe complex layer is formed through electron donor–acceptor interactions with Fe. This layer effectively blocks the interaction between the steel and acid media and reduces the chance of the further oxidation of iron. The uniform corrosion of MS and pitting corrosion of STS in the 1 M HCl solution can effectively be inhibited by the protective organic-Fe complex layer.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Flores, E.A.; Olivares, O.; Likhanova, N.V.; Domínguez-Aguilar, M.A.; Nava, N.; Guzman-Lucero, D.; Corrales, M. Sodium phthalamates as corrosion inhibitors for carbon steel in aqueous hydrochloric acid solution. Corros. Sci. 2011, 53, 3899–3913. [Google Scholar] [CrossRef]
- Dehghani, A.; Poshtiban, F.; Bahlakeh, G.; Ramezanzadeh, B. Fabrication of metal-organic based complex film based on three-valent samarium ions-[bis (phosphonomethyl) amino] methylphosphonic acid (ATMP) for effective corrosion inhibition of mild steel in simulated seawater. Constr. Build. Mater. 2020, 239, 117812. [Google Scholar] [CrossRef]
- Lin, B.; Zuo, Y. Corrosion inhibition of carboxylate inhibitors with different alkylene chain lengths on carbon steel in an alkaline solution. RSC Adv. 2019, 9, 7065–7077. [Google Scholar] [CrossRef] [Green Version]
- Bokati, K.S.; Dehghanian, C.; Yari, S. Corrosion inhibition of copper, mild steel and galvanically coupled copper-mild steel in artificial sea water in presence of 1H-benzotriazole, sodium molybdate and sodium phosphate. Corros. Sci. 2017, 126, 272–285. [Google Scholar] [CrossRef]
- Khan, G.; Basirun, W.J.; Kazi, S.N.; Ahmed, P.; Magaji, L.; Ahmed, S.M.; Khan, G.M.; Rehman, M.A.; Badry, A.B.B.M. Electrochemical investigation on the corrosion inhibition of mild steel by Quinazoline Schiff base compounds in hydrochloric acid solution. J. Colloid Interface Sci. 2017, 502, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Finšgar, M.; Jackson, J. Application of corrosion inhibitors for steels in acidic media for the oil and gas industry: A review. Corros. Sci. 2014, 86, 17–41. [Google Scholar] [CrossRef] [Green Version]
- Onyeachu, I.B.; Solomon, M.M.; Umoren, S.A.; Obot, I.B.; Sorour, A.A. Corrosion inhibition effect of a benzimidazole derivative on heat exchanger tubing materials during acid cleaning of multistage flash desalination plants. Desalination 2020, 479, 114283. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. A critical review on the recent studies on plant biomaterials as corrosion inhibitors for industrial metals. J. Ind. Eng. Chem. 2019, 76, 91–115. [Google Scholar] [CrossRef]
- Verma, C.; Olasunkanmi, L.O.; Ebenso, E.E.; Quraishi, M.A. Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: A review. J. Mol. Liq. 2018, 251, 100–118. [Google Scholar] [CrossRef]
- M’hiri, N.; Veys-Renaux, D.; Rocca, E.; Ioannou, I.; Boudhrioua, N.M.; Ghoul, M. Corrosion inhibition of carbon steel in acidic medium by orange peel extract and its main antioxidant compounds. Corros. Sci. 2016, 102, 55–62. [Google Scholar] [CrossRef]
- Ostovari, A.; Hoseinieh, S.M.; Peikari, M.; Shadizadeh, S.R.; Hashemi, S.J. Corrosion inhibition of mild steel in 1 M HCl solution by henna extract: A comparative study of the inhibition by henna and its constituents (Lawsone, Gallic acid, α-d-Glucose and Tannic acid). Corros. Sci. 2009, 51, 1935–1949. [Google Scholar] [CrossRef]
- Behpour, M.; Ghoreishi, S.M.; Khayatkashani, M.; Soltani, N. Green approach to corrosion inhibition of mild steel in two acidic solutions by the extract of Punica granatum peel and main constituents. Mater. Chem. Phys. 2012, 131, 621–633. [Google Scholar] [CrossRef]
- Garai, S.; Garai, S.; Jaisankar, P.; Singh, J.K.; Elango, A. A comprehensive study on crude methanolic extract of Artemisia pallens (Asteraceae) and its active component as effective corrosion inhibitors of mild steel in acid solution. Corros. Sci. 2012, 60, 193–204. [Google Scholar] [CrossRef]
- Faiz, M.; Zahari, A.; Awang, K.; Hussin, H. Corrosion inhibition on mild steel in 1 M HCl solution by Cryptocarya nigra extracts and three of its constituents (alkaloids). RSC Adv. 2020, 10, 6547–6562. [Google Scholar] [CrossRef] [Green Version]
- Faustin, M.; Maciuk, A.; Salvin, P.; Roos, C.; Lebrini, M. Corrosion inhibition of C38 steel by alkaloids extract of Geissospermum laeve in 1 M hydrochloric acid: Electrochemical and phytochemical studies. Corros. Sci. 2015, 92, 287–300. [Google Scholar] [CrossRef]
- Mourya, P.; Banerjee, S.; Singh, M.M. Corrosion inhibition of mild steel in acidic solution by Tagetes erecta (Marigold flower) extract as a green inhibitor. Corros. Sci. 2014, 85, 352–363. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Guo, L.; Jiang, H.; Ji, Q. In vitro evaluation of antioxidant and antimicrobial activities of Melaleuca alternifolia essential oil. BioMed Res. Int. 2018, 2018, 2396109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shapira, S.; Pleban, S.; Kazanov, D.; Tirosh, P.; Arber, N. Terpinen-4-ol: A novel and promising therapeutic agent for human gastrointestinal cancers. PLoS ONE 2016, 11, e0156540. [Google Scholar] [CrossRef] [Green Version]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A review of applications of tea tree oil in dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef]
- Tankeu, S.; Vermaak, I.; Kamatou, G.; Viljoen, A. Vibrational spectroscopy as a rapid quality control method for Melaleuca alternifolia Cheel (tea tree oil). Phytochem. Anal. 2014, 25, 81–88. [Google Scholar] [CrossRef]
- Haque, J.; Srivastava, V.; Quraishi, M.A.; Chauhan, D.S.; Lgaz, H.; Chung, I.M. Polar group substituted imidazolium zwitterions as eco-friendly corrosion inhibitors for mild steel in acid solution. Corros. Sci. 2020, 172, 108665. [Google Scholar] [CrossRef]
- Salehi, E.; Naderi, R.; Ramezanzadeh, B. Synthesis and characterization of an effective organic/inorganic hybrid green corrosion inhibitive complex based on zinc acetate/Urtica Dioica. Appl. Surf. Sci. 2017, 396, 1499–1514. [Google Scholar] [CrossRef]
- Arellanes-Lozada, P.; Díaz-Jiménez, V.; Hernández-Cocoletzi, H.; Nava, N.; Olivares-Xometl, O.; Likhanova, N.V. Corrosion inhibition properties of iodide ionic liquids for API 5L X52 steel in acid medium. Corros. Sci. 2020, 175, 108888. [Google Scholar] [CrossRef]
- Mohammed, A.R.I.; Solomon, M.M.; Haruna, K.; Umoren, S.A.; Saleh, T.A. Evaluation of the corrosion inhibition efficacy of Cola acuminata extract for low carbon steel in simulated acid pickling environment. Environ. Sci. Pollut. Res. 2020, 27, 34270–34288. [Google Scholar] [CrossRef]
- Roy, P.; Karfa, P.; Adhikari, U.; Sukul, D. Corrosion inhibition of mild steel in acidic medium by polyacrylamide grafted Guar gum with various grafting percentage: Effect of intramolecular synergism. Corros. Sci. 2014, 88, 246–253. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared spectra, a practical approach. Encycl. Anal. Chem. 2006, 1–23. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M.; Solomon, M.M.; Udoh, A.P. Corrosion inhibition by leaves and stem extracts of Sida acuta for mild steel in 1 M H2SO4 solutions investigated by chemical and spectroscopic techniques. Arab. J. Chem. 2016, 9, S209–S224. [Google Scholar] [CrossRef] [Green Version]
- Larkin, P. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation, 2nd ed.; Elsevier: Stamford, CT, USA, 2017. [Google Scholar]
- Daferera, D.J.; Tarantilis, P.A.; Polissiou, M.G. Characterization of essential oils from Lamiaceae species by Fourier transform Raman spectroscopy. J. Agric. Food Chem. 2002, 50, 5503–5507. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nawaz, H.; Naz, S.; Mukhtar, R.; Rashid, N.; Bhatti, I.A.; Saleem, M. Raman spectroscopy for the characterization of different fractions of hemp essential oil extracted at 130 °C using steam distillation method. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2017, 182, 168–174. [Google Scholar] [CrossRef]
- Alcántara, J.; Chico, B.; Simancas, J.; Díaz, I.; De la Fuente, D.; Morcillo, M. An attempt to classify the morphologies presented by different rust phases formed during the exposure of carbon steel to marine atmospheres. Mater. Charact. 2016, 118, 65–78. [Google Scholar] [CrossRef]
- De Faria, D.L.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, K.; Dong, C.; Wu, J.; Li, X.; Huang, Y. In situ Raman spectroscopy study of corrosion products on the surface of carbon steel in solution containing Cl− and SO42−. Eng. Fail. Anal. 2011, 18, 1981–1989. [Google Scholar] [CrossRef]
- Huang, H.C.; Wang, C.H.; Shown, I.; Chang, S.T.; Hsu, H.C.; Du, H.Y.; Chen, L.C.; Chen, K.H. High-performance pyrolyzed iron corrole as a potential non-precious metal catalyst for PEMFCs. J. Mater. Chem. A Mater. 2013, 1, 14692–14699. [Google Scholar] [CrossRef]
- Chang, S.T.; Wang, C.H.; Du, H.Y.; Hsu, H.C.; Kang, C.M.; Chen, C.C.; Wu, J.C.S.; Yen, S.C.; Huang, W.F.; Chen, L.C.; et al. Vitalizing fuel cells with vitamins: Pyrolyzed vitamin B12 as a non-precious catalyst for enhanced oxygen reduction reaction of polymer electrolyte fuel cells. Energy Environ. Sci. 2012, 5, 5305–5314. [Google Scholar] [CrossRef]
- Wang, C.H.; Chang, S.T.; Hsu, H.C.; Du, H.Y.; Wu, J.C.S.; Chen, L.C.; Chen, K.H. Oxygen reducing activity of methanol-tolerant catalysts by high-temperature pyrolysis. Diam. Relat. Mater. 2011, 20, 322–329. [Google Scholar] [CrossRef]
- Palomäki, T.; Wester, N.; Johansson, L.S.; Laitinen, M.; Jiang, H.; Arstila, K.; Sajavaara, T.; Han, J.G.; Koskinenb, J.; Laurila, T. Characterization and electrochemical properties of oxygenated amorphous carbon (a-C) films. Electrochim. Acta 2016, 220, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Pardanaud, C.; Martin, C.; Roubin, P.; Giacometti, G.; Hopf, C.; Schwarz-Selinger, T.; Jacob, W. Raman spectroscopy investigation of the H content of heated hard amorphous carbon layers. Diam. Relat. Mater. 2013, 34, 100–104. [Google Scholar] [CrossRef] [Green Version]
- El-Khodary, S.A.; El-Enany, G.M.; El-Okr, M.; Ibrahim, M. Preparation and characterization of microwave reduced graphite oxide for high-performance supercapacitors. Electrochim. Acta 2014, 150, 269–278. [Google Scholar] [CrossRef]
- Dong, X.; Li, L.; Zhao, C.; Liu, H.K.; Guo, Z. Controllable synthesis of RGO/FexOy nanocomposites as high-performance anode materials for lithium ion batteries. J. Mater. Chem. A Mater. 2014, 2, 9844–9850. [Google Scholar] [CrossRef]
- Albrimi, Y.A.; Addi, A.A.; Douch, J.; Souto, R.M.; Hamdani, M. Inhibition of the pitting corrosion of 304 stainless steel in 0.5 M hydrochloric acid solution by heptamolybdate ions. Corros. Sci. 2015, 90, 522–528. [Google Scholar] [CrossRef]
- Ilevbare, G.O.; Burstein, G.T. The inhibition of pitting corrosion of stainless steels by chromate and molybdate ions. Corros. Sci. 2003, 45, 1545–1569. [Google Scholar] [CrossRef]
- Aljourani, J.; Raeissi, K.; Golozar, M.A. Benzimidazole and its derivatives as corrosion inhibitors for mild steel in 1M HCl solution. Corros. Sci. 2009, 51, 1836–1843. [Google Scholar] [CrossRef]
- Popova, A.; Christov, M.; Zwetanova, A. Effect of the molecular structure on the inhibitor properties of azoles on mild steel corrosion in 1 M hydrochloric acid. Corros. Sci. 2007, 49, 2131–2143. [Google Scholar] [CrossRef]
- Ramya, K.; Mohan, R.; Joseph, A. Interaction of benzimidazoles and benzotriazole: Its corrosion protection properties on mild steel in hydrochloric acid. J. Mater. Eng. Perform. 2014, 23, 4089–4101. [Google Scholar] [CrossRef]
- Selvi, S.T.; Raman, V.; Rajendran, N. Corrosion inhibition of mild steel by benzotriazole derivatives in acidic medium. J. Appl. Electrochem. 2003, 33, 1175–1182. [Google Scholar] [CrossRef]
- Farhadian, A.; Rahimi, A.; Safaei, N.; Shaabani, A.; Abdouss, M.; Alavi, A. A theoretical and experimental study of castor oil-based inhibitor for corrosion inhibition of mild steel in acidic medium at elevated temperatures. Corros. Sci. 2020, 175, 108871. [Google Scholar] [CrossRef]
- Soltani, N.; Tavakkoli, N.; Kashani, M.K.; Mosavizadeh, A.E.E.O.; Oguzie, E.E.; Jalali, M.R. Silybum marianum extract as a natural source inhibitor for 304 stainless steel corrosion in 1.0 M HCl. J. Ind. Eng. Chem. 2014, 20, 3217–3227. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. A combined experimental and theoretical study of green corrosion inhibition of mild steel in HCl solution by aqueous Citrullus lanatus fruit (CLF) extract. J. Mol. Liq. 2019, 279, 603–624. [Google Scholar] [CrossRef]
- Kowsari, E.; Payami, M.; Amini, R.; Ramezanzadeh, B.; Javanbakht, M. Task-specific ionic liquid as a new green inhibitor of mild steel corrosion. Appl. Surf. Sci. 2014, 289, 478–486. [Google Scholar] [CrossRef]
- Zhang, Q.B.; Hua, Y.X. Corrosion inhibition of mild steel by alkylimidazolium ionic liquids in hydrochloric acid. Electrochim. Acta 2009, 54, 1881–1887. [Google Scholar] [CrossRef]




| Type | C | Si | Mn | Cr | Ni | Cu | Al | Nb | P | S | Fe |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | 0.07 | 0.02 | 0.7 | 0.005 | 0.005 | 0.02 | 0.03 | 0.01 | 0.009 | 0.003 | Bal. |
| STS | 0.04 | 0.42 | 1.15 | 18.19 | 8.08 | - | - | - | 0.031 | 0.001 | Bal. |
| Gas Chromatograph (HP 6890, Hewlett-Packard Co., USA) | |||
|---|---|---|---|
| Analytical column | HP-5MS (30 m × 0.25 mm × 0.25 μm) | ||
| Inlet temperature | 493 K | ||
| Injection type | Split (5:1) | ||
| Injection volume | 1 μL | ||
| Carrier gas | He (1 mL/min) | ||
| Oven program | Temperature | Hold Time | Rate |
| 323 K | 2 min | 283 K/min | |
| 523 K | 3 min | ||
| Mass Spectrometer (HP 5973, Hewlett-Packard Co., USA) | |||
| Ionization | Electron ionization (EI), 70 eV | ||
| Ion source temperature | 203 K | ||
| Quadrupole temperature | 423 K | ||
| MS transfer line temperature | 553 K | ||
| Mass range | SCAN mode (m/z 40–550) | ||
| Solvent delay | 3 min | ||
| Extract (g/L) | Ecorr (V vs. SCE) | Icorr (A/cm2) | IEPD (%) |
|---|---|---|---|
| Uninhibited | −0.492 | 1.79 × 10−4 | |
| 0.15 | −0.484 | 0.78 × 10−4 | 56.5 |
| 0.30 | −0.467 | 0.64 × 10−4 | 64.0 |
| 0.75 | −0.461 | 0.44 × 10−4 | 75.6 |
| 2.25 | −0.482 | 0.38 × 10−4 | 78.6 |
| Inhibitor | Specimen | Temperature (K) | IEWL (%) | ID/IG | G-Peak Position (cm−1) |
|---|---|---|---|---|---|
| tea tree extract | MS | 298 | 77 | 0.92 | 1576 |
| 323 | 48 | 0.67 | 1560 | ||
| 333 | 36 | 0.66 | 1558 | ||
| tea tree extract | STS | 298 | 86 | 0.88 | 1568 |
| α-terpineol | 83 | 0.77 | 1579 | ||
| 1,8-cineole | 65 | 0.71 | 1571 | ||
| terpinen-4-ol | 55 | 0.7 | 1520 | ||
| benzimidazole | 83 | 0.83 | 1588 | ||
| benzotriazole | 88 | 0.91 | 1583 |
| Extract (g/L) | RS (Ω·cm2) | RCT (Ω·cm2) | CPEdl (μs/Ω·cm2) | n | Cdl (μF/cm2) | IEEIS (%) |
|---|---|---|---|---|---|---|
| Uninhibited | 4.9 | 91 | 128 | 0.8281 | 51.8 | |
| 0.15 | 2.5 | 224 | 110 | 0.8315 | 52.0 | 59.3 |
| 0.30 | 4.2 | 280 | 106 | 0.8395 | 54.1 | 67.4 |
| 0.75 | 4.6 | 432 | 70 | 0.8447 | 36.8 | 78.9 |
| 2.25 | 4.2 | 515 | 65 | 0.8523 | 36.1 | 82.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-Y.; Shin, I.; Byeon, J.-W. Corrosion Inhibition of Mild Steel and 304 Stainless Steel in 1 M Hydrochloric Acid Solution by Tea Tree Extract and Its Main Constituents. Materials 2021, 14, 5016. https://doi.org/10.3390/ma14175016
Kim J-Y, Shin I, Byeon J-W. Corrosion Inhibition of Mild Steel and 304 Stainless Steel in 1 M Hydrochloric Acid Solution by Tea Tree Extract and Its Main Constituents. Materials. 2021; 14(17):5016. https://doi.org/10.3390/ma14175016
Chicago/Turabian StyleKim, Jae-Yeon, Inji Shin, and Jai-Won Byeon. 2021. "Corrosion Inhibition of Mild Steel and 304 Stainless Steel in 1 M Hydrochloric Acid Solution by Tea Tree Extract and Its Main Constituents" Materials 14, no. 17: 5016. https://doi.org/10.3390/ma14175016
APA StyleKim, J.-Y., Shin, I., & Byeon, J.-W. (2021). Corrosion Inhibition of Mild Steel and 304 Stainless Steel in 1 M Hydrochloric Acid Solution by Tea Tree Extract and Its Main Constituents. Materials, 14(17), 5016. https://doi.org/10.3390/ma14175016







