Bacterial Exposure to Nickel: Influence on Adhesion and Biofilm Formation on Orthodontic Archwires and Sensitivity to Antimicrobial Agents
Abstract
1. Introduction
- bacteria adapted to lower concentrations of nickel ions will adhere better to NiTi wires;
- bacteria adapted to lower concentrations of nickel ions will demonstrate better biofilm formation to NiTi wires;
- bacteria adapted to lower concentrations will show a decreased sensitivity to antimicrobial agents.
2. Materials and Methods
2.1. Strain and Growth Media
2.2. Preparation of Reagents
2.3. Adaptation of S. aureus to Nickel
2.4. Minimal Inhibitory Concentrations and Disk Diffusion
- Benzylpenicillin—P—1 unit (12–18)
- Clindamycin—CMN—2 µg (23–29)
- Erythromycin—ERY—15 µg (23–29)
- Gentamicin—GMN—10 µg (19–25)
- Cefoxitin—FOX—30 µg (24–30)
- Teicoplanin—TEC—30 µg (10–14)
- Linezolid—LIN—10 µg (21–27)
- Ciprofloxacin—CIP—5 µg (21–27)
- Rifampicin—RIF—5 µg (30–36)
- Trimethoprim-sulfamethoxazole—SXT—1.25–23.75 µg (26–32)
- Moxifloxacin—MXF—5 µg (25–31)
2.5. Growth Curve Assays
2.6. Adhesion, Early Biofilm and Biofilm Biomass Tests
2.7. Statistics
2.8. Ethics
3. Results
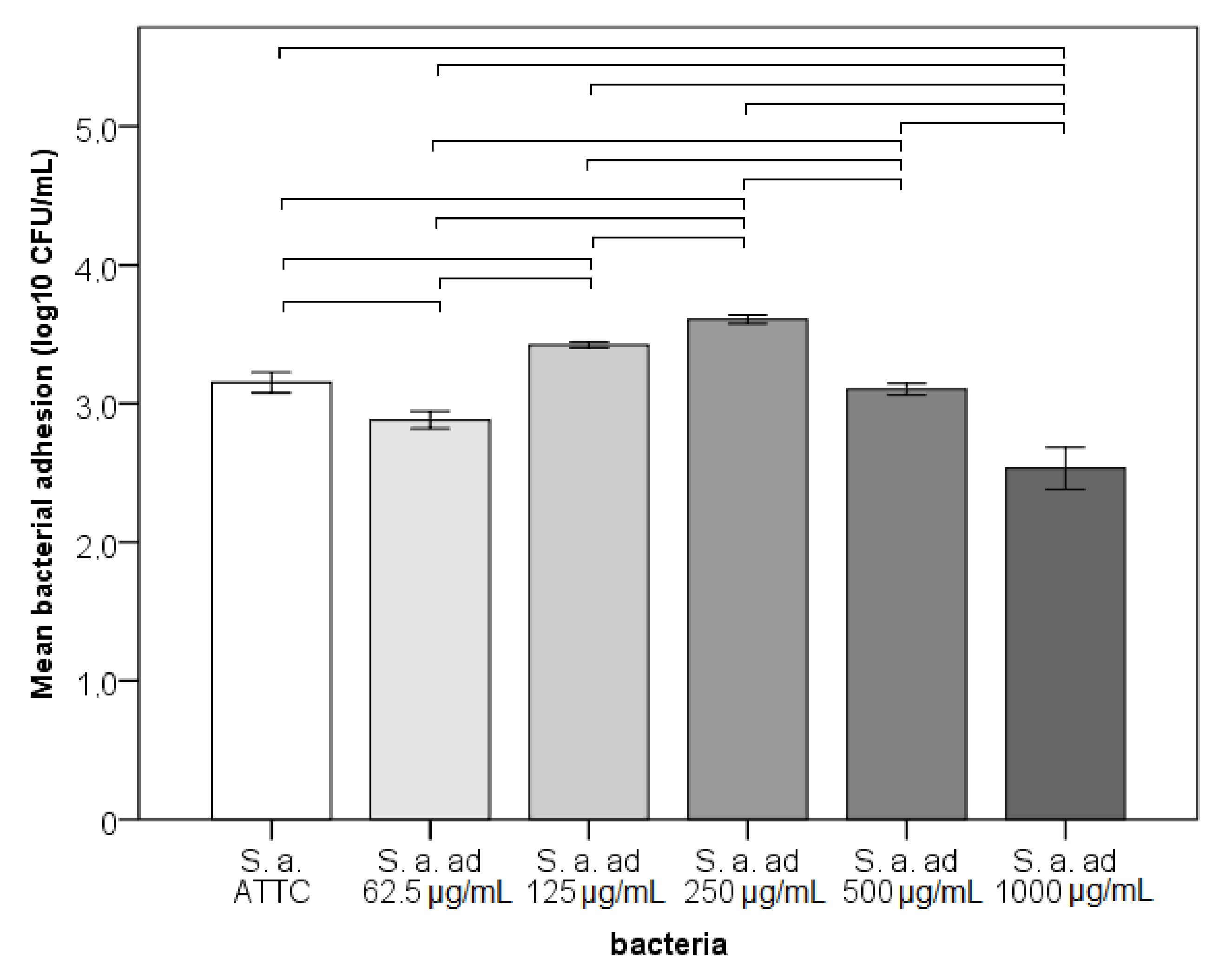
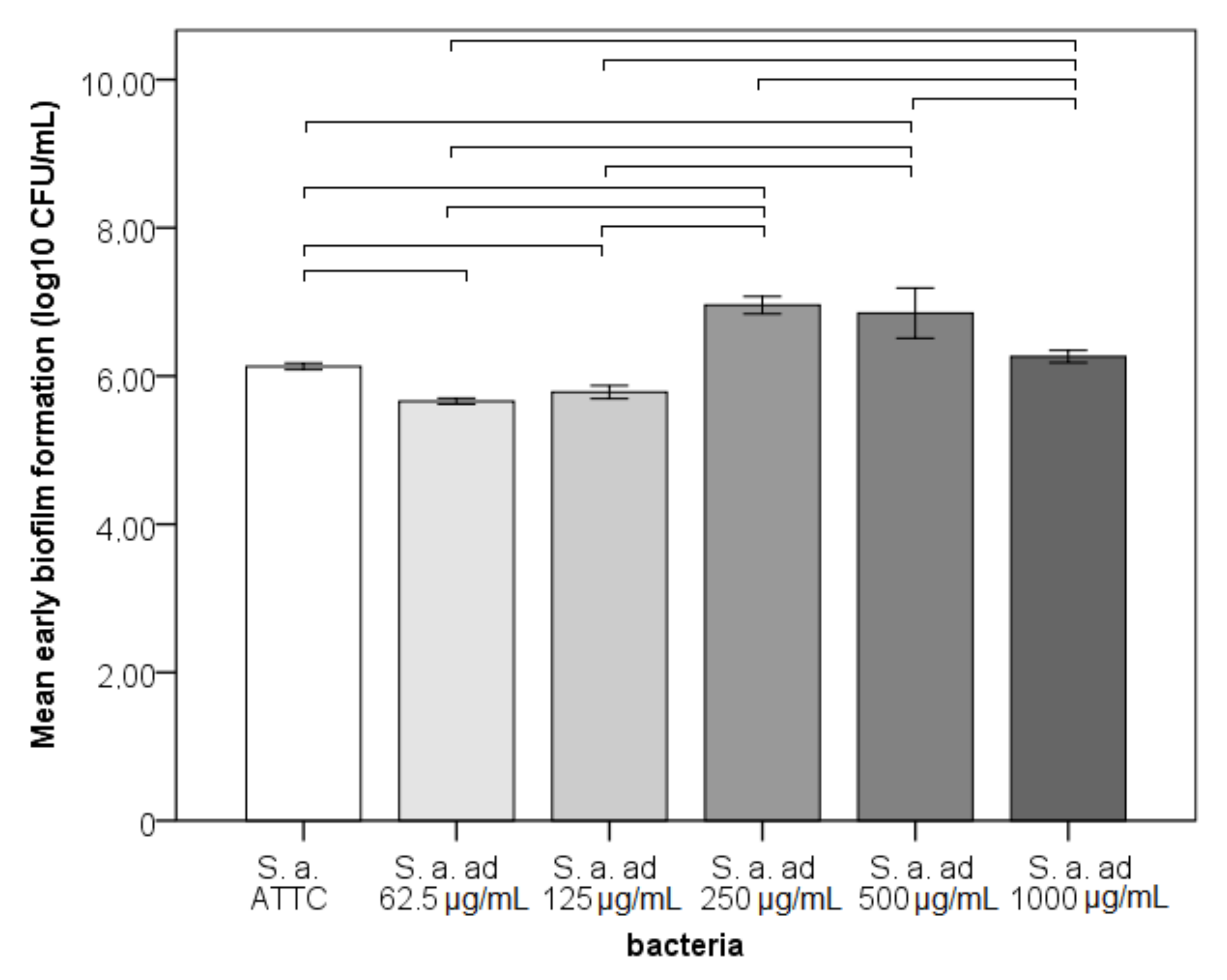
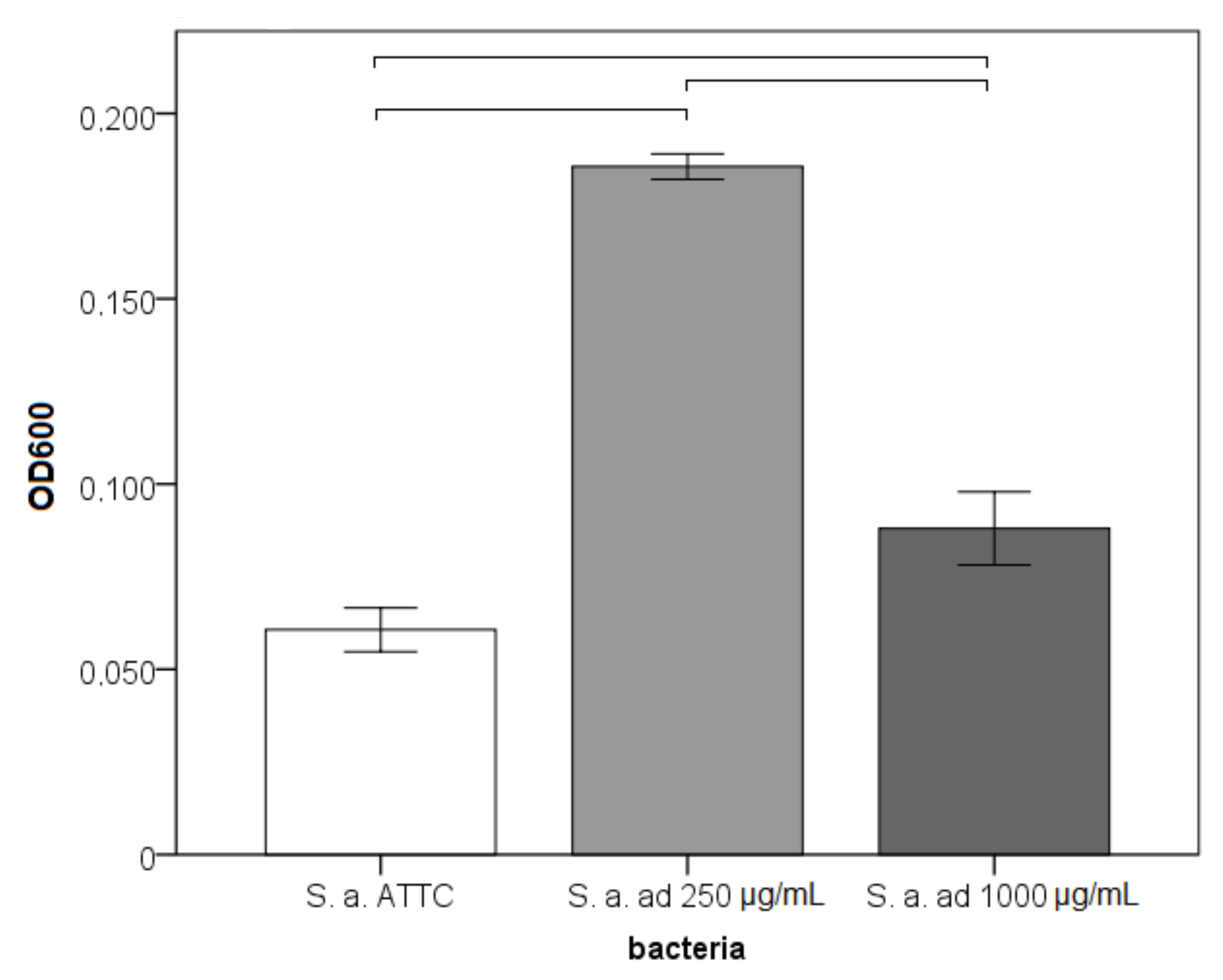
3.1. Adhesion, Early Biofilm and Biomass Tests
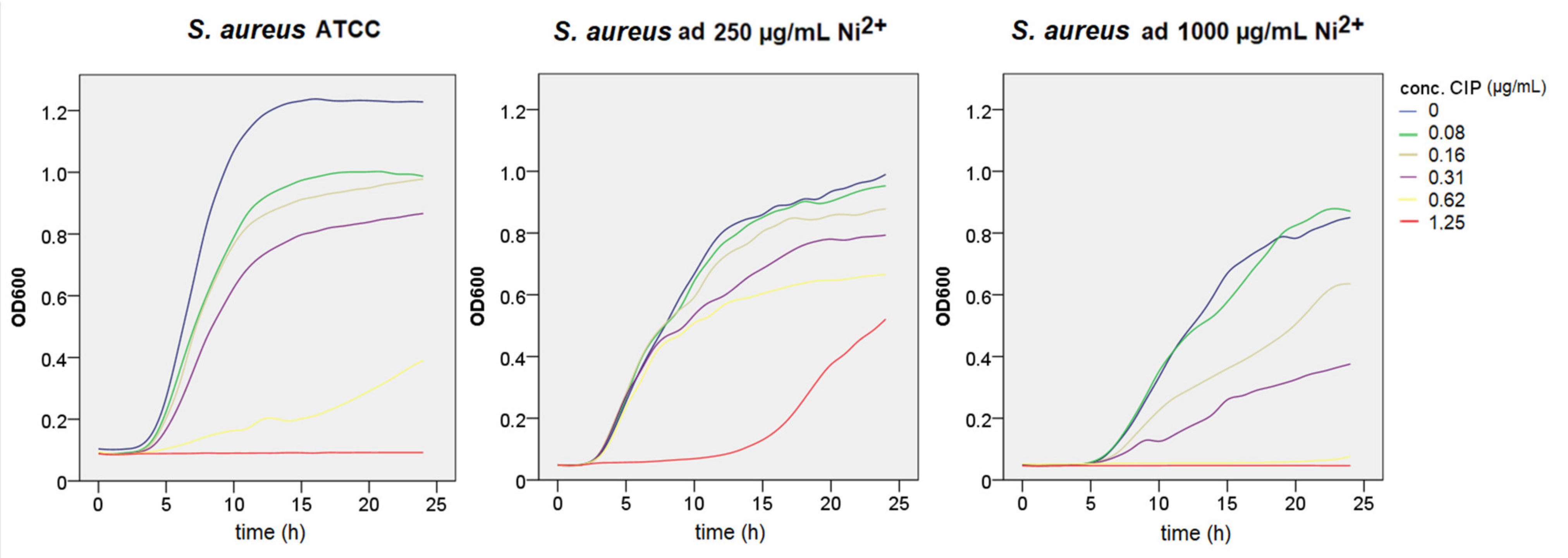
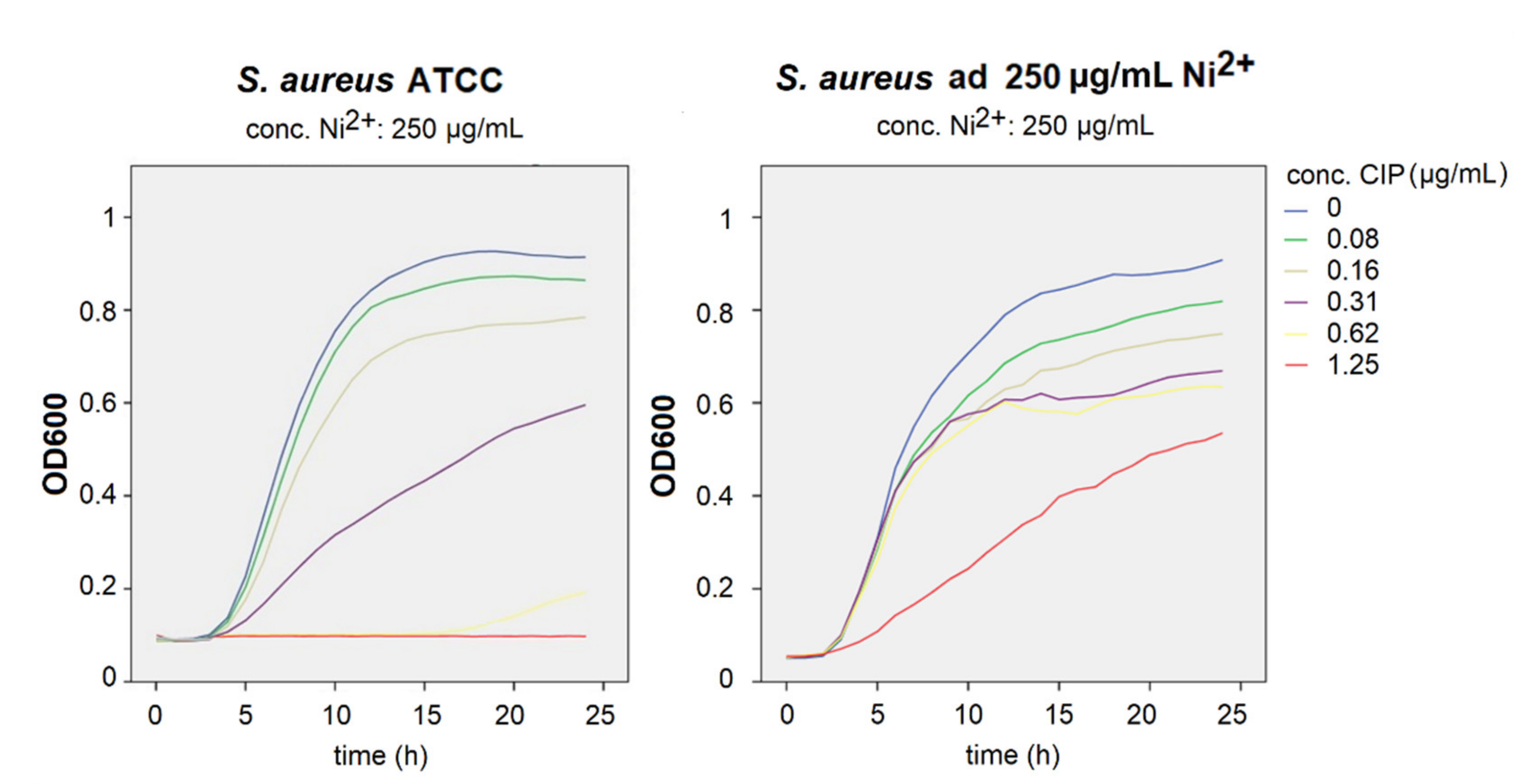
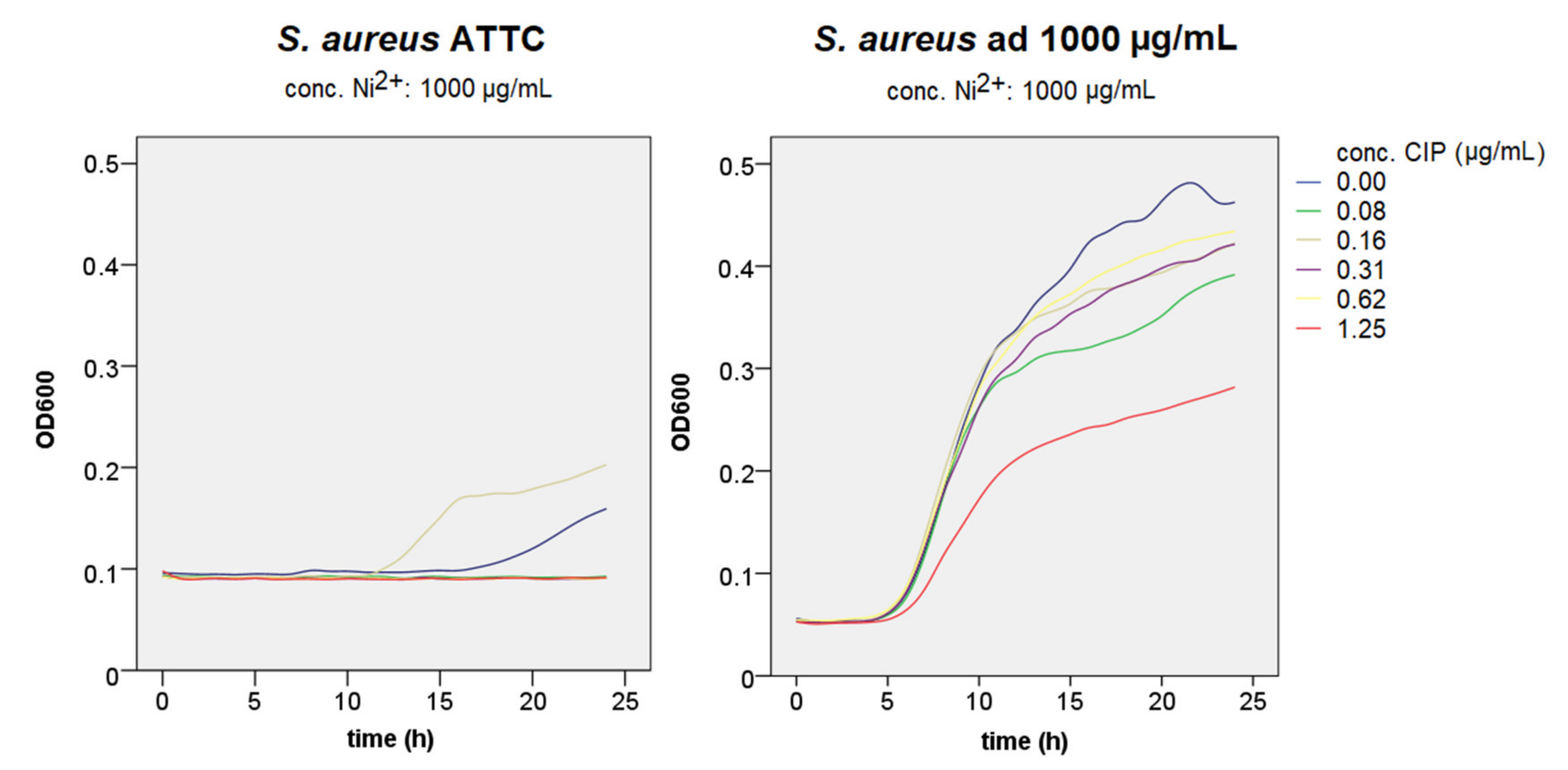
3.2. Growth Curve Assays
3.3. Antimicrobial Susceptibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wendl, B.; Wiltsche, H.; Lankmayr, E.; Winsauer, H.; Walter, A.; Muchitsch, A.; Jakse, N.; Wendl, M.; Wendl, T. Metal release profiles of orthodontic bands, brackets, and wires: An in vitro study. J. Orofac. Orthop. 2017, 78, 494–503. [Google Scholar] [CrossRef]
- Downarowicz, P.; Mikulewicz, M. Trace metal ions release from fixed orthodontic appliances and DNA damage in oral mucosa cells by in vivo studies: A literature review. Adv. Clin. Exp. Med. 2017, 26, 1155–1162. [Google Scholar] [CrossRef]
- Park, H.; Shearer, T. In vitro release of nickel and chromium from simulated orthodontic appliances. Am. J. Orthod. 1983, 84, 156–159. [Google Scholar] [CrossRef]
- Huang, H.H.; Chiu, Y.H.; Lee, T.H.; Wu, S.C.; Yang, H.W.; Su, K.H.; Hsu, C.C. Ion release from NiTi orthodontic wires in artificial saliva with various acidities. Biomaterials 2003, 24, 3585–3592. [Google Scholar] [CrossRef]
- Petoumenou, E.; Arndt, M.; Keilig, L.; Reimann, S.; Hoederath, H.; Eliades, T.; Jäger, A.; Bourauel, C. Nickel concentration in the saliva of patients with nickel-titanium orthodontic appliances. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Kerosuo, H.; Moe, G.; Kleven, E. In vitro release of nickel and chromium from different types of simulated orthodontic appliances. Angle Orthod. 1995, 65, 111–116. [Google Scholar] [CrossRef]
- Rincic Mlinaric, M.; Karlovic, S.; Ciganj, Z.; Pop Acev, D.; Pavlic, A.; Spalj, S. Oral antiseptics and orthodontic appliances: Mechanical and chemical effect of interaction. Odontology 2019, 107, 150–157. [Google Scholar] [CrossRef]
- Jamshidi, S.; Rahmati-Kamel, M.; Mirzaie, M.; Sarrafan, A.; Khafri, S.; Parsian, H. Evaluation of scalp hair nickel and chromium level changes in patients with fixed orthodontic appliance: A one-year follow-up study. Acta Odontol. Scand. 2017, 76, 1–5. [Google Scholar] [CrossRef]
- Kaluarachchi, H.; Chung, K.C.C.; Zamble, D. Microbial nickel proteins. Nat. Prod. Rep. 2010, 27, 681–694. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Hausinger, R.P. Mechanisms of nickel toxicity in microorganisms. Metallomics 2011, 3, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Wang, J.-T.; Li, J.; Shi, X.-Z.; Ma, Y.; Chen, D.; He, J.-Z. Long-term nickel contamination increases the occurrence of antibiotic resistance genes in agricultural soils. Environ. Sci. Technol. 2017, 51, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, X.; Sun, G.; Zhang, Y.; Matsuoka, M.; Ye, J. Heavy metal induced antibiotic resistance in bacterium LSJC7. Int. J. Mol. Sci. 2015, 16, 23390–23404. [Google Scholar] [CrossRef]
- Laila, M.A.; Wagdy, K.B.; Thanaa, H.A. Heavy metal resistance and gene expression analysis of metal resistance gene in gram- positive and gram- negative bacteria present in Egyptian soils. J. Appl. Sci. Environ. Sanit. 2011, 6, 201–211. [Google Scholar]
- Narasimhulu, K.; Sreenivasa, P.; Vinod, A. Isolation and identification of bacterial strains and study of their resistance to heavy metals and antibiotics. J. Microbial. Biochem. Technol. 2010, 2, 74–76. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Theophel, K.; Schacht, V.J.; Schlüter, M.; Schnell, S.; Stingu, C.-S.; Schaumann, R.; Bunge, M. The importance of growth kinetic analysis in determining bacterial susceptibility against antibiotics and silver nanoparticles. Front. Microbiol. 2014, 5, 544. [Google Scholar] [CrossRef]
- Mei, L.; Chieng, J.; Wong, C.; Benic, G.; Farella, M. Factors affecting dental biofilm in patients wearing fixed orthodontic appliances. Prog. Orthod. 2017, 18, 1–6. [Google Scholar] [CrossRef]
- Nanda, R.S.; Ghosh, J. Biomechanical considerations in sliding mechanics. In Biomechanics in Clinical Orthodontics; Nanda, R., Ed.; W.B. Saunders: Philadelphia, PA, USA, 1997; pp. 188–217. [Google Scholar]
- Levin, L.; Samorodnitzky-Naveh, G.R.; Machtei, E.E. The association of orthodontic treatment and fixed retainers with gingival health. J. Periodontol. 2008, 79, 2087–2092. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.; Greenberg, E. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Izumida, F.E.; Moffa, E.B.; Vergani, C.E.; Machado, A.L.; Jorge, J.H.; Giampaolo, E.T. In vitro evaluation of adherence of Candida albicans, Candida glabrata, and Streptococcus mutans to an acrylic resin modified by experimental coatings. Biofouling 2014, 30, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, M.E.; McLoughlin, R.M. Host–bacterial crosstalk determines Staphylococcus aureus nasal colonization. Trends Microbiol. 2016, 24, 872–886. [Google Scholar] [CrossRef] [PubMed]
- Dumitrescu, O.; Dauwalder, O.; Boisset, S.; Reverdy, M.É.; Tristan, A.; Vandenesch, F. Staphylococcus aureus resistance to antibiotics: Key points in 2010. Med. Sci. 2010, 26, 943–949. [Google Scholar]
- Goldberg, M.H. Infections of the salivary glands. In Management of Infections in the Oral and Maxillofacial Regions; Topazian, R.G., Goldberg, M.H., Eds.; Saunders: Philadelphia, PA, USA, 1981. [Google Scholar]
- Bagg, J.; Sweeney, M.P.; Harvey-Wood, K.; Wiggins, A. Possible role of Staphylococcus aureus in severe oral mucositis among elderly dehydrated patients. Microbiol. Ecol. Health Dis. 1995, 8, 51–56. [Google Scholar] [CrossRef]
- Matuschek, E.; Brown, D.F.J.; Kahlmeter, G. Development of the EUCAST disk diffusion antimicrobial susceptibility testing method and its implementation in routine microbiology laboratories. Clin. Microbiol. Infect. 2014, 20, O255–O266. [Google Scholar] [CrossRef]
- Millhouse, E.; Jose, A.; Sherry, L.; Lappin, D.F.; Patel, N.; Middleton, A.M.; Pratten, J.; Culshaw, S.; Ramage, G. Development of an in vitro periodontal biofilm model for assessing antimicrobial and host modulatory effects of bioactive molecules. BMC Oral Health 2014, 14, 80. [Google Scholar] [CrossRef]
- Pitts, B.; Hamilton, M.A.; Zelver, N.; Stewart, P. A microtiter-plate screening method for biofilm disinfection and removal. J. Microbiol. Methods 2003, 54, 269–276. [Google Scholar] [CrossRef]
- Silver, S.; Phung, L.T. Bacterial heavy metal resistance: New surprises. Annu. Rev. Microbiol. 1996, 50, 753–789. [Google Scholar] [CrossRef]
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef] [PubMed]
- Nies, D.H.; Silver, S. Ion efflux systems involved in bacterial metal resistances. J. Ind. Microbiol. Biotechnol. 1995, 14, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Paul, A.K. Nickel uptake and intracellular localization in Cupriavidus pauculus KPS 201, native to ultramafic ecosystem. Adv. Biosci. Biotechnol. 2010, 1, 276–280. [Google Scholar] [CrossRef][Green Version]
- Harrison, J.; Ceri, H.; Turner, R.J. Multimetal resistance and tolerance in microbial biofilms. Nat. Rev. Genet. 2007, 5, 928–938. [Google Scholar] [CrossRef]
- Hoffman, D.A.; Clark, A.E.; Rody, W.J., Jr.; McGorray, S.P.; Wheeler, T.T. A prospective randomized clinical trial into the capacity of a toothpaste containing NovaMin to prevent white spot lesions and gingivitis during orthodontic treatment. Prog. Orthod. 2015, 16, 25. [Google Scholar] [CrossRef]
- Aithal, P.R.V.; Shetty, K.R.A.; Dinesh, M.R.; Amarnath, B.C.; Prashanth, C.S.; Roopak, M.D. In vitro evaluation of microbial contamination and the disinfecting efficacy of chlorhexidine on orthodontic brackets. Prog. Orthod. 2019, 20, 17. [Google Scholar] [CrossRef]
- Katic, V.; Curkovic, L.; Bosnjak, M.U.; Peros, K.; Mandic, D.; Spalj, S. Effect of pH, fluoride and hydrofluoric acid concentration on ion release from NiTi wires with various coatings. Dent. Mater. J. 2017, 36, 149–156. [Google Scholar] [CrossRef][Green Version]
- Smith, A.J.; Robertson, D.; Tang, M.K.; Jackson, M.S.; MacKenzie, D.; Bagg, J. Staphylococcus aureus in the oral cavity: A three-year retrospective analysis of clinical laboratory data. Br. Dent. J. 2003, 195, 701–703. [Google Scholar] [CrossRef] [PubMed]
- McCormack, M.; Smith, A.; Akram, A.; Jackson, M.; Robertson, D.; Edwards, G. Staphylococcus aureus and the oral cavity: An overlooked source of carriage and infection? Am. J. Infect. Control. 2015, 43, 35–37. [Google Scholar] [CrossRef]
- Raji, S.H.; Shojaei, H.; Ghorani, P.S.; Rafiei, E. Bacterial colonization on coated and uncoated orthodontic wires: A prospective clinical trial. Dent. Res. J. 2014, 11, 680–683. [Google Scholar]
- Teitzel, G.M.; Parsek, M.R. Heavy metal resistance of biofilm and planktonic pseudomonas aeruginosa. Appl. Environ. Microbiol. 2003, 69, 2313–2320. [Google Scholar] [CrossRef] [PubMed]
- Taha, M.; El-Fallal, A.; Degla, H. In vitro and in vivo biofilm adhesion to esthetic coated arch wires and its correlation with surface roughness. Angle Orthod. 2015, 86, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Sapata, D.M.; Ramos, A.L.; Sábio, S.; Normando, D.; Pascotto, R.C. Evaluation of biofilm accumulation on and deactivation force of orthodontic Ni-Ti archwires before and after exposure to an oral medium: A prospective clinical study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2020, 14, 41–47. [Google Scholar] [CrossRef]
- Resende, J.A.; Silva, V.L.; Fontes, C.O.; Souza-Filho, J.A.; de Oliveira, T.L.R.; Coelho, C.M.; César, D.E.; Diniz, C.G. Multidrug-resistance and toxic metal tolerance of medically important bacteria isolated from an aquaculture system. Microbes Environ. 2012, 27, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Calomiris, J.J.; Armstrong, J.L.; Seidler, R.J. Association of metal tolerance with multiple antibiotic resistance of bacteria isolated from drinking water. Appl. Environ. Microbiol. 1984, 47, 1238–1242. [Google Scholar] [CrossRef] [PubMed]
- Sabry, S.A.; Ghozlan, H.A.; Abou-Zeid, D.M. Metal tolerance and antibiotic resistance patterns of a bacterial population isolated from sea water. J. Appl. Microbiol. 1997, 82, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, A.Z.; Cen, T.; Li, X.; He, M.; Li, D.; Chen, J. Sub-inhibitory concentrations of heavy metals facilitate the horizontal transfer of plasmid-mediated antibiotic resistance genes in water environment. Environ. Pollut. 2018, 237, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.J.; Decker, E.A.; Goddard, J.M. Metal-chelating active packaging film enhances lysozyme inhibition of listeria monocytogenes. J. Food Prot. 2014, 77, 1153–1160. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlic, A.; Begic, G.; Tota, M.; Abram, M.; Spalj, S.; Gobin, I. Bacterial Exposure to Nickel: Influence on Adhesion and Biofilm Formation on Orthodontic Archwires and Sensitivity to Antimicrobial Agents. Materials 2021, 14, 4603. https://doi.org/10.3390/ma14164603
Pavlic A, Begic G, Tota M, Abram M, Spalj S, Gobin I. Bacterial Exposure to Nickel: Influence on Adhesion and Biofilm Formation on Orthodontic Archwires and Sensitivity to Antimicrobial Agents. Materials. 2021; 14(16):4603. https://doi.org/10.3390/ma14164603
Chicago/Turabian StylePavlic, Andrej, Gabrijela Begic, Marin Tota, Maja Abram, Stjepan Spalj, and Ivana Gobin. 2021. "Bacterial Exposure to Nickel: Influence on Adhesion and Biofilm Formation on Orthodontic Archwires and Sensitivity to Antimicrobial Agents" Materials 14, no. 16: 4603. https://doi.org/10.3390/ma14164603
APA StylePavlic, A., Begic, G., Tota, M., Abram, M., Spalj, S., & Gobin, I. (2021). Bacterial Exposure to Nickel: Influence on Adhesion and Biofilm Formation on Orthodontic Archwires and Sensitivity to Antimicrobial Agents. Materials, 14(16), 4603. https://doi.org/10.3390/ma14164603






