Effect of Platelet Concentrates on Marginal Bone Loss of Immediate Implant Procedures: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Focused Question
2.2. Eligibility Criteria
2.2.1. Inclusion Criteria
2.2.2. Exclusion Criteria
2.3. Information Sources and Search Strategy
2.4. Study Records
2.5. Data Collection
2.6. Risk of Bias in Individual Studies
2.7. Assessment of Evidence Levels
2.8. Synthesis of Results
2.9. Sensitivity Analysis
3. Results
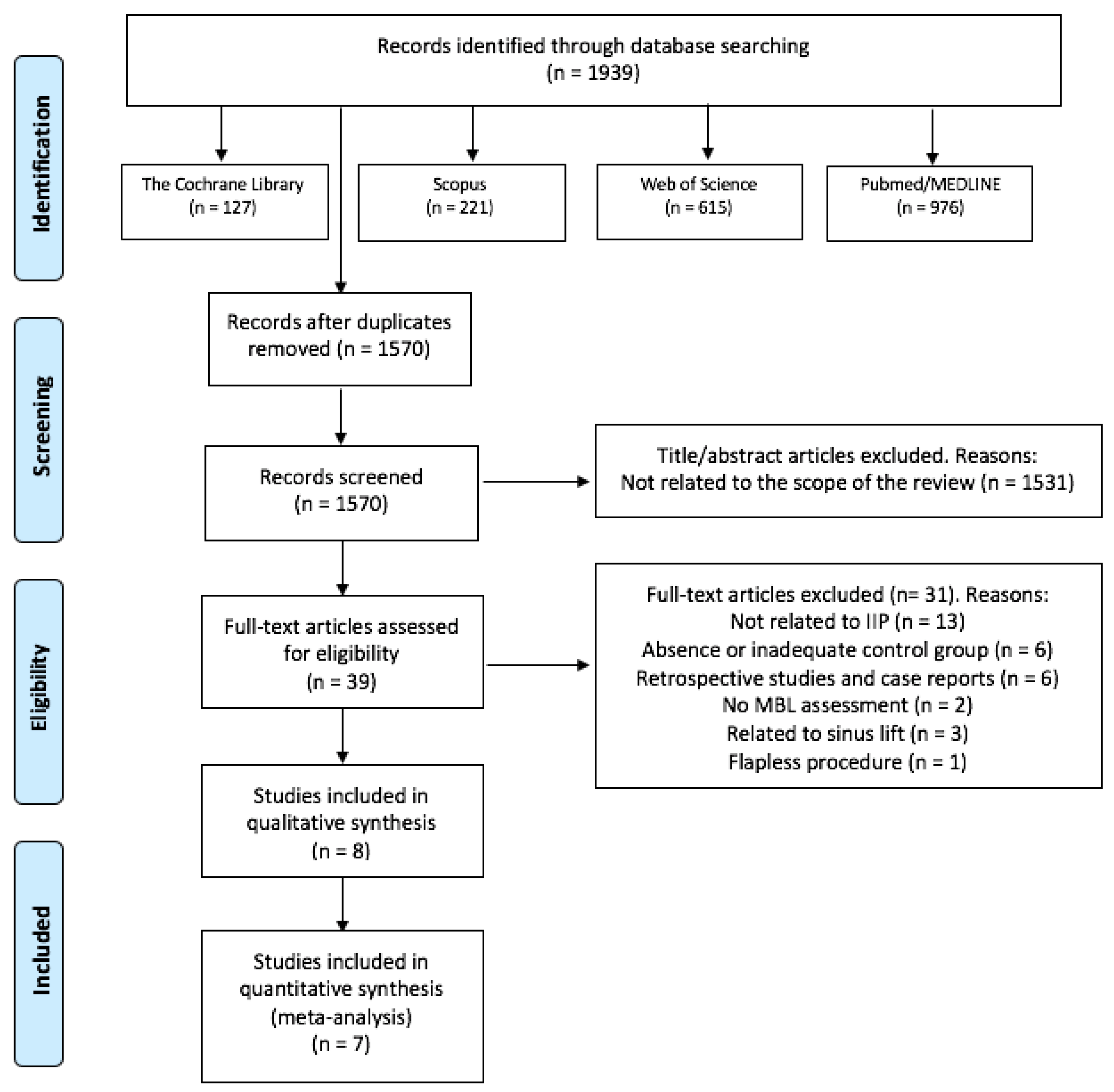
3.1. Study Selection
3.2. Study Characteristics
3.2.1. Patients’ Characteristics
3.2.2. Implants and Bone Characteristics
3.2.3. Radiographic Evaluation
3.2.4. Platelet Concentrates (PCs) Protocols
3.3. Main Findings
3.3.1. Marginal Bone Loss (MBL)
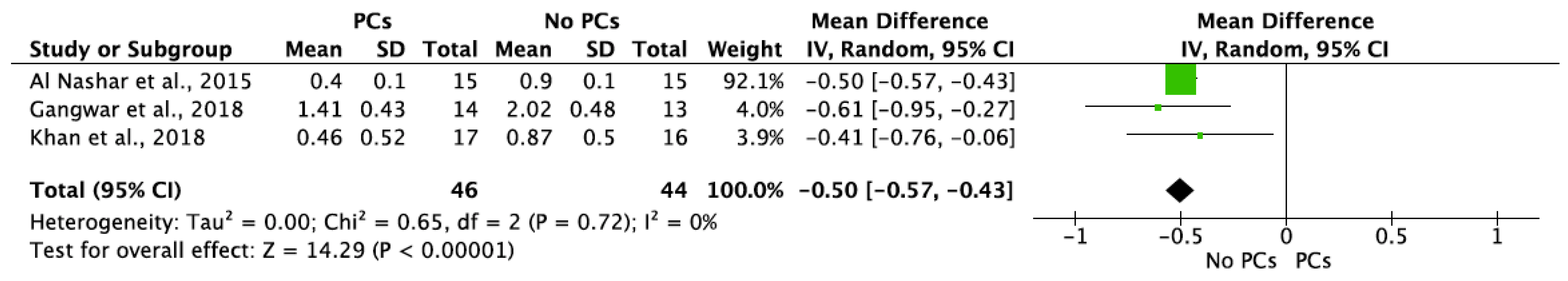
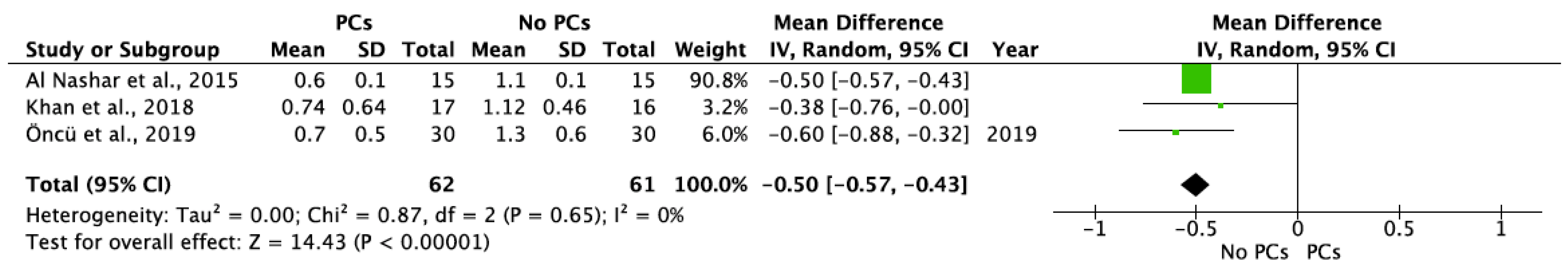
- PCs vs. non-PCs
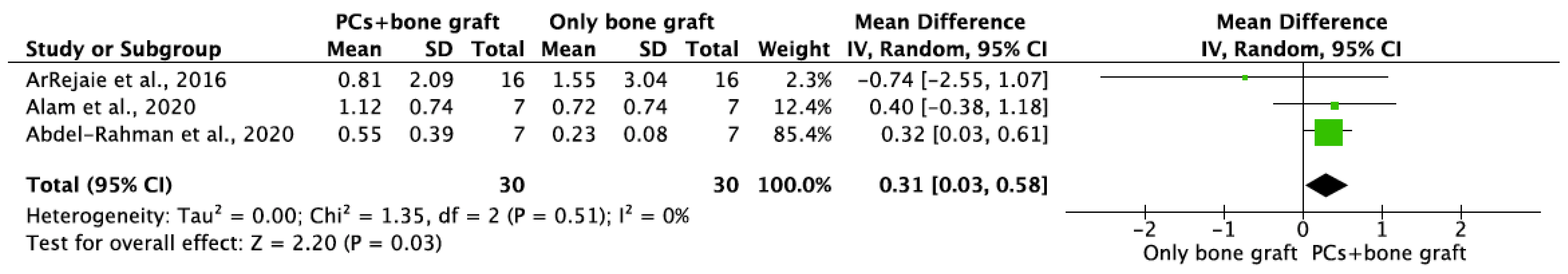
- PCs + bone graft vs. only bone graft
3.3.2. Implant Survival Rates
3.4. Risk of Bias within Studies
3.5. Evaluation of Evidence Levels
3.6. Synthesis of Results: Meta-Analysis
3.7. Sensitivity Analysis
4. Discussion
4.1. Summary of Evidence
4.2. Strength and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IIP | immediate implant procedures |
| PCs | platelet concentrates |
| MBL | marginal bone loss |
| PRF | platelet-rich fibrin |
| PRP | platelet-rich plasma |
| L-PRF | leucocyte platelet-rich fibrin |
| L-PRP | leucocyte platelet-rich plasma |
| P-PRF | pure platelet-rich fibrin |
| P-PRP | pure platelet-rich plasma |
| PRGF | plasma rich in growth factors |
| PICO | population, intervention, comparison, outcome |
| RCT | randomized controlled trial |
| CCT | clinical controlled trial |
| SD | standard deviation |
| SE | standard error |
References
- Mello, C.C.; Lemos, C.A.A.; Verri, F.R.; Dos Santos, D.M.; Goiato, M.C.; Pellizzer, E.P. Immediate implant placement into fresh extraction sockets versus delayed implants into healed sockets: A systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2017, 46, 1162–1177. [Google Scholar] [CrossRef] [Green Version]
- Kan, J.Y.; Rungcharassaeng, K.; Lozada, J. Immediate placement and provisionalization of maxillary anterior single implants: 1-year prospective study. Int. J. Oral Maxillofac. Implants 2003, 18, 31–39. [Google Scholar]
- Schropp, L.; Wenzel, A.; Kostopoulos, L.; Karring, T. Bone healing and soft tissue contour changes following single-tooth extraction: A clinical and radiographic 12-month prospective study. Int. J. Periodontics Restor. Dent. 2003, 23, 313–323. [Google Scholar]
- Chen, S.T.; Buser, D. Clinical and esthetic outcomes of implants placed in postextraction sites. Int. J. Oral Maxillofac. Implants 2009, 24, 186–217. [Google Scholar] [PubMed]
- Ferrus, J.; Cecchinato, D.; Pjetursson, E.B.; Lang, N.P.; Sanz, M.; Lindhe, J. Factors influencing ridge alterations following immediate implant placement into extraction sockets. Clin. Oral Implants Res. 2010, 21, 22–29. [Google Scholar] [CrossRef]
- Del Fabbro, M.; Boggian, C.; Taschieri, S. Immediate implant placement into fresh extraction sites with chronic periapical pathologic features combined with plasma rich in growth factors: Preliminary results of single-cohort study. J. Oral Maxillofac. Surg. 2009, 67, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Maia, L.P.; Reino, D.M.; Muglia, V.A.; Almeida, A.L.; Nanci, A.; Wazen, R.M.; de Oliveira, P.T.; Palioto, D.B.; Novaes, A.B., Jr. Influence of periodontal tissue thickness on buccal plate remodelling on immediate implants with xenograft. J. Clin. Periodontol. 2015, 42, 590–598. [Google Scholar] [CrossRef]
- Al-Hamed, F.S.; Mahri, M.; Al-Waeli, H.; Torres, J.; Badran, Z.; Tamimi, F. Regenerative Effect of Platelet Concentrates in Oral and Craniofacial Regeneration. Front. Cardiovasc. Med. 2019, 6, 126. [Google Scholar] [CrossRef] [Green Version]
- Dohan, D.M.; Bielecki, T.; Mishra, A.; Borzini, P.; Inchingolo, F.; Sammartino, G.; Rasmusson, L.; Evert, P.A. In search of a consensus terminology in the field of platelet concentrates for surgical use: Platelet-rich plasma (PRP), plateletrich fibrin (PRF), fibrin gel polymerization and leukocytes. Curr. Pharm. Biotechnol. 2012, 13, 1131–1137. [Google Scholar]
- Miron, R.J.; Pinto, N.R.; Quirynen, M.; Ghanaati, S. Standardization of relative centrifugal forces in studies related to platelet-rich fibrin. J. Periodontol. 2019, 90, 817–820. [Google Scholar] [CrossRef]
- Bennardo, F.; Liborio, F.; Barone, S.; Antonelli, A.; Buffone, C.; Fortunato, L.; Giudice, A. Efficacy of platelet-rich fibrin compared with triamcinolone acetonide as injective therapy in the treatment of symptomatic oral lichen planus: A pilot study. Clin. Oral Investig. 2021, 25, 3747–3755. [Google Scholar] [CrossRef]
- Anitua, E.; Murias-Freijo, A.; Alkhraisat, M.H.; Orive, G. Clinical, radiographical, and histological outcomes of plasma rich in growth factors in extraction socket: A randomized controlled clinical trial. Clin. Oral Investig. 2015, 19, 589–600. [Google Scholar] [CrossRef]
- Kobayashi, E.; Fujioka-Kobayashi, M.; Sculean, A.; Chappuis, V.; Buser, D.; Schaller, B.; Dőri, F.; Miron, R.J. Effects of platelet rich plasma (PRP) on human gingival fibroblast, osteoblast and periodontal ligament cell behaviour. BMC Oral Health 2017, 17, 91. [Google Scholar]
- Giudice, A.; Antonelli, A.; Muraca, D.; Fortunato, L. Usefulness of advanced-platelet rich fibrin (A-PRF) and injectable-platelet rich fibrin (i-PRF) in the management of a massive medication-related osteonecrosis of the jaw (MRONJ): A 5-years follow-up case report. Indian J. Dent. Res. 2020, 31, 813–818. [Google Scholar]
- Brancaccio, Y.; Antonelli, A.; Barone, S.; Bennardo, F.; Fortunato, L.; Giudice, A. Evaluation of local hemostatic efficacy after dental extractions in patients taking antiplatelet drugs: A randomized clinical trial. Clin. Oral Investig. 2021, 25, 1159–1167. [Google Scholar] [CrossRef]
- Annunziata, M.; Guida, L.; Nastri, L.; Piccirillo, A.; Sommese, L.; Napoli, C. The role of autologous platelet concentrates in alveolar socket preservation: A systematic review. Transfus. Med. Hemother. 2018, 45, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Strauss, F.J.; Stähli, A.; Gruber, R. The use of platelet-rich fibrin to enhance the outcomes of implant therapy: A systematic review. Clin. Oral Implants Res. 2018, 29, 6–19. [Google Scholar] [CrossRef] [PubMed]
- Kotsovilis, S.; Markou, N.; Pepelassi, E.; Nikolidakis, D. The adjunctive use of platelet-rich plasma in the therapy of periodontal intraosseous defects: A systematic review. J. Periodontal Res. 2010, 45, 428–443. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, L.; Bennardo, F.; Buffone, C.; Giudice, A. Is the application of platelet concentrates effective in the prevention and treatment of medication-related osteonecrosis of the jaw? A systematic review. J. Craniomaxillofac. Surg. 2020, 48, 268–285. [Google Scholar] [CrossRef]
- Galindo-Moreno, P.; León-Cano, A.; Ortega-Oller, I.; Monje, A.; O Valle, F.; Catena, A. Marginal bone loss as success criterion in implant dentistry: Beyond 2 mm. Clin. Oral Implants Res. 2015, 26, e28–e34. [Google Scholar] [CrossRef]
- Attanasio, F.; Antonelli, A.; Brancaccio, Y.; Averta, F.; Figliuzzi, M.M.; Fortunato, L.; Giudice, A. Primary Stability of Three Different Osteotomy Techniques in Medullary Bone: An in Vitro Study. Dent. J. 2020, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Sánchez, R.M.; Delgado-Muñoz, J.M.; Hita-Iglesias, P.; Pullen, K.T.; Serrera-Figallo, M.Á.; Torres-Lagares, D. Improvement in the Initial Implant Stability Quotient Through Use of a Modified Surgical Technique. J. Oral Implantol. 2017, 43, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Peng, B.Y.; Wang, P.D.; Feng, S.W. Evaluation of the implant stability and the marginal bone level changes during the first three months of dental implant healing process: A prospective clinical study. J. Mech. Behav. Biomed. Mater. 2020, 110, 103899. [Google Scholar] [CrossRef]
- Kutkut, A.; Andreana, S.; Monaco, E., Jr. Clinical and radiographic evaluation of single-tooth dental implants placed in grafted extraction sites: A one-year report. J. Int. Acad. Periodontol. 2013, 15, 113–124. [Google Scholar]
- Rosano, G.; Taschieri, S.; Del Fabbro, M. Immediate postextraction implant placement using plasma rich in growth factors technology in maxillary premolar region: A new strategy for soft tissue management. J. Oral Implantol. 2013, 39, 98–102. [Google Scholar] [CrossRef]
- Taschieri, S.; Lolato, A.; Ofer, M.; Testori, T.; Francetti, L.; Del Fabbro, M. Immediate post-extraction implants with or without pure platelet-rich plasma: A 5-year follow-up study. Oral Maxillofac. Surg. 2017, 21, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Badran, Z.; Abdallah, M.N.; Torres, J.; Tamimi, F. Platelet concentrates for bone regeneration: Current evidence and future challenges. Platelets 2018, 29, 105–112. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Altman, D.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.T.; Green, S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2; The Cochrane Collaboration: London, UK, 2021. [Google Scholar]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.0; The Cochrane Collaboration: London, UK, 2019. [Google Scholar]
- Al Nashar, A.; Yakoob, H. Evaluation of the use of plasma rich in growth factors with immediate implant placement in periodontally compromised extraction sites: A controlled prospective study. Int. J. Oral Maxillofac. Surg. 2015, 44, 507–512. [Google Scholar] [CrossRef]
- ArRejaie, A.; Al-Harbi, F.; Alagl, A.S.; Hassan, K.S. Platelet-rich plasma gel combined with bovine-derived xenograft for the treatment of dehiscence around immediately placed conventionally loaded dental implants in humans: Cone beam computed tomography and three-dimensional image evaluation. Int. J. Oral Maxillofac. Implants 2016, 31, 431–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gangwar, S.; Pal, U.S.; Singh, S.; Singh, R.K.; Kumar, L. Immediately placed dental implants in smokers with plasma rich in growth factor versus without plasma rich in growth factor: A comparison. Natl. J. Maxillofac. Surg. 2018, 9, 39–47. [Google Scholar] [PubMed]
- Khan, Z.A.; Jhingran, R.; Bains, V.K.; Madan, R.; Srivastava, R.; Rizvi, I. Evaluation of peri-implant tissues around nanopore surface implants with or without platelet rich fibrin: A clinico-radiographic study. Biomed. Mater. 2018, 13, 25002. [Google Scholar] [CrossRef] [Green Version]
- Öncü, E.; Erbeyoğlu, A.A. Enhancement of immediate implant stability and recovery using platelet-rich fibrin. Int. J. Periodontics Restor. Dent. 2019, 39, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Soni, R.; Priya, A.; Agrawal, R.; Bhatnagar, A.; Kumar, L. Evaluation of efficacy of platelet-rich fibrin membrane and bone graft in coverage of immediate dental implant in esthetic zone: An in vivo study. Natl. J. Maxillofac. Surg. 2020, 11, 67–75. [Google Scholar] [CrossRef]
- Alam, S.; Dhiman, M.; Jain, A.V.; Buthia, O.; Pruthi, G. Vertical bone implant contact around anterior immediate implants and their stability after using either alloplast or L-PRF or both in peri-Implant gap: A prospective randomized trial. J. Maxillofac. Oral Surg. 2020. [Google Scholar] [CrossRef]
- Abdel-Rahman, F.H.; Salem, A.S.; El-Shinnawi, U.M.; Hammouda, N.I.; El-Kenawy, M.H. Efficacy of autogenous platelet-rich fibrin versus slowly resorbable collagen membrane with immediate implants in the esthetic zone. J. Oral Implantol. 2020. [Google Scholar] [CrossRef]
- Fabbro, M.D.; Bortolin, M.; Taschieri, S.; Ceci, C.; Weinstein, R.L. Antimicrobial properties of platelet-rich preparations. A systematic review of the current preclinical evidence. Platelets 2016, 27, 276–285. [Google Scholar] [CrossRef]
- Becker, W.; Lynch, S.E.; Lekholm, U.; Becker, B.E.; Caffesse, R.; Donath, K.; Sanchez, R. A comparison of PTFE membranes alone or in combination with platelet-derived growth factors and insulin-like growth factor-I or demineralized freeze-dried bone in promoting bone formation around immediate extraction socket implants. J. Periodontol. 1992, 63, 929–940. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Orive, G.; Pla, R.; Roman, P.; Serrano, V.; Andía, I. The effects of PRGF on bone regeneration and on titanium implant osseointegration in goats: A histologic and histomorphometric study. J. Biomed. Mater. Res. A 2009, 91, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Öncü, E.; Alaaddinoğlu, E.E. The effect of platelet-rich fibrin on implant stability. Int. J. Oral Maxillofac. Implants 2015, 30, 578–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anitua, E.; Orive, G.; Aguirre, J.J.; Andía, I. Clinical outcome of immediately loaded dental implants bioactivated with plasma rich in growth factors: A 5-year retrospective study. J. Periodontol. 2008, 79, 1168–1176. [Google Scholar] [CrossRef] [PubMed]
- Moraschini, V.; Barboza, E.S. Effect of autologous platelet concentrates for alveolar socket preservation: A systematic review. Int. J. Oral Maxillofac. Surg. 2015, 44, 632–641. [Google Scholar] [CrossRef] [PubMed]
- Zeytinoğlu, M.; İlhan, B.; Dündar, N.; Boyacioğlu, H. Fractal analysis for the assessment of trabecular peri-implant alveolar bone using panoramic radiographs. Clin. Oral Investig. 2015, 19, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Cardaropoli, D.; Tamagnone, L.; Roffredo, A.; Gaveglio, L. Relationship between the buccal bone plate thickness and the healing of postextraction sockets with/without ridge preservation. Int. J. Periodontics Restor. Dent. 2014, 34, 211–217. [Google Scholar] [CrossRef]
- Araújo, M.G.; da Silva, J.C.C.; de Mendonça, A.F.; Lindhe, J. Ridge alterations following grafting of fresh extraction sockets in man. A randomized clinical trial. Clin. Oral Implants Res. 2015, 26, 407–412. [Google Scholar] [CrossRef] [PubMed]
- Grassi, F.R.; Grassi, R.; Rapone, B.; Alemanno, G.; Balena, A.; Kalemaj, Z. Dimensional changes of buccal bone plate in immediate implants inserted through open flap, open flap and bone grafting and flapless techniques: A cone-beam computed tomography randomized controlled clinical trial. Clin. Oral Implants Res. 2019, 30, 1155–1164. [Google Scholar] [CrossRef]

| Keywords Employed Using Boolean Operator “OR” | Boolean Operator “AND” | Keywords Employed Using Boolean Operator “OR” |
| “Immediate implant”, “immediate implantation”, “post-extraction implant”, “immediate implant procedure”, “immediate implant placement” | “Platelet concentrates”, “platelet-rich fibrin”, “PRF”, “platelet-rich plasma”, “PRP”, “leukocyte platelet-rich fibrin”, “L-PRF”, “leukocyte platelet-rich plasma”, “L-PRP”, “pure platelet-rich fibrin”, “P-PRF”, “pure platelet-rich plasma”, “P-PRP”, “plasma-rich in growth factors”, “PRGF”, “injectable platelet-rich fibrin”, “I-PRF”, “growth factors”, “platelet-derived growth factors” |
| Author, Year | Country | Type of Study | Patients | Implants | Age (Years) | Sex | Inclusion Criteria | Exclusion Criteria | Periodontal Therapy | Clinician | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCs | Non-PCs | ||||||||||
| Al Nashar et al., 2015 | Syria | CCT with split-mouth design | 15 | 15 | 15 | 30–55 | Male: 7 Female: 8 | Patients with good health, with no chronic disease, non-smoking, physically able, with chronic periodontitis in the anterior region of the mandible which had lost 75% of the supporting bone or had a probing depth >8 mm of four bony walls of the remaining alveolus with at least 5 mm depth on both sides and the presence of 5 mm of bone beyond root apex | Patients with any disease, condition, or medication that might compromise healing or osseointegration, unable/unwilling to return for follow-up visits, or needing grafting of the implant site | NA | NA |
| ArRejaie et al., 2016 | Saudi Arabia | RCT with split-mouth design | 16 | 16 | 16 | NA | NA | Patients with either tooth fracture, endodontic failure, or badly decayed teeth in the anterior and premolar regions of the maxilla with previous buccal bone loss | Patients with systemic contraindications to treatment, pregnants, smokers, having received systemic antibiotics or non-steroidal anti-inflammatory drugs within the last 3 months prior to treatment, or having received surgical treatment in the selected sites within the year before the initiation of the study | Oral hygiene instructions and SRP Surgical phase: < 10% O’Leary Plaque Index | The same surgeon |
| Gangwar et al., 2018 | India | RCT | 27 | 14 | 13 | NA | NA | Patients >18 years with missing maxillary or mandibular tooth, adequate bone volume to accommodate an implant of appropriate size and with good oral hygiene | Pathological radiolucency in jaw bone of more than 1 cm, chronic inflammatory rheumatoid disease, uncontrolled diabetes, osteoporosis, Systemic corticosteroid treatment of more than 1 month within 1 year, and severe disease with a life expectancy <1 year | NA | NA |
| Khan et al., 2018 | India | RCT | 14 | 17 | 16 | PCs 32.59 ± 1.65 Non-PCs 33.25 ± 1.83 | NA | Patients > 18 years, ASA I, non-smokers (>2 months), with monoradicular tooth indicated for extraction (trauma, endodontic failure and non-restorable carious lesion, root/crown fracture), intact bone in all dimensions, jumping gap < 2 mm, with a suitable occlusion | Pregnant women or nursing mother, smokers, patients with localised infection (presence of chronic pain, purulent periodontal and endodontic lesion, severe periodontal bone loss with a remaining alveolar height < 7 mm), Patients taking medication that may affect the clinical outcome in last 6 months, extraction site of mobile teeth | Preliminary phase: hygiene instructions, SRP Before surgical phase: complete SRP and polishing | NA |
| Öncü et al., 2019 | Turkey | RCT with split-mouth design | 26 | 30 | 30 | 40.2 ± 11.5 | Male 16 Female 10 | No systemic health problems; no need of sinus floor augmentation, distraction osteogenesis, or bone grafting, and having at least two adjacent or contralateral premolar or molar teeth that needed extraction in the mandible/maxilla | Insufficient bone volume, parafunctional habits, smoking more than 10 cigarettes per day, systemic disorders, and poor oral hygiene | During the study, patients enrolled in an individually maintenance care program for professional cleaning and examinations | The same surgeon |
| Soni et al., 2020 | India | RCT | 16 | 8 | 8 | PCs: 21–45 Non-PCs: 18–45 | NA | Patients >18 years; good oral hygiene and satisfactory periodontal status of the remaining dentition; presence of a single failing tooth in anterior maxilla; patients who gave positive informed consent and patients available for follow-up | Metabolic or systemic disease affecting the integration of implant or connective tissue health surrounding implant; history of irradiation in the head-and-neck area; smokers; pregnant women; parafunctional habits such as bruxism, tongue thrust, and teeth clenching; untreated generalised periodontitis; psychiatric disorders or unrealistic expectations and acute infection (abscess) at the intended site for implant placement | Initial periodontal therapy was done before the surgical procedure | NA |
| Alam et al., 2020 | India | RCT | 20 * | 10 | 10 | 18–45 | NA | Patients with single maxillary anterior tooth with poor prognosis and indicated for extraction (root fractures, endodontic failures, root caries, internal or external resorption, and over retained deciduous tooth); with good oral hygiene and periodontal status of remaining dentition; without any metabolic and systemic disease; with sufficient quality and quantity of bone and available for follow-up visits. | Teeth with associated periapical pathology, patients with parafunctional habits, history of smoking, diabetes, any other systemic health problems, immunocompromised state, pregnant females, peri-implant gaps <2 mm or >5 mm and primary stability <35 N/cm. | Patients recalled at regular intervals to evaluate oral hygiene | The same surgeon |
| Abdel-Rahman et al., 2020 | Egypt | CCT | 14 | 7 | 7 | 22.54 ± 4.97 (18–36) | Male: 9 Female: 5 | Patients needing a extraction of a non-restorable maxillary incisor or premolar, with adequate horizontal and vertical bone, with opposing occlusion, good oral hygiene and no medical limiting conditions. | Teeth adjacent to the future implant that are periodontally or endodontically compromised, no opposing dentition; inadequate oral hygiene; chronic medical conditions (hemorrhagic disease, uncontrolled diabetes), smokers, alcohol abusers and bruxers. | All patients were instructed for oral hygiene and received adequate periodontal scaling prior to implant placement | NA |
| Author, Year | Al Nashar et al., 2015 | ArRejaie et al., 2016 | Gangwar et al., 2018 | Khan et al., 2018 | Öncü et al., 2019 | Soni et al., 2020 | Alam et al., 2020 | Abdel-Rahman et al., 2020 |
|---|---|---|---|---|---|---|---|---|
| Types of PCs | PRGF | PRP | PRGF | PRF | L-PRF | A-PRF | L-PRF | PRF |
| PCs preparation protocol | 10 mL of peripheral blood 3.8% trisodium citrate 270 rpm for 7 min (PRGF System; BTI, Álava, Spain) The middle layer was collected Leukocytes were not collected 50 µL of 10% CaCl2 solution was added to coagulation | 10 mL of blood 0.5 mL sodium citrate 200 G for 20 min Plasma: 400 G for 10 min Bovine thrombin and 10% calcium chloride were added to coagulation | 8 mL of blood 0.2 mL of 3.2% sodium citrate 270 G (3500 rpm) for 10 min PRGF located above the red clot was used | 10 mL of blood without any anticoagulant 3000 rpm for 12 min (REMI, Laboratories, India). PRF in central clot was collected | 9 mL of blood without anti-clotting agent (Becton Dickinson Vacutainer) 2700 rpm for 12 min (PC-02, Process Ltd., Nice, France) The middle fibrin clot was transferred to the L-PRF box (Process Ltd., Nice, France) and compressed to obtain L-PRF membranes | 10 mL tubes No anticoagulant Centrifugation (DUO Quattro PRF Centrifuge, Nice, France) at 1300 rpm for 8 min. The middle layer was collected and placed in the PRF box for A-PRF membrane formation | 5 mL of venous blood Centrifuge machine (REMI R-8C, Maharashtra, India) PRF was obtained | 10 mL tubes No anticoagulant Centrifuged at 3000 rpm and 400 g for 10 min. The middle layer was collected for PRF membranes formation |
| PCs application form | Liquid: PRGF injected into the drill holes immediately before implant placement. Implants were dipped in PRGF before seating | Gel: PRP gel combined with bovine-derived xenograft | Liquid: Implants dipped in PRGF before placing it | Liquid and solid: Implants bio-activated and covered with PRF membrane | Solid: L-PRF membrane applied inside the implant cavity | Solid: Xenograft was placed into the defects and then covered with A-PRF membranes | Solid: L-PRF combined with synthetic bone graft | Solid: PRF membranes combined with bovine-derived xenograft |
| Premedication | 600 mg clindamycin 1 h before surgery | NA | NA | 500 mg amoxicillin and 125 mg of clavulanic acid a day before surgery | NA | 2 g amoxicillin with potassium clavulanate (augmentin) 1 h before surgery, and rinse with chlorhexidine (0.2%) for 1 min before intervention. | 2 g amoxicillin with potassium clavulanate | NA |
| Local anesthetic | 3.6–5.4 mL of mepivacaine HCl 2% with vasoconstrictor (levonordefrin) 1:20.000 | NA | 2% xylocaine hydrochloride with 1:20.0000 adrenaline | 2% xylocaine, with 1:80.000 adrenaline | Ultracaine | 2% lignocaine, with 1:80.000 adrenaline | Lignocaine 2% with 1:200.000 adrenaline | Local anesthesia |
| Surgical procedure | Full-thickness flap Teeth extracted gently with minimum trauma Sockets carefully debrided and irrigated with sterile saline. Osteotomies according to standard protocols, with slow-speed sequential drills and copious irrigation Closure of the wound was obtained by coronal repositioning of the flap | Full-thickness flap Vertical releasing incisions when needed. Teeth carefully extracted Infected granulation tissues were removed All implants were placed completely into the extraction socket with primary stability | Mucoperiosteal flap reflected. Extraction was performed with the help of root forceps, bur, periotome, etc. Drilling procedure 2 mm beyond the apex of tooth, with internal irrigation. Flap sutured to achieve primary tension-free closure | Full-thickness flap. Atraumatic extractions. Socket debrided using surgical curette. Implants placed at a speed of 30 rpm. All implants had a primary stability of at least 35 N/cm | Mucoperiosteal flap. Carefully extractions. Sockets cleaned and rinsed with saline. The implant sites 5 mm apart. Flap sutures to original position. Healing caps were not covered | Crestal incision with releasing incision Full-thickness mucoperiosteal flap Tooth extracted and socket debrided and irrigated with saline Implants placed with cover screws Primary closure of the wound | A traumatic extraction, socket cleaned and irrigated. Implants placed in the palatal wall and left for submerged healing. Primary closure | Intrasulcular incision full thickness mucoperiosteal flap Atraumatic extraction Socket curetted and irrigated with saline Implant placed with a customised surgical guide. Implants placed 2–3 mm past the extracted tooth apex Cover screw and primary closure with 4.0 silk suture |
| Implant position | At the crestal ridge | At the crestal bone level | NA | Slightly below the bone crest level | Submerged 2 mm below the margins of the socket | NA | 2 mm below the line joining the cementum-enamel junction of adjacent teeth | 1 mm sub crestal in all cases |
| Inclusion sockets | Presence of four bony walls | Buccal bone loss | Adequate bone volume | All four walls intact | Sufficient bone volume | Buccal bone defect | Sufficient quantity of bone | Adequate horizontal and vertical bone |
| Included gaps | NA | NA | NA | >2 mm | About 1 mm | NA | Between 2–5 mm | Nr |
| Implants characteristics | Length: 10–12 mm Diameter: 3.6 mm (Euroteknika, Sallanches, France) | Length: 10–14 mm Diameter: 3.4–3.8 mm (Friadent, Dentsply) | NA | The Myriad Plus (MyriadTM Plus Implant System) | Length: 12 mm Diameter: 4.1 mm (ITI SLActive, Straumann) | Double piece, (ADIN; Touareg™-S) | Tapered implants with internal trilobed (Myriad plus, Equinox Medical Technologies B.V, Netherlands) | Length: 14 mm Diameter: 3.6, 4 and 4.5 mm (Dentium system, Superline, Seoul, Korea) |
| Regions of implants insertion | Mandibular lateral incisors | Maxillary anterior and premolar regions | Anterior and posterior maxilla. Anterior and posterior mandible | PCs: 5 maxilla, 12 mandible Non-PCs: 5 maxilla, 11 mandible | PCs: 12 maxilla; 18 mandible Non-PCs: 14 maxilla, 16 mandible | All in the anterior maxilla | Maxillary anterior region | Maxillary incisors and premolars |
| Postsurgical medication | 0.2% chlorhexidine HCl twice daily for 7 days 300 mg clindamycin orally every 6 h for 5 days and ibuprofen 600 mg twice daily for 7–10 days | NA | The patient is discharged after prescribing antibiotic and analgesic and hexidine mouthwash. The patient was seen after 7 days postoperatively for suture removal | 500 mg amoxicillin and 125 mg clavulanate acid twice daily during 6 days, Zerodol every 12 h and 0.2% chlorhexine mouth wash | 1 g amoxicillin and clavulanic acid 2 times/day, flurbiprofen 2 times/day and chlorhexidine gluconate 3 times/day | Antibiotics, analgesics, anti-inflammatory and chlorhexidine mouth rinses for 7 days | 400 mg ibuprofen and 325 mg paracetamol 3 times per day for 3 days | 500 mg amoxicillin every 8 h for 1 week |
| Prosthetic procedure | Healing period of 3 months. Then, healing abutments were placed. Prosthetic rehabilitation started 2 weeks later, where crowns were cemented with temporary cement | After 6 months, healing abutments were connected to the implants, and the prosthetic procedures were performed. Crowns were cemented using implant cement materials | NA | NA | The healing caps were placed in the 3rd month | Second-stage: 4 months Healing cap placed for 2 weeks | Second-stage: 3 months Finally restored with porcelain fused to metal crowns, luted with temporary cement | Second-stage: 6 months Healing cap placed for 2 weeks 1 week later: final cemented crown |
| MBL evaluation | An independent radiologist blinded to the study groups Digital panoramic radiographs The implant length fixture was measured and compared to the real implant length to determine the magnification factor in the image | A standardised long cone parallel technique was used to record the radiographic parameters. Three CBCT images (baseline, 6 and 12 months post-surgery) | Intraoral periapical radiograph with XCP extension cone paralleling film-holding device. The implant-abutment junction was used as a reference point for all measurements | An independent investigator unaware of the treatment modality. Radiovisiography (RVG) The images were calibrated geometrically based on implant length. The radiograph was obtained in a constant and reproducible plane, using film holder and a template | Periapical radiographs using long cone paralleling technique and employing a positioner (X-ray Holders, KerrHawe). Upper corner of the coronal shoulder of the implant as reference point. Measurements from reference point to the first bone–implant contact. Image J software (version 1.49 m, National Institutes of Health) | Periapical radiographs using a digital intraoral sensor (Sirona Dental system, Bensheim, Germany), an X-ray positioner with an individually customised acrylic positioning jig Measurements with Sirona software from the shoulder of the implant to the first bone–implant contact Images were calibrated with the known size of implant | Periapical radiographs taken with long cone paralleling technique using positioner The images were calibrated by the known length of the dental implant Distance from the implant shoulder to the first bone-to implant was measured using contact Image J software (1.47 V Wayne Rasband, National Institutes of Health, Bethesda, MD, USA) by two calibrated examiners | Periapical radiographs taken with the long cone parallel technique, using Rinn XCP (Dentsply, Friadent Schweiz, Nidau, Switzerland) and a customised bite-block. The known implant length was used as reference. The distance from the implant–abutment connection to the marginal bone level was measured. Digital tracing was conducted with Scanora 5.2 software (Tuusula, Finland) |
| Complications | without infections or complications | NA | NA | NA | No complications were observed. | Only one patient with cover screw exposure | NA | No signs of dehiscence, infection or mobility |
| Marginal Bone Loss (mm) | Survival Rates (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PCs | No PCs | PCs | No PCs | ||||||||
| Follow-Up After IIP (Months) | Implants (n) | Mesial Aspect | Distal Aspect | Both Aspects | Implants (n) | Mesial Aspect | Distal Aspect | Both Aspects | |||
| Al Nashar et al., 2015 | 3 6 12 | 15 | - | 0.2 ± 0.1 0.4 ± 0.1 0.6 ± 0.1 | 15 | - | 0.6 ± 0.0 0.9 ± 0.1 1.1 ± 0.1 | 15/15 (100%) | 15/15 (100%) | ||
| ArRejaie et al., 2016 | 3 6 9 12 | 16 | 1.66 ± 0.96 † 1.30 ± 0.96 † 0.83 ± 1.16 † 0.80 ± 0.96 † | 1.76 ± 0.96 † 1.40 ± 1.24 † 0.87 ± 1.16 † 0.82 ± 2.84 † | 1.71 ± 0.95 * 1.35 ± 1.09 * 0.85 ± 1.14 * 0.81 ± 2.09 * | 16 | 2.27 ± 1.8 † 2.76 ± 1.24 † 1.66 ± 1.12 † 1.60 ± 1.04 † | 2.17 ± 1.64 † 2.56 ± 1.4 † 1.69 ± 1.12 1.50 ± 4.24 † | 2.22 ± 1.71 * 2.66 ± 1.31 * 1.68 ± 1.10 * 1.55 ± 3.04 * | U/16 | U/16 |
| Gangwar et al., 2018 | 6 | 14 | 1.36 ± 0.42 | 1.45 ± 0.45 | 1.41 ± 0.43 * | 13 | 1.90 ± 0.43 | 2.14 ± 0.52 | 2.02 ± 0.48 * | U/14 | U/13 |
| (90%) | |||||||||||
| Khan et al., 2018 | 4–5 6 7–8 10–11 12 13–14 | 17 | 0.34 ± 0.49 † 0.44 ± 0.52 * 0.53 ± 0.54 † 0.66 ± 0.62 † 0.72 ± 0.65 * 0.78 ± 0.7 † | 0.37 ±0.49 † 0.48 ± 0.54 * 0.59 ±0.58 † 0.70 ±0.62 † 0.76 ± 0.65 * 0.82 ± 0.7 † | 0.36 ± 0.48 * 0.46 ± 0.52 * 0.56 ± 0.55 * 0.68 ± 0.61 * 0.74 ± 0.64 * 0.8 ± 0.69 * | 16 | 0.74 ± 0.48 † 0.82 ± 0.48 * 0.89 ± 0.48 † 1.00 ± 0.48 † 1.07 ± 0.46 * 1.14 ± 0.44 † | 0.83 ± 0.56 † 0.92 ± 0.54 * 1.00 ± 0.52 † 1.10 ± 0.48 † 1.18 ± 0.48 * 1.25 ± 0.48 † | 0.79 ± 0.52 * 0.87 ± 0.5 * 0.95 ± 0.5 * 1.05 ± 0.48 * 1.12 ± 0.46 * 1.2 ± 0.46 * | 17/17 (100%) | 16/16 (100%) |
| Öncü et al., 2019 | 12 | 30 | - | 0.7 ± 0.5 | 30 | - | 1.3 ± 0.6 | 30/30 (100%) | 30/30 (100%) | ||
| Soni et al., 2020 | 4 | 8 | −0.03 ± 0.56 | −0.18 ± 0.3 | −0.11 ± 0.44 * | 8 | −0.09 ± 0.76 | −0.17 ± 0.42 | −0.13 ± 0.6 * | 8/8 (100%) | 8/8 (100%) |
| Alam et al., 2020 | 3 6 12 + | 10 | 3.18 ± 2.36 2.03 ± 0.95 1.25 ± 0.85 | 2.19 ± 1.75 1.38 ± 0.64 0.99 ± 0.66 | 2.69 ± 2.09 * 1.71 ± 0.86 * 1.12 ± 0.74 * | 10 | 2.57 ± 0.94 1.83 ± 0.46 0.86 ± 0.71 | 1.96 ± 1.27 1.56 ± 1.1 0.58 ± 0.79 | 2.27 ± 1.13 * 1.7 ± 0.83 * 0.72 ± 0.74 * | 100% | 100% |
| Abdel-Rahman et al., 2020 | 6 12 18 | 7 | 0.36 ± 0.32 0.49 ± 0.36 0.54 ± 0.32 | 0.47 ± 0.38 0.6 ± 0.44 0.67 ± 0.49 | 0.42 ± 0.34 * 0.55 ± 0.39 * 0.61 ± 0.4 * | 7 | 0.16 ± 0.08 0.24 ± 0.09 0.23 ± 0.09 | 0.2 ± 0.06 0.21 ± 0.07 0.24 ± 0.08 | 0.18 ± 0.07 * 0.23 ± 0.08 * 0.24 ± 0.08 * | 7/7 (100%) | 7/7 (100%) |
| Author, Year | Possible Source of Bias (Type of Bias) | |||||||
|---|---|---|---|---|---|---|---|---|
| Random Sequence Generation (Selection) | Allocation Concealment (Selection) | Blinding of Participants and Personnel (Performance) | Blinding of Outcome Assessment (Detection) | Incomplete Outcome Data (Attrition) | Selective Reporting (Reporting) | Other Bias | Overall Assessment | |
| Al Nashar et al., 2015 | Unclear risk | High risk | High risk | Low risk | Low risk | Low risk | Low risk | High risk |
| ArRejaie et al., 2016 | Low risk | Low risk | High risk | Unclear risk | Unclear risk | Unclear risk | Low risk | High risk |
| Gangwar et al., 2018 | Low risk | Unclear risk | High risk | Unclear risk | Unclear risk | Unclear risk | Low risk | High risk |
| Khan et al., 2018 | Low risk | Low risk | High risk | Low risk | Low risk | Low risk | Low risk | High risk |
| Öncü et al., 2019 | Low risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Low risk | High risk |
| Soni et al., 2020 | Low risk | Unclear risk | High risk | Unclear risk | Low risk | High risk | Low risk | High risk |
| Alam et al., 2020 | Low risk | Unclear risk | High risk | Low risk | Unclear risk | Low risk | Low risk | High risk |
| Abdel-Rahman et al., 2020 | Low risk | Unclear risk | High risk | Unclear risk | Low risk | Low risk | Low risk | High risk |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Serrano, J.; Vallina, C.; González-Serrano, C.; Sánchez-Monescillo, A.; Torres, J.; Hernández, G.; López-Pintor, R.M. Effect of Platelet Concentrates on Marginal Bone Loss of Immediate Implant Procedures: A Systematic Review and Meta-Analysis. Materials 2021, 14, 4582. https://doi.org/10.3390/ma14164582
González-Serrano J, Vallina C, González-Serrano C, Sánchez-Monescillo A, Torres J, Hernández G, López-Pintor RM. Effect of Platelet Concentrates on Marginal Bone Loss of Immediate Implant Procedures: A Systematic Review and Meta-Analysis. Materials. 2021; 14(16):4582. https://doi.org/10.3390/ma14164582
Chicago/Turabian StyleGonzález-Serrano, José, Carmen Vallina, Carlos González-Serrano, Andrés Sánchez-Monescillo, Jesús Torres, Gonzalo Hernández, and Rosa María López-Pintor. 2021. "Effect of Platelet Concentrates on Marginal Bone Loss of Immediate Implant Procedures: A Systematic Review and Meta-Analysis" Materials 14, no. 16: 4582. https://doi.org/10.3390/ma14164582
APA StyleGonzález-Serrano, J., Vallina, C., González-Serrano, C., Sánchez-Monescillo, A., Torres, J., Hernández, G., & López-Pintor, R. M. (2021). Effect of Platelet Concentrates on Marginal Bone Loss of Immediate Implant Procedures: A Systematic Review and Meta-Analysis. Materials, 14(16), 4582. https://doi.org/10.3390/ma14164582







