Prognosis of Regenerative Endodontic Procedures in Mature Teeth: A Systematic Review and Meta-Analysis of Clinical and Radiographic Parameters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Question
2.2. Eligibility Criteria
2.3. Information Sources
2.4. Search Strategy
2.5. Study Selection
2.6. Data Extraction
2.7. Quality Assessment
2.8. Synthesis of Results
2.9. Certainty of Evidence
3. Results
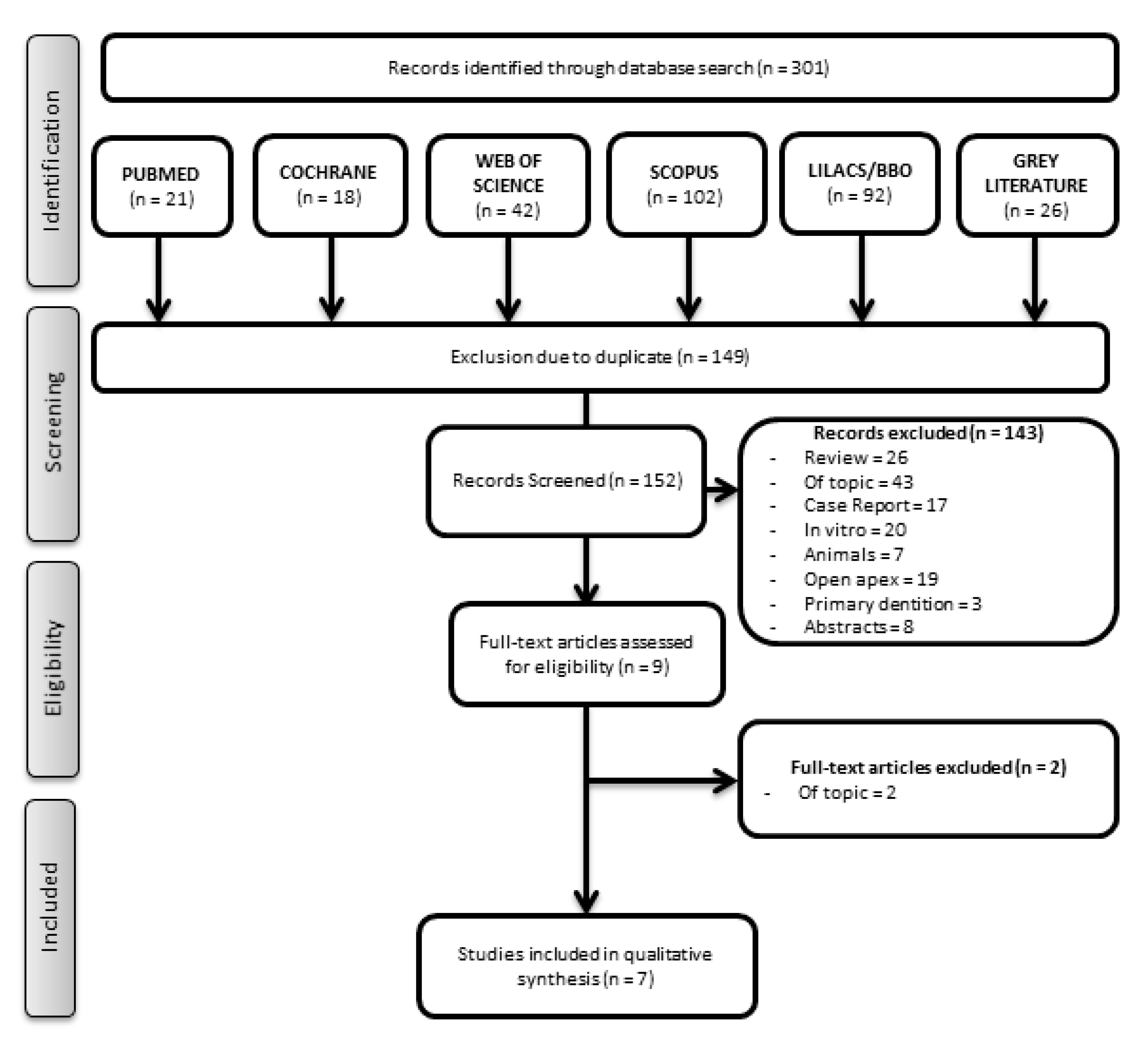
3.1. Study Selection
3.2. Main Characteristics of the Included Articles
3.3. Quality Assessment
3.4. Synthesis of Results
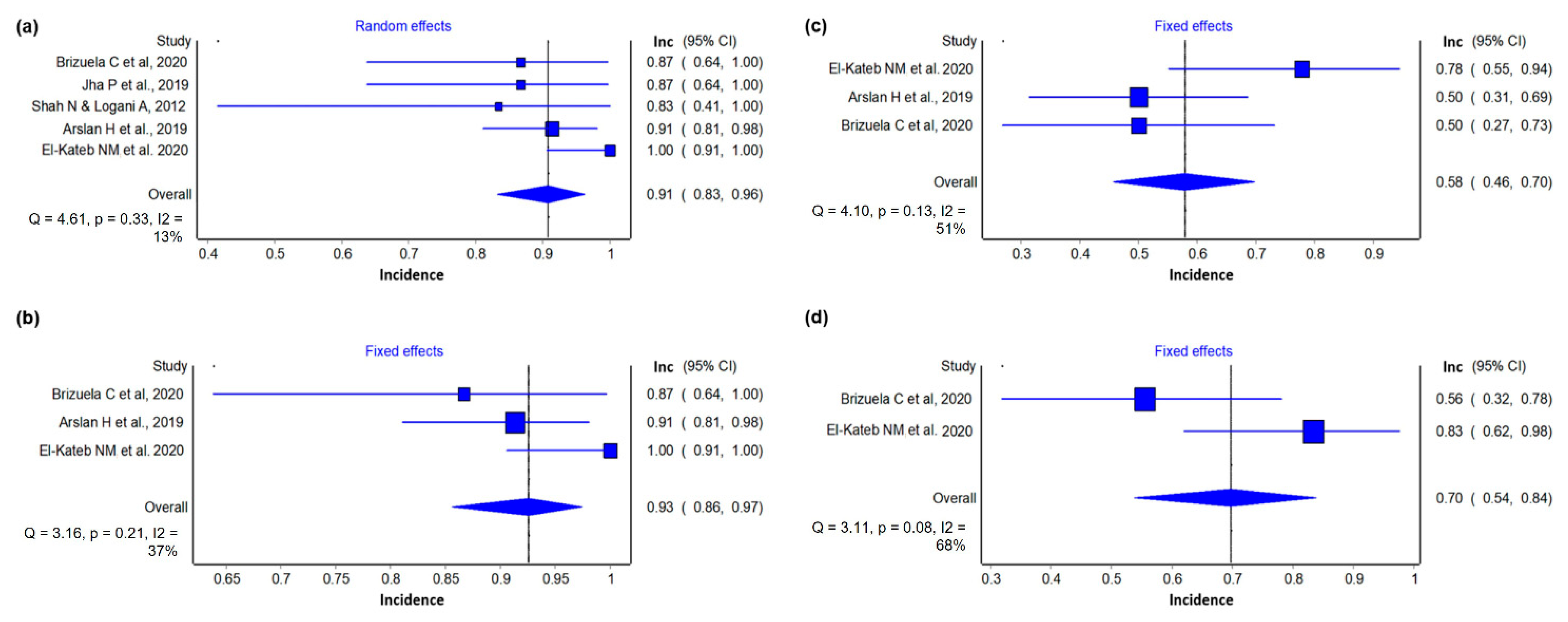
3.4.1. Success Incidence
3.4.2. Reduction of Periapical Lesion
3.4.3. Electrical Test
3.4.4. Cold Test
4. Discussion
4.1. Patients’ Age and REP
4.2. Management of Blood Clot and Derivatives
4.3. Root Canal Irrigants
4.4. Root Canal Medication
4.5. Quality Assessment
4.6. Outcomes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seltzer, S.; Bender, I.B.; Ziontz, M. The dynamics of pulp inflammation: Correlations between diagnostic data and actual histologic findings in the pulp. Oral Surg. Oral Med. Oral Pathol. 1963, 16, 969–977. [Google Scholar] [CrossRef]
- González-Plata-R, R.; González-Plata-E, W. Conventional and surgical treatment of a two-rooted maxillary central incisor. J. Endod. 2003, 29, 422–424. [Google Scholar] [CrossRef] [PubMed]
- Badole, G.P.; Warhadpande, M.M.; Bahadure, R.N.; Badole, S.G. Nonsurgical endodontic treatment of permanent maxillary incisors with immature apex and a large periapical lesion: A case report. Gen. Dent. 2015, 63, 58–60. [Google Scholar] [PubMed]
- He, L.; Kim, S.G.; Gong, Q.; Zhong, J.; Wang, S.; Zhou, X.; Ye, L. Regenerative Endodontics for Adult Patients. J. Endod. 2017, 43, S57–S64. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Wang, X.; Zhu, J.; Su, C.; Yang, Y.; Meng, L. Influence of Apical Diameter on the Outcome of Regenerative Endodontic Treatment in Teeth with Pulp Necrosis: A Review. J. Endod. 2018, 44, 414–431. [Google Scholar] [CrossRef]
- Ostby, B.N. The role of the blood clot in endodontic therapy. An experimental histologic study. Acta Odontol. Scand. 1961, 19, 324–353. [Google Scholar] [CrossRef]
- American Association of Endodontists. Endodontists: Colleagues for Excellence. J. Am. Dent. Assoc. 2013, 1–8. [Google Scholar] [CrossRef]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative Endodontics: A Review of Current Status and a Call for Action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.T.P.; Valera, M.C.; Nakashima, M.; Nör, J.E.; Bottino, M.C. Tissue-engineering-based strategies for regenerative endodontics. J. Dent. Res. 2014, 93, 1222–1231. [Google Scholar] [CrossRef]
- Nakashima, M.; Akamine, A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J. Endod. 2005, 31, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Marrelli, M.; Codispoti, B.; Shelton, R.M.; Scheven, B.A.; Cooper, P.R.; Tatullo, M.; Paduano, F. Dental Pulp Stem Cell Mechanoresponsiveness: Effects of Mechanical Stimuli on Dental Pulp Stem Cell Behavior. Front. Physiol. 2018, 9, 1685. [Google Scholar] [CrossRef]
- El Moshy, S.; Radwan, I.A.; Rady, D.; Abbass, M.M.S.; El-Rashidy, A.A.; Sadek, K.M.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Dental Stem Cell-Derived Secretome/Conditioned Medium: The Future for Regenerative Therapeutic Applications. Stem Cells Int. 2020, 2020, 7593402. [Google Scholar] [CrossRef] [Green Version]
- Ballini, A.; Boccaccio, A.; Saini, R.; Van Pham, P.; Tatullo, M. Dental-Derived Stem Cells and Their Secretome and Interactions with Bioscaffolds/Biomaterials in Regenerative Medicine: From the In Vitro Research to Translational Applications. Stem Cells Int. 2017, 2017, 6975251. [Google Scholar] [CrossRef]
- Tatullo, M.; Spagnuolo, G.; Codispoti, B.; Zamparini, F.; Zhang, A.; Esposti, M.D.; Aparicio, C.; Rengo, C.; Nuzzolese, M.; Manzoli, L.; et al. PLA-Based Mineral-Doped Scaffolds Seeded with Human Periapical Cyst-Derived MSCs: A Promising Tool for Regenerative Healing in Dentistry. Materials 2019, 12, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diogenes, A.; Henry, M.A.; Teixeira, F.B.; Hargreaves, K.M. An update on clinical regenerative endodontics. Endod. Top. 2013, 28, 2–23. [Google Scholar] [CrossRef]
- Galler, K.M.; Widbiller, M. Perspectives for Cell-homing Approaches to Engineer Dental Pulp. J. Endod. 2017, 43, S40–S45. [Google Scholar] [CrossRef] [PubMed]
- Rizk, H.M.; Salah Al-Deen, M.S.M.; Emam, A.A. Comparative evaluation of Platelet Rich Plasma (PRP) versus Platelet Rich Fibrin (PRF) scaffolds in regenerative endodontic treatment of immature necrotic permanent maxillary central incisors: A double blinded randomized controlled trial. Saudi Dent. J. 2020, 32, 224–231. [Google Scholar] [CrossRef]
- Huang, F.M.; Yang, S.F.; Zhao, J.H.; Chang, Y.C. Platelet-rich fibrin increases proliferation and differentiation of human dental pulp cells. J. Endod. 2010, 36, 1628–1632. [Google Scholar] [CrossRef]
- Tong, H.J.; Rajan, S.; Bhujel, N.; Kang, J.; Duggal, M.; Nazzal, H. Regenerative Endodontic Therapy in the Management of Nonvital Immature Permanent Teeth: A Systematic Review—Outcome Evaluation and Meta-analysis. J. Endod. 2017, 43, 1453–1464. [Google Scholar] [CrossRef]
- American Association of Endodontists. Clinical Considerations for a Regenerative Procedure; American Association of Endodontists: Chicago, IL, USA, 2018; pp. 1–6. [Google Scholar]
- Ong, T.K.; Lim, G.S.; Singh, M.; Fial, A.V. Quantitative Assessment of Root Development after Regenerative Endodontic Therapy: A Systematic Review and Meta-Analysis. J. Endod. 2020, 46, 1856–1866.e2. [Google Scholar] [CrossRef] [PubMed]
- Galler, K.M.; Krastl, G.; Simon, S.; Van Gorp, G.; Meschi, N.; Vahedi, B.; Lambrechts, P. European Society of Endodontology position statement: Revitalization procedures. Int. Endod. J. 2016, 49, 717–723. [Google Scholar] [CrossRef]
- Anna, D.; Dimitra, S.; Kleoniki, L. Regenerative procedures in mature teeth a new era in endodontics? A systematic review. Int. J. Dent. Oral Health 2020, 6, 1–13. [Google Scholar]
- Iohara, K.; Murakami, M.; Nakata, K.; Nakashima, M. Age-dependent decline in dental pulp regeneration after pulpectomy in dogs. Exp. Gerontol. 2014, 52, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Glynis, A.; Foschi, F.; Kefalou, I.; Koletsi, D.; Tzanetakis, G.N. Regenerative Endodontic Procedures for the Treatment of Necrotic Mature Teeth with Apical Periodontitis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Endod. 2021, 47, 873–882. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Sideri, S.; Papageorgiou, S.N.; Eliades, T. Registration in the international prospective register of systematic reviews (PROSPERO) of systematic review protocols was associated with increased review quality. J. Clin. Epidemiol. 2018, 100, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Kelly, A.M.; Cronin, P. How to perform a critically appraised topic: Part 1, ask, search, and apply. Am. J. Roentgenol. 2011, 197, 1039–1047. [Google Scholar] [CrossRef]
- Mohadeb, J.V.N.; Somar, M.; He, H. Effectiveness of decoronation technique in the treatment of ankylosis: A systematic review. Dent. Traumatol. 2016, 32, 255–263. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Chichester, UK, 2019. [Google Scholar]
- Lisboa, C.O.; Martins, M.M.; Ruellas, A.C.O.; Ferreira, D.M.T.P.; Maia, L.C.; Mattos, C.T. Soft tissue assessment before and after mandibular advancement or setback surgery using three-dimensional images: Systematic review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 1389–1397. [Google Scholar] [CrossRef]
- Borenstein, M. Common Mistakes in Meta-Analysis and How to Avoid Them; Biostat Inc.: Englewood, NJ, USA, 2019. [Google Scholar]
- Iorio, A.; Spencer, F.A.; Falavigna, M.; Alba, C.; Lang, E.; Burnand, B.; McGinn, T.; Hayden, J.; Williams, K.; Shea, B.; et al. Use of GRADE for assessment of evidence about prognosis: Rating confidence in estimates of event rates in broad categories of patients. BMJ 2015, 350, h870. [Google Scholar] [CrossRef] [Green Version]
- Ryan, R.; Hill, S. How to GRADE the Quality of the Evidence 2016. Available online: http://cccrg.cochraneorg/author-resources (accessed on 24 November 2020).
- Shah, N.; Logani, A. SealBio: A novel, non-obturation endodontic treatment based on concept of regeneration. J. Conserv. Dent. 2012, 15, 328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrepa, V.; Henry, M.A.; Daniel, B.J.; Diogenes, A. Delivery of Apical Mesenchymal Stem Cells into Root Canals of Mature Teeth. J. Dent. Res. 2015, 94, 1653–1659. [Google Scholar] [CrossRef]
- Shah, N. A regeneration-based, nonobturation root-canal treatment for fully-mature teeth: Six years experience with «sealBio». Contemp. Clin. Dent. 2016, 7, 296–301. [Google Scholar] [CrossRef]
- Nageh, M.; Ahmed, G.M.; El-baz, A.A. Assessment of Regaining Pulp Sensibility in Mature Necrotic Teeth Using a Modified Revascularization Technique with Platelet-rich Fibrin: A Clinical Study. J. Endod. 2018, 44, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.; Ahmed, H.M.A.; Şahin, Y.; Doğanay Yıldız, E.; Gündoğdu, E.C.; Güven, Y.; Khalilov, R. Regenerative Endodontic Procedures in Necrotic Mature Teeth with Periapical Radiolucencies: A Preliminary Randomized Clinical Study. J. Endod. 2019, 45, 863–872. [Google Scholar] [CrossRef] [PubMed]
- Jha, P.; Virdi, M.S.; Nain, S. A Regenerative Approach for Root Canal Treatment of Mature Permanent Teeth: Comparative Evaluation with 18 Months Follow-up. Int. J. Clin. Pediatr. Dent. 2019, 12, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Brizuela, C.; Meza, G.; Urrejola, D.; Quezada, M.A.; Concha, G.; Ramírez, V.; Angelopoulos, I.; Cadiz, M.I.; Tapia-Limonchi, R.; Khoury, M. Cell-Based Regenerative Endodontics for Treatment of Periapical Lesions: A Randomized, Controlled Phase I/II Clinical Trial. J. Dent. Res. 2020, 99, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Doumani, M.; Seirawan, M.Y.; Layous, K.; Seirawan, M.K. Coronal Discoloration Related to Bioceramic and Mineral Trioxide Aggregate Coronal Barrier in Non-vital Mature Teeth Undergoing Regenerative Endodontic Procedures. World J. Dent. 2020, 11, 52–60. [Google Scholar] [CrossRef]
- El-Kateb, N.M.; El-Backly, R.N.; Amin, W.M.; Abdalla, A.M. Quantitative Assessment of Intracanal Regenerated Tissues after Regenerative Endodontic Procedures in Mature Teeth Using Magnetic Resonance Imaging: A Randomized Controlled Clinical Trial. J. Endod. 2020, 46, 563–574. [Google Scholar] [CrossRef]
- Estefan, B.S.; El Batouty, K.M.; Nagy, M.M.; Diogenes, A. Influence of Age and Apical Diameter on the Success of Endodontic Regeneration Procedures. J. Endod. 2016, 42, 1620–1625. [Google Scholar] [CrossRef]
- Huang, G.T.J.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/Progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.J. Characterization of the Apical Papilla and Its Residing Stem Cells from Human Immature Permanent Teeth: A Pilot Study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chng, H.S.; Pitt Ford, T.R.; McDonald, F. Effects of Prilocaine local anaesthetic solutions on pulpal blood flow in maxillary canines. Endod. Dent. Traumatol. 1996, 12, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, E.S.; Mourão, C.F.d.A.B.; Leite, P.E.C.; Granjeiro, J.M.; Calasans-Maia, M.D.; Alves, G.G. The in vitro release of cytokines and growth factors from fibrin membranes produced through horizontal centrifugation. J. Biomed. Mater. Res. A 2018, 106, 1373–1380. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; Inchingolo, A.D.; Dipalma, G.; Flace, P.; Girolamo, F.; Tarullo, A.; Laino, L.; et al. Regenerative surgery performed with platelet-rich plasma used in sinus lift elevation before dental implant surgery: An useful aid in healing and regeneration of bone tissue. Eur. Revr. Med. Pharmacol. Sci. 2012, 16, 1222–1226. [Google Scholar]
- Blokhuis, T.J.; Arts, J.J.C. Bioactive and osteoinductive bone graft substitutes: Definitions, facts and myths. Injury 2011, 42, 26–29. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, Y.; Chen, Y.; Chen, S.; Lyu, H.; Cai, Z.; Huang, X. Radiographic, Histologic, and Biomechanical Evaluation of Combined Application of Platelet-rich Fibrin with Blood Clot in Regenerative Endodontics. J. Endod. 2017, 43, 2034–2040. [Google Scholar] [CrossRef]
- Galler, K.M.; D’Souza, R.N.; Federlin, M.; Cavender, A.C.; Hartgerink, J.D.; Hecker, S.; Schmalz, G. Dentin conditioning codetermines cell fate in regenerative endodontics. J. Endod. 2011, 37, 1536–1541. [Google Scholar] [CrossRef]
- Pang, N.S.; Lee, S.J.; Kim, E.; Shin, D.M.; Cho, S.W.; Park, W.; Zhang, X.; Jung, I.Y. Effect of EDTA on attachment and differentiation of dental pulp stem cells. J. Endod. 2014, 40, 811–817. [Google Scholar] [CrossRef]
- Trevino, E.G.; Patwardhan, A.N.; Henry, M.A.; Perry, G.; Dybdal-Hargreaves, N.; Hargreaves, K.M.; Diogenes, A. Effect of irrigants on the survival of human stem cells of the apical papilla in a platelet-rich plasma scaffold in human root tips. J. Endod. 2011, 37, 1109–1115. [Google Scholar] [CrossRef]
- Begue-Kirn, C.; Smith, A.J.; Ruch, J.V.; Wozney, J.M.; Purchio, A.; Hartmann, D.; Lesot, H. Effects of dentin proteins, transforming growth factor β1 (TGFβ1) and bone morphogenetic protein 2 (BMP2) on the differentiation of odontoblast in vitro. Int. J. Dev. Biol. 1992, 36, 491–503. [Google Scholar] [CrossRef]
- Roberts-Clark, D.J.; Smith, A.J. Angiogenic growth factors in human dentine matrix. Arch. Oral. Biol. 2000, 45, 1013–1016. [Google Scholar] [CrossRef]
- Zhao, S.; Sloan, A.J.; Murray, P.E.; Lumley, P.J.; Smith, A.J. Ultrastructural localisation of TGF-β exposure in dentine by chemical treatment. Histochem. J. 2000, 32, 489–494. [Google Scholar] [CrossRef]
- Murvindran, V.; Raj, J.D. Antibiotics as an intracanal medicament in endodontics. J. Pharm Sci. Res. 2014, 6, 297–301. [Google Scholar]
- Hoshino, E.; Kurihara-Ando, N.; Sato, I.; Uematsu, H.; Sato, M.; Kota, K.; Iwaku, M. In-vitro antibacterial susceptibility of bacteria taken from infected root dentine to a mixture of ciprofloxacin, metronidazole and minocycline. Int. Endod. J. 1996, 29, 125–130. [Google Scholar] [CrossRef]
- Althumairy, R.I.; Teixeira, F.B.; Diogenes, A. Effect of Dentin Conditioning with Intracanal Medicaments on Survival of Stem Cells of Apical Papilla. J. Endod. 2014, 40, 521–525. [Google Scholar] [CrossRef]
- Montero-Miralles, P.; Martín-González, J.; Alonso-Ezpeleta, O.; Jiménez-Sánchez, M.C.; Velasco-Ortega, E.; Segura-Egea, J.J. Effectiveness and clinical implications of the use of topical antibiotics in regenerative endodontic procedures: A review. Int. Endod. J. 2018, 51, 981–988. [Google Scholar] [CrossRef] [Green Version]
- Song, M.; Cao, Y.; Shin, S.J.; Shon, W.J.; Chugal, N.; Kim, R.H.; Kim, E.; Kang, M.K. Revascularization-associated Intracanal Calcification: Assessment of Prevalence and Contributing Factors. J. Endod. 2017, 43, 2025–2033. [Google Scholar] [CrossRef] [PubMed]
- Guidance Before-and-after Study: Comparative Studies. Available online: https://www.gov.uk/guidance/before-and-after-study-comparativestudies (accessed on 20 November 2020).
- Alghaithy, R.A.; Qualtrough, A.J. Pulp sensibility and vitality tests for diagnosing pulpal health in permanent teeth: A critical review. Int. Endod. J. 2017, 50, 135–142. [Google Scholar] [CrossRef] [Green Version]

| Author/Year of Publication | N. of Participants | Tooth | Control | Regerating Material | Irrigating Solution | Intracanal Medication | Apical File | Internal Sealing | External Sealing | Follow-Up | At 2nd Visit | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anesthetic | Period | ||||||||||||
| Brizuela C et al., 2020 a | 36 11♂ 25♀ (16–58 y) | Incisors, canines, mandibular premolars | Guta percha conventional obturation | PPP umbilical cord mesenchymal stem cells | 2.5% NaOCl EDTA | Calcium hydroxide | #8 K-file (bleeding) | Absorbable gelatin sponge hemostats (Gelita-Spon® GmbH, Eberbach, Germany) and Biodentine (Septodont, France) | Resin (Filtek™ Z350 XT Universal Restorative; 3M ESPE, St Paul, MN, USA) | 6 and 12 months | Not reported | 3 weeks | Radiography and Cone-beam computed tomography (CBCT). Vitality by Laser Doppler Flowmetry (LDF) and the Perfusion Unit (PU) percentage. Cold Test: 56% were responsive. Hot test: 28% were responsive. Eletric Test: 50% responsive. Cortical compromise: 89% not present. |
| El-Kateb NM et al., 2020 a | 18 (20–34 y) | 17 maxillary central incisors and 1 lateral incisor | X5 Blood clot | X3 Blood clot | 1.5% NaOCl 17% EDTA | Ultracal XS calcium hydroxide (Ultradent Products GmbH, South Jordan, UT) | Widening Protaper next X3 and Protaper next X5 K-file #25 (bleeding) | Biodentine (Septodont, Saint-Maur-des-Fosses, France) | GIC + resin composite | 1, 3, 6, 9, and 12 months | 3% mepivacaine without vasoconstrictor | Not reported | Clinical evaluation, digital radiographs, magnetic resonance imaging (MRI), sensitivity test (cold test and electrical test). 60% of the cases regained sensitivity after 12 months. |
| Arslan H et al., 2019 a | 46 35♂ 11♀ (18–30 y) | 32 maxillary anterior teeth + 16 mandibular anterior teeth | Guta percha+ epoxy resin– based sealer (2Seal; VDW, Munich, Germany) | Blood clot | 1% NaOCl 2 mL 5% EDTA distilled water | Triple antibiotic paste (doxycycline, metronidazole, and ciprofloxacin) | K-file #25 (bleeding) | MTA | MTA + resin composite material (Universal Restorative 200, 3M ESPE) | 12 months | Isocaine 3%; (Novocol, ON, Canada) | 3 weeks | Clinical and radiograph evaluation, EletricalTest. 92.3% successful cases and 50% positive response to EPT in REP. |
| Jha P et al., 2019 a | 30 (9–15 y) | 30 | Guta percha conventional obturation | SealBio | 2.5% NaOCl EDTA | Triple antibiotic paste (ciprofloxacin, metronidazole and minocycline) | Widening K-files #25–#30 #20 K-file (bleeding) | Calcium sulfate-based cement (Cavit G) | Not reported | 6, 12, and 18 months | 3% mepivacaine without adrenaline | 1 or 2 weeks | Clinical and radiographic evaluation. 86.66% of the teeth were considered completely cured in group I (SealBio) and 80% in group II (obturation). |
| Nageh M et al., 2018 b | 15 (18–40 y) | Maxillary central incisor | No | PRF e Blood clot | 1.5% NaOCl 17% EDTA | Double antibiotic paste (DAP) (metronidazol, ciprofloxacin) | K-files #20–#40 (Widening and bleeding) | MTA | GIC base and resin composite | Every 3 months for a follow-up of 1 year | 3% mepivacaine | 3 weeks | Thermal (cold) and electrical tests were used. 60% of the teeth had vital pulp and 40% partial vitality after 12 months. |
| Shah N, 2016 b | 116 76♂ 40♀ (12–80 y) | 134 | No | SealBio | 2.5% NaOCl. Final wash with Betadine | Triple antibiotic paste (ciprofloxacin, metronidazole, tetracycline) or calcium hydroxide | Widening K-files #25 K-files #20 (bleeding) | Calcium sulfate-based cement (Cavit, 3M ESPE USA) | Silver amalgam/ composite/and full coverage coronal restoration | Every 6 months for a follow-up of 6 years | Not reported | 5–7 days | Clinical and radiographic evaluation. 16 cases were lost to follow-up. Approximately 97% of cases treated with the new technique were successful. |
| Shah N and Logani A 2012 b | 18 11♂ 7♀ (15–76 y) | Not reported | No | SealBio | 2.5% NaOCl | Triple antibiotic paste (metrogyl, ciprofloxacin and tetracycline) | Widening K-files #25-#30 K-files #20(bleeding) | Calcium sulfate-based cement (Cavit, 3M ESPE USA) | Not reported | Every 6 months for a follow-up of 3 years | Not reported | Not reported | Lesion size, bone, and cementum density in HU and periapical index (CBCT-PAI). Remarkable decrease in the lesion size and increase in bone and cementum density were documented. |
| Scheme 2020. | Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall |
|---|---|---|---|---|---|---|
| Brizuela et al. 2020 [37] |  |  |  |  |  |  |
| El-Kateb et al. 2020 [39] |  |  |  |  |  |  |
| Arslan et al. 2019 [35] |  |  |  |  |  |  |
| Jha et al. 2019 [36] |  |  |  |  |  |  |
 Low risk Low risk  Some concerns Some concerns  High risk High risk | ||||||
| Certainty Assessment | Certainty | |||||||
|---|---|---|---|---|---|---|---|---|
| No. of Studies | Study Design | Risk of Bias | Inconsistency | Indirectness | Imprecision | Other Considerations | No. of Mature Necrotic Teeth | |
| Success | ||||||||
| 7 | Before and after | Serious a | Serious b | Not serious | Not serious | None | 228 | ⨁◯◯◯ VERY LOW |
| Reduction of periapical lesion (last follow-up) | ||||||||
| 5 | Before and after | Serious a | Not serious | Not serious | Serious c | None | 100 | ⨁◯◯◯ VERY LOW |
| Reduction of periapical lesion (12 months) | ||||||||
| 3 | Before and after | Serious a | Serious b | Serious d | Serious c | None | 79 | ⨁◯◯◯ VERY LOW |
| Electrical pulp test | ||||||||
| 3 | Before and after | Serious a | Very serious b,e | Serious d | Serious c | None | 64 | ⨁◯◯◯ VERY LOW |
| Cold pulp test | ||||||||
| 2 | Before and after | Serious a | Very serious b,e | Serious f | Serious c | None | 36 | ⨁◯◯◯ VERY LOW |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scelza, P.; Gonçalves, F.; Caldas, I.; Nunes, F.; Lourenço, E.S.; Tavares, S.; Magno, M.; Pintor, A.; Montemezzi, P.; Edoardo, E.D.; et al. Prognosis of Regenerative Endodontic Procedures in Mature Teeth: A Systematic Review and Meta-Analysis of Clinical and Radiographic Parameters. Materials 2021, 14, 4418. https://doi.org/10.3390/ma14164418
Scelza P, Gonçalves F, Caldas I, Nunes F, Lourenço ES, Tavares S, Magno M, Pintor A, Montemezzi P, Edoardo ED, et al. Prognosis of Regenerative Endodontic Procedures in Mature Teeth: A Systematic Review and Meta-Analysis of Clinical and Radiographic Parameters. Materials. 2021; 14(16):4418. https://doi.org/10.3390/ma14164418
Chicago/Turabian StyleScelza, Pantaleo, Fabiano Gonçalves, Isleine Caldas, Fernanda Nunes, Emanuelle Stellet Lourenço, Sandro Tavares, Marcela Magno, Andrea Pintor, Pietro Montemezzi, Emanuele Di Edoardo, and et al. 2021. "Prognosis of Regenerative Endodontic Procedures in Mature Teeth: A Systematic Review and Meta-Analysis of Clinical and Radiographic Parameters" Materials 14, no. 16: 4418. https://doi.org/10.3390/ma14164418
APA StyleScelza, P., Gonçalves, F., Caldas, I., Nunes, F., Lourenço, E. S., Tavares, S., Magno, M., Pintor, A., Montemezzi, P., Edoardo, E. D., Mourão, C. F. d. A. B., Alves, G., & Scelza, M. Z. (2021). Prognosis of Regenerative Endodontic Procedures in Mature Teeth: A Systematic Review and Meta-Analysis of Clinical and Radiographic Parameters. Materials, 14(16), 4418. https://doi.org/10.3390/ma14164418







