Unprecedented Use of NHC Gold (I) Complexes as Catalysts for the Selective Oxidation of Ethane to Acetic Acid
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
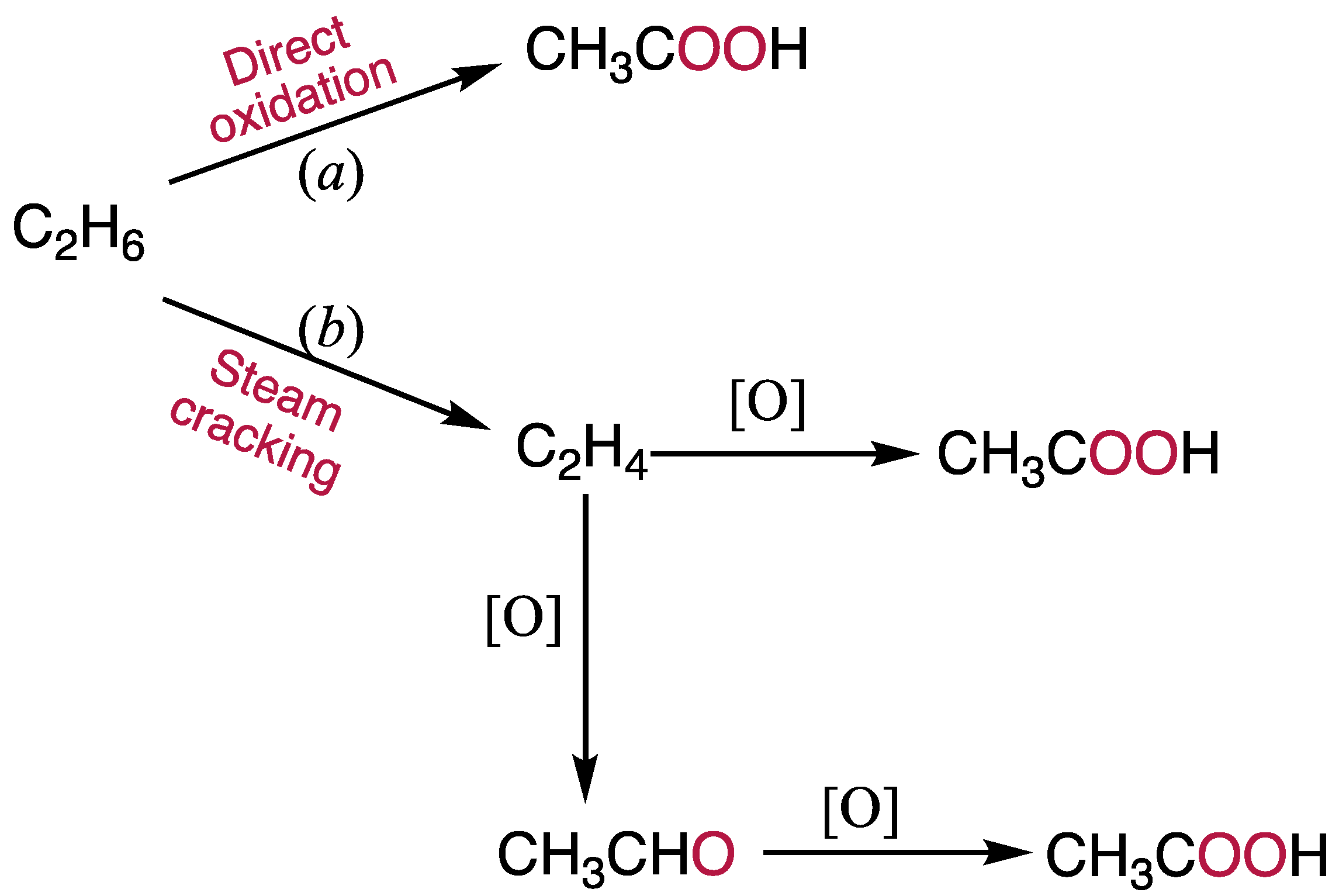
3.1. Direct Oxidation of Ethane to Acetic Acid
3.2. Green Metrics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peris, E. Smart N-heterocyclic carbene ligands in catalysis. Chem. Rev. 2018, 118, 9988–10031. [Google Scholar] [CrossRef]
- Marion, N.; Nolan, S.P. N-Heterocyclic carbenes in gold catalysis. Chem. Soc. Rev. 2008, 37, 1776–1782. [Google Scholar] [CrossRef] [PubMed]
- Tavakkolifard, S.; Sekine, K.; Reichert, L.; Ebrahimi, M.; Museridz, K.; Michel, E.; Rominger, F.; Babaahmadi, R.; Ariafard, A.; Yates, B.; et al. Gold-Catalyzed Regiospecific Annulation of Unsymmetrically Substituted 1, 5-Diynes for the Precise Synthesis of Bispentalenes. Chemistry 2019, 25, 12180–12186. [Google Scholar] [CrossRef] [Green Version]
- Plajer, A.; Ahrens, L.; Wieteck, M.; Lustosa, D.M.; Ahmadi, R.B.; Yates, B.; Ariafard, A.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. Different Selectivities in the Insertions into C (sp2)− H Bonds: Benzofulvenes by Dual Gold Catalysis Competition Experiments. Chem. Eur. J. 2018, 24, 10766–10772. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Lauterbach, T.; Nösel, P.; Vilhelmsen, M.H.; Rudolph, M.; Rominger, F. Dual Gold Catalysis: σ, π-Propyne Acetylide and Hydroxyl-Bridged Digold Complexes as Easy-To-Prepare and Easy-To-Handle Precatalysts. Chem. Eur. J. 2013, 19, 1058–1065. [Google Scholar] [CrossRef]
- Martins, L.M. Alkane oxidation with C-scorpionate metal complexes. In Alkane Functionalization; Pombeiro, A.J.L., de Silva, M.d.F.C.G., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2019; Chapter 6; pp. 173–187. [Google Scholar]
- Martins, L.M. C-scorpionate complexes: Ever young catalytic tools. Coord. Chem. Rev. 2019, 396, 89–102. [Google Scholar] [CrossRef]
- Global Acetic Acid Market to Reach 11.85 Million Tons by 2026. Available online: https://www.expertmarketresearch.com/pressrelease/global-acetic-acid-market (accessed on 3 June 2021).
- Bohnet, M. Ullmann’s Encyclopedia of Industrial Chemistry, 6th ed.; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Sunley, G.J.; Watson, D.J. High productivity methanol carbonylation catalysis using iridium: The Cativa™ process for the manufacture of acetic acid. Catal. Today 2000, 58, 293–307. [Google Scholar] [CrossRef]
- Schultz, R.G.; Montgome, P.D. Vapor phase carbonylation of methanol to acetic acid. J. Catal. 1969, 13, 105–106. [Google Scholar] [CrossRef]
- Yoneda, N.; Kusano, S.; Yasui, M.; Pujado, P.; Wilcher, S. Recent advances in processes and catalysts for the production of acetic acid. Appl. Catal. A Gen. 2001, 221, 253–265. [Google Scholar] [CrossRef]
- Boronat, M.; Martínez-Sánchez, C.; Law, D.; Corma, A. Enzyme-like specificity in zeolites: A unique site position in mordenite for selective carbonylation of methanol and dimethyl ether with CO. J. Am. Chem. Soc. 2008, 130, 16316–16323. [Google Scholar] [CrossRef]
- Cheung, P.; Bhan, A.; Sunley, G.J.; Iglesia, E. Selective carbonylation of dimethyl ether to methyl acetate catalyzed by acidic zeolites. Angew. Chem. Int. Ed. 2006, 45, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Cheung, P.; Bhan, A.G.; Sunley, J.; Law, D.J.; Iglesia, E. Site requirements and elementary steps in dimethyl ether carbonylation catalyzed by acidic zeolites. J. Catal. 2007, 245, 110–123. [Google Scholar] [CrossRef]
- Nishiguchi, T.; Nakata, K.; Takaki, K.; Fujiwara, Y. Transition metal catalyzed acetic acid synthesis from methane and CO. Chem. Lett. 1992, 21, 1141–1142. [Google Scholar] [CrossRef]
- Silva, T.F.; Luzyanin, K.V.; Kirillova, M.V.; Silva, M.F.G.; Martins, L.M.; Pombeiro, A.J. Novel scorpionate and pyrazole dioxovanadium complexes, catalysts for carboxylation and peroxidative oxidation of alkanes. Adv. Synth. Catal. 2010, 352, 171–187. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; Kuznetsov, M.L.; Silva, J.A.; da Silva, J.J.F.; Pombeiro, A.J. Alkanes to carboxylic acids in aqueous medium: Metal-free and metal-promoted highly efficient and mild conversions. Chem. Commun. 2009, 17, 2353–2355. [Google Scholar] [CrossRef]
- Kirillova, M.V.; da Silva, J.A.; da Silva, J.J.F.; Pombeiro, A.J. Direct and efficient transformation of gaseous alkanes into carboxylic acids catalyzed by vanadium containing heteropolyacids. Appl. Catal. A Gen. 2007, 332, 159–165. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y.T.; Fung, V.; Jiang, D.E.; Huang, W.X.; Zhang, S.R.; Iwasawa, Y.; Sakata, T.; Nguyen, L.; Zhang, X.Y.; et al. Single rhodium atoms anchored in micropores for efficient transformation of methane under mild conditions. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Smejkal, Q.; Linke, D.; Baerns, M. Energetic and economic evaluation of the production of acetic acid via ethane oxidation. Chem. Eng. Process. Process Intensif. 2005, 44, 421–428. [Google Scholar] [CrossRef]
- Alegria, E.C.B.A.; Kirillova, M.V.; Martins, L.M.; Pombeiro, A.J.L. Pyrazole and trispyrazolylmethane rhenium complexes as catalysts for ethane and cyclohexane oxidations. Appl. Catal. A Gen. 2007, 317, 43–52. [Google Scholar] [CrossRef]
- Armstrong, R.D.; Hutchings, G.J.; Taylor, S.H. An overview of recent advances of the catalytic selective oxidation of ethane to oxygenates. Catalysts 2016, 6, 71. [Google Scholar] [CrossRef] [Green Version]
- Jin, R.; Peng, M.; Li, A.; Deng, Y.; Jia, Z.; Huang, F.; Ling, Y.; Yang, F.; Fu, H.; Xie, J.; et al. Low temperature oxidation of ethane to oxygenates by oxygen over iridium-cluster catalysts. J. Am. Chem. Soc. 2019, 141, 18921–18925. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, T.; Sato, K.; Tsunoda, T.; Suzuki, K.; Shimizu, M.; Takehira, K.J. Partial oxidation of ethane into acetaldehyde by active oxygen generated electrochemically on gold through yttria-stabilized zirconia. Chem. Soc. Chem. Commun. 1994, 1743–1744. [Google Scholar] [CrossRef]
- Strassner, T. The Role of NHC Ligands in Oxidation Catalysis. In Organometallic Oxidation Catalysis; Meyer, F., Limberg, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 22, pp. 125–148. [Google Scholar]
- Zargaran, P.; Wurm, T.; Zahner, D.; Schiessl, J.; Rudolph, M.; Rominger, F.; Hashmi, A.S.K. A Structure-Based Activity Study of Highly Active Unsymmetrically Substituted NHC Gold (I) Catalysts. Adv. Synth. Catal. 2018, 360, 106–111. [Google Scholar] [CrossRef]
- Burkert, U.; Allinger, N. Molecular Mechanics; (ACS Mongraph 177); ACS: Washington, DC, USA, 1982. [Google Scholar]
- Bourissou, D.; Guerret, O.; Gabbai, F.P.; Bertrand, G. Stable carbenes. Chem. Rev. 2000, 100, 39–92. [Google Scholar] [CrossRef]
- Kirillov, A.M.; Haukka, M.; Kirillova, M.V.; Pombeiro, A.J. Single-Pot Ethane Carboxylation Catalyzed by New Oxorhenium (V) Complexes with N, O Ligands. Adv. Synth. Catal. 2005, 347, 1435–1446. [Google Scholar] [CrossRef]
- Kirillova, M.V.; Kirillov, A.M.; Reis, P.M.; Silva, J.A.; Silva, J.J.F.; Pombeiro, A.J.L. Group 5–7 transition metal oxides as efficient catalysts for oxidative functionalization of alkanes under mild conditions. J. Catal. 2007, 248, 130–136. [Google Scholar] [CrossRef]
- Anastas, P.T. Green Metrics. In Handbook of Green Chemistry; Constable, D.J.C., Gonzales, C.J., Eds.; Wiley-VCH: Weinheim, Germany, 2018; Volume 11. [Google Scholar]
- Sheldon, R.A. The E factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Constable, D.J.; Curzons, A.D.; Cunningham, V.L. Metrics to ‘green’ chemistry—Which are the best? Green Chem. 2002, 4, 521–527. [Google Scholar] [CrossRef]
- Phan, T.T.; Gallardo, C.; Mane, J. GREEN MOTION: A new and easy to use green chemistry metric from laboratories to industry. Green Chem. 2015, 17, 2846–2852. [Google Scholar] [CrossRef]
- Dunn, P.J. The importance of green chemistry in process research and development. Chem. Soc. Rev. 2012, 41, 1452–1461. [Google Scholar] [CrossRef]

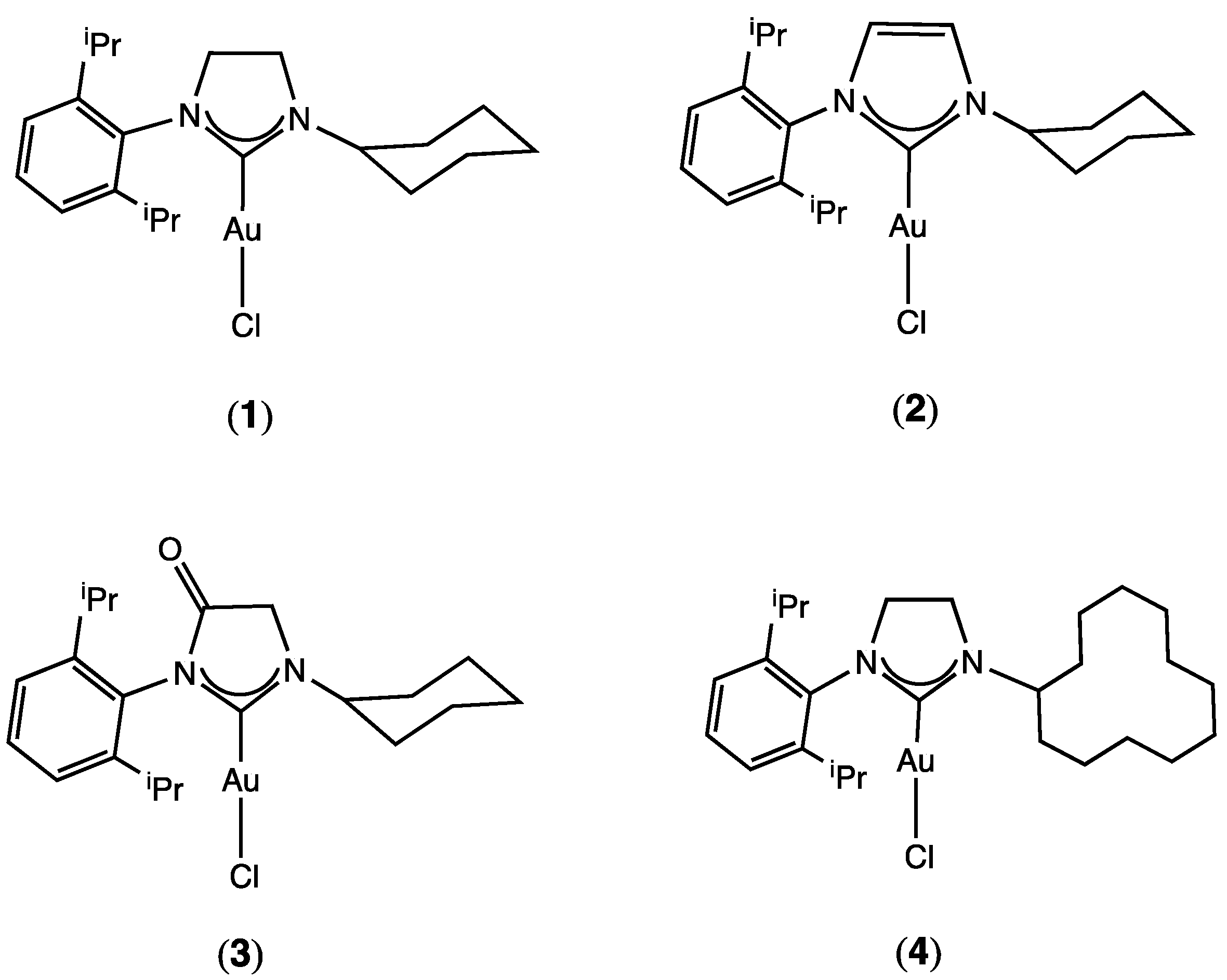

| Entry | Catalyst | C2H6 | Catalyst Amount (μmol) | Yield (%) a | TON b | ||
|---|---|---|---|---|---|---|---|
| (atm) | Acetic Acid | Propionic Acid | Acetic Acid | Propionic Acid | |||
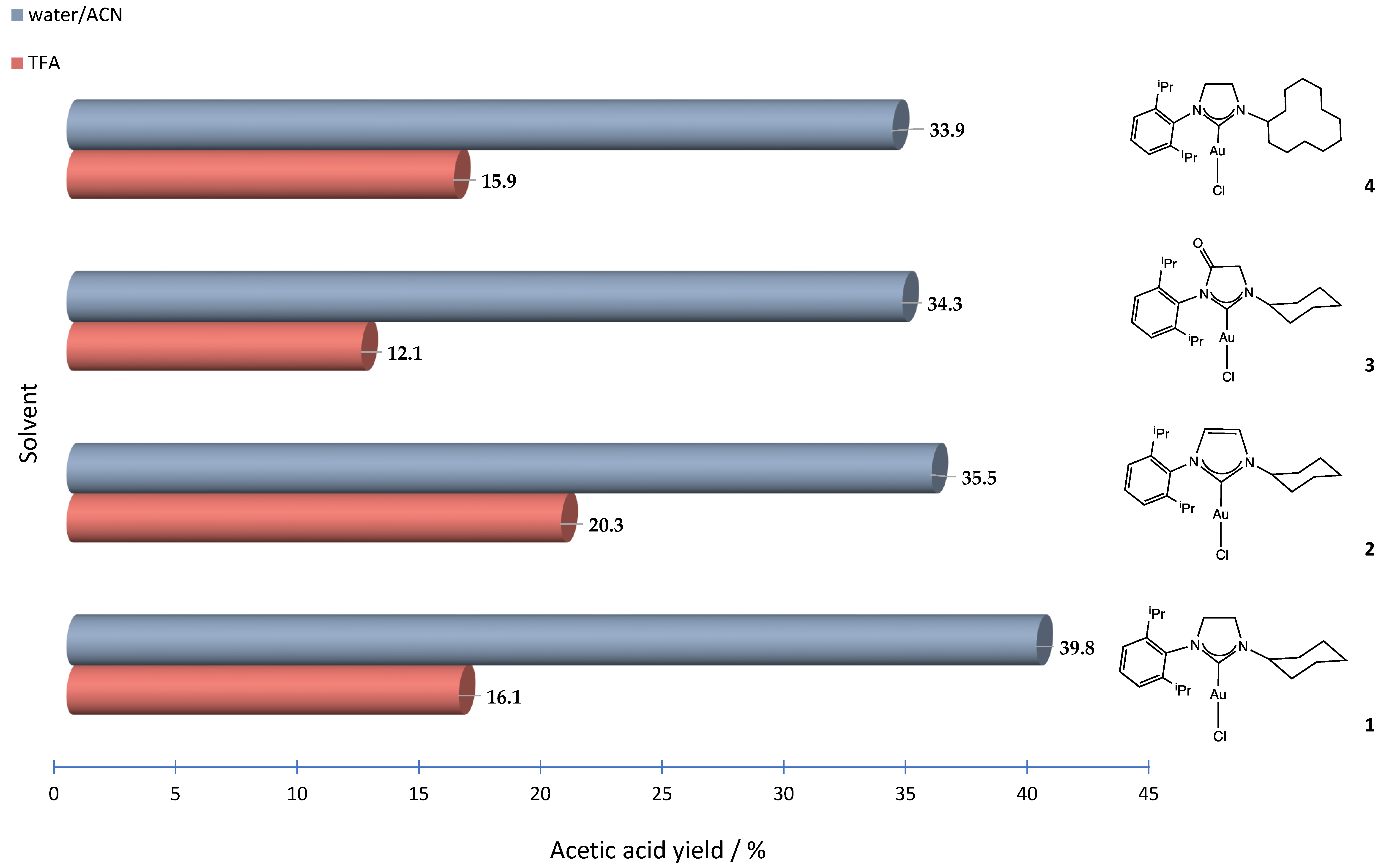
| 1 | 1 | 3 | 1.5 | 39.8 | 210 | ||
| 2 | 2 | 3 | 1.5 | 35.5 | 187 | ||
| 3 | 3 | 3 | 1.5 | 34.3 | 181 | ||
| 4 | 4 | 3 | 1.5 | 33.9 | 178 | ||
| 5 | 3 | 2.2 | |||||
| 6 c | 1 | 3 | 1.5 | ||||
| 7 d | 1 | 3 | 1.5 | 13.4 | 67 | ||
| Entry | Catalyst | C2H6 | Catalyst Amount (μmol) | Yield (%) a | TON b | Ref. | ||
|---|---|---|---|---|---|---|---|---|
| (atm) | Acetic Acid | Propionic Acid | Acetic Acid | Propionic Acid | ||||
| 1 | 1 | 3 | 1.5 | 16.1 | 18 | this work | ||
| 2 | 2 | 3 | 1.5 | 20.3 | 22 | |||
| 3 | 3 | 3 | 1.5 | 12.1 | 13 | |||
| 4 | 4 | 3 | 1.5 | 15.9 | 18 | |||
| 5 | [ReCl2{κ2-N,O-N2C(O)Ph}(PPh3)2] | 3 | 20 | 3.3 | 2 | 22 | ||
| 6 | [ReCl2{N2C(O)Ph}(Hpz)(PPh3)2] | 3 | 20 | 3.7 | 7 | |||
| 7 | [ReCl2{N2C(O)Ph}(Hpz)2(PPh3)] | 3 | 10 | 24.1 | 0.7 | 19 | 0.6 | |
| 8 | [ReOCl3(PPh3)2] | 3 | 20 | 14.0 | 3.0 | 6 | 1.4 | |
| 9 | [ReCl3{κ3-HC(pz)3}] | 3 | 20 | 5.1 | 2 | |||
| 10 | [ReO3{κ3-SO3C(pz)3}] | 3 | 20 | 27.9 | 1.0 | 13 | 0.4 | |
| 12 | [ReClF{N2C(O)Ph}(Hpz)2(PPh3)] | 5 | 20 | 40.9 | 5.4 | 31 | 4.0 | |
| 13 | [ReOCl2(C5H4N(COO))(PPh3)] | 10 | 20 | 5.0 | 1.3 | 7 | 2.9 | 30 |
| 14 | Na2[MoO4] | 3 | 20 | 25.0 | 63 | 31 | ||
| 15 | Nb2O5 | 1.5 | 20 | 18.0 | 10 | |||
| 16 | H4[PMo11VO40]·34H2O | 5 | 2.5 | 36.3 | 2.8 | 222 | 17 | 19 |
| 17 | H5[PMo10V2O40]·32H2O | 5 | 2.5 | 20.2 | 3.2 | 124 | 20 | |
| 18 | H6[PMo9V3O40]·34H2O | 5 | 2.5 | 35.2 | 2.9 | 216 | 18 | |
| Entry | Solvent | Catalyst | Theoretical E-Factor | E-Factor | AE (%) | MI | RME | AU (%) | f |
|---|---|---|---|---|---|---|---|---|---|
| 1 | H2O/ACN | 1 | 0.2 | 0.5 | 20.0 | 36.5 | 66.5 | 100 | 107.1 |
| 2 | TFA | 2.7 | 90.3 | 26.9 | 696.8 | ||||
| 3 | H2O/ACN | 2 | 0.2 | 0.7 | 20.0 | 41.0 | 59.4 | 100 | 120.1 |
| 4 | TFA | 1.9 | 71.6 | 34.0 | 552.6 | ||||
| 5 | H2O/ACN | 3 | 0.2 | 0.8 | 20.0 | 42.4 | 57.1 | 100 | 124.3 |
| 6 | TFA | 4.0 | 120.2 | 20.1 | 927.2 | ||||
| 7 | H2O/ACN | 4 | 0.2 | 0.8 | 20.0 | 42.9 | 55.3 | 100 | 125.8 |
| 8 | TFA | 2.9 | 91.6 | 25.9 | 705.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.P.C.; Matias, I.A.S.; Zargaran, P.; Hashmi, A.S.K.; Martins, L.M.D.R.S. Unprecedented Use of NHC Gold (I) Complexes as Catalysts for the Selective Oxidation of Ethane to Acetic Acid. Materials 2021, 14, 4294. https://doi.org/10.3390/ma14154294
Ribeiro APC, Matias IAS, Zargaran P, Hashmi ASK, Martins LMDRS. Unprecedented Use of NHC Gold (I) Complexes as Catalysts for the Selective Oxidation of Ethane to Acetic Acid. Materials. 2021; 14(15):4294. https://doi.org/10.3390/ma14154294
Chicago/Turabian StyleRibeiro, Ana P. C., Inês A. S. Matias, Poorya Zargaran, A. Stephen K. Hashmi, and Luísa M. D. R. S. Martins. 2021. "Unprecedented Use of NHC Gold (I) Complexes as Catalysts for the Selective Oxidation of Ethane to Acetic Acid" Materials 14, no. 15: 4294. https://doi.org/10.3390/ma14154294
APA StyleRibeiro, A. P. C., Matias, I. A. S., Zargaran, P., Hashmi, A. S. K., & Martins, L. M. D. R. S. (2021). Unprecedented Use of NHC Gold (I) Complexes as Catalysts for the Selective Oxidation of Ethane to Acetic Acid. Materials, 14(15), 4294. https://doi.org/10.3390/ma14154294








