Characteristics of Impulse Carburization LPC Process
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
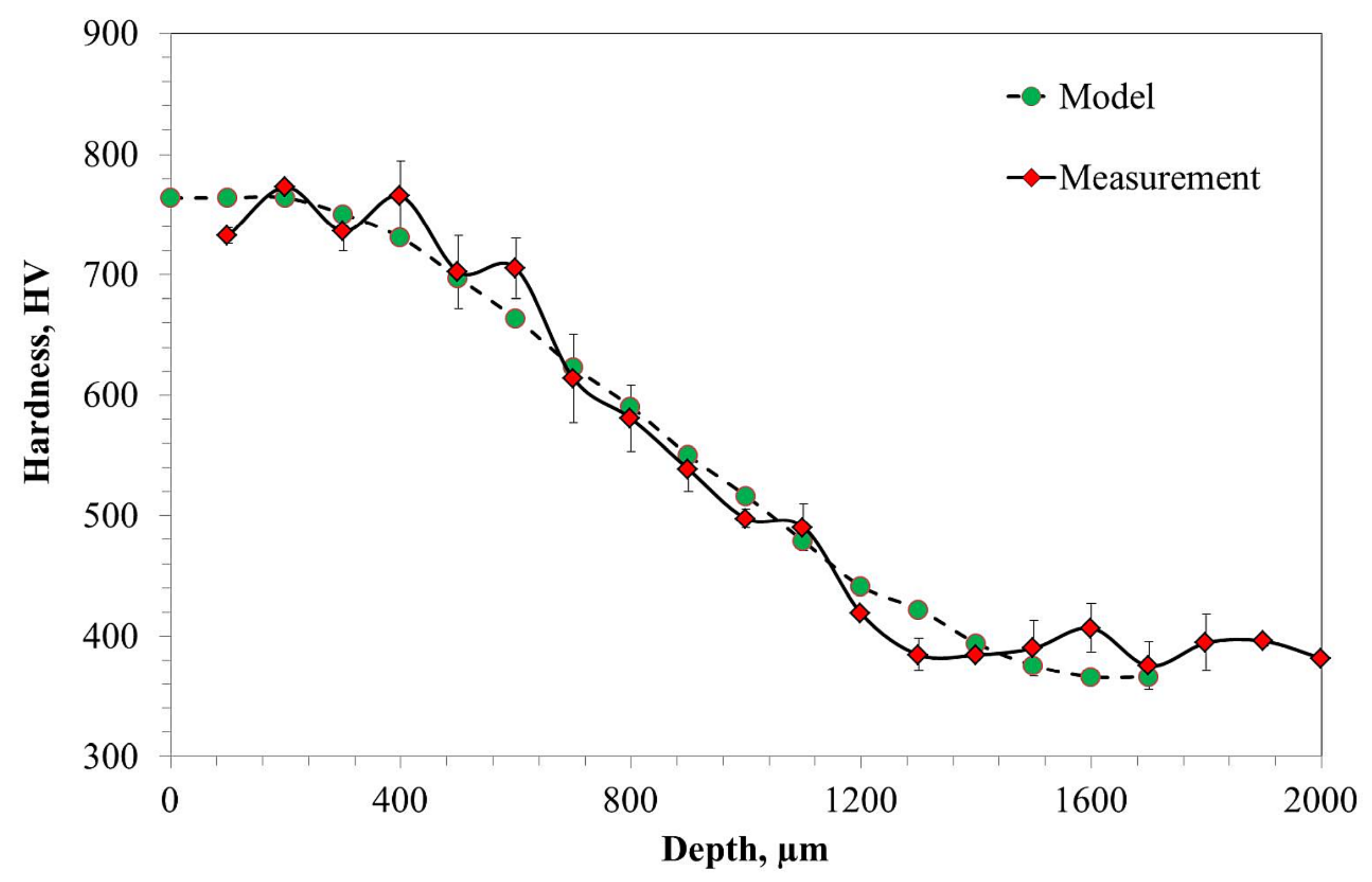
- Carburizing, quenching, and tempering processes increased the hardness of Pyrowear53 from 400 HV up to 750 HV in the carburized layer.
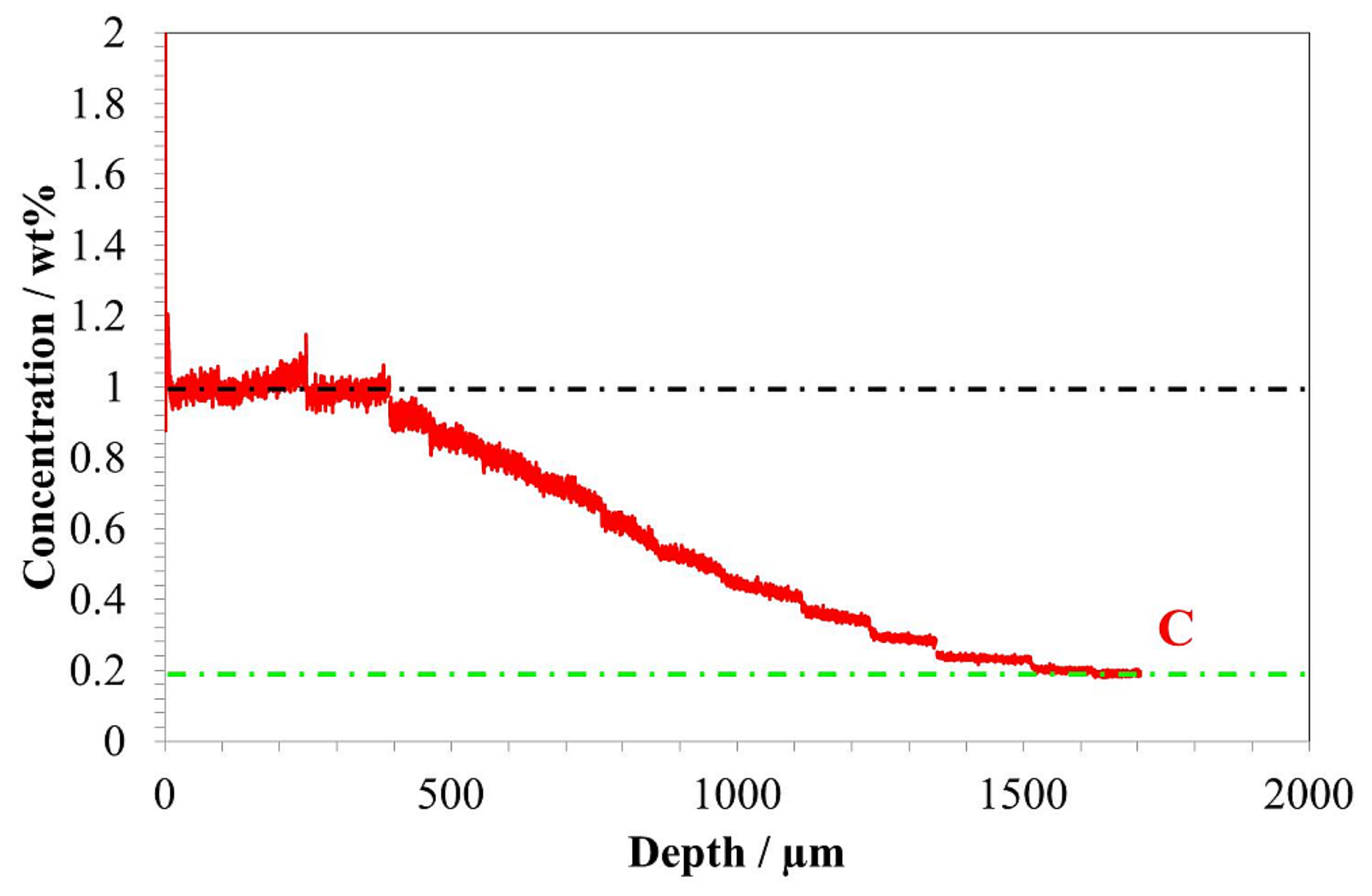
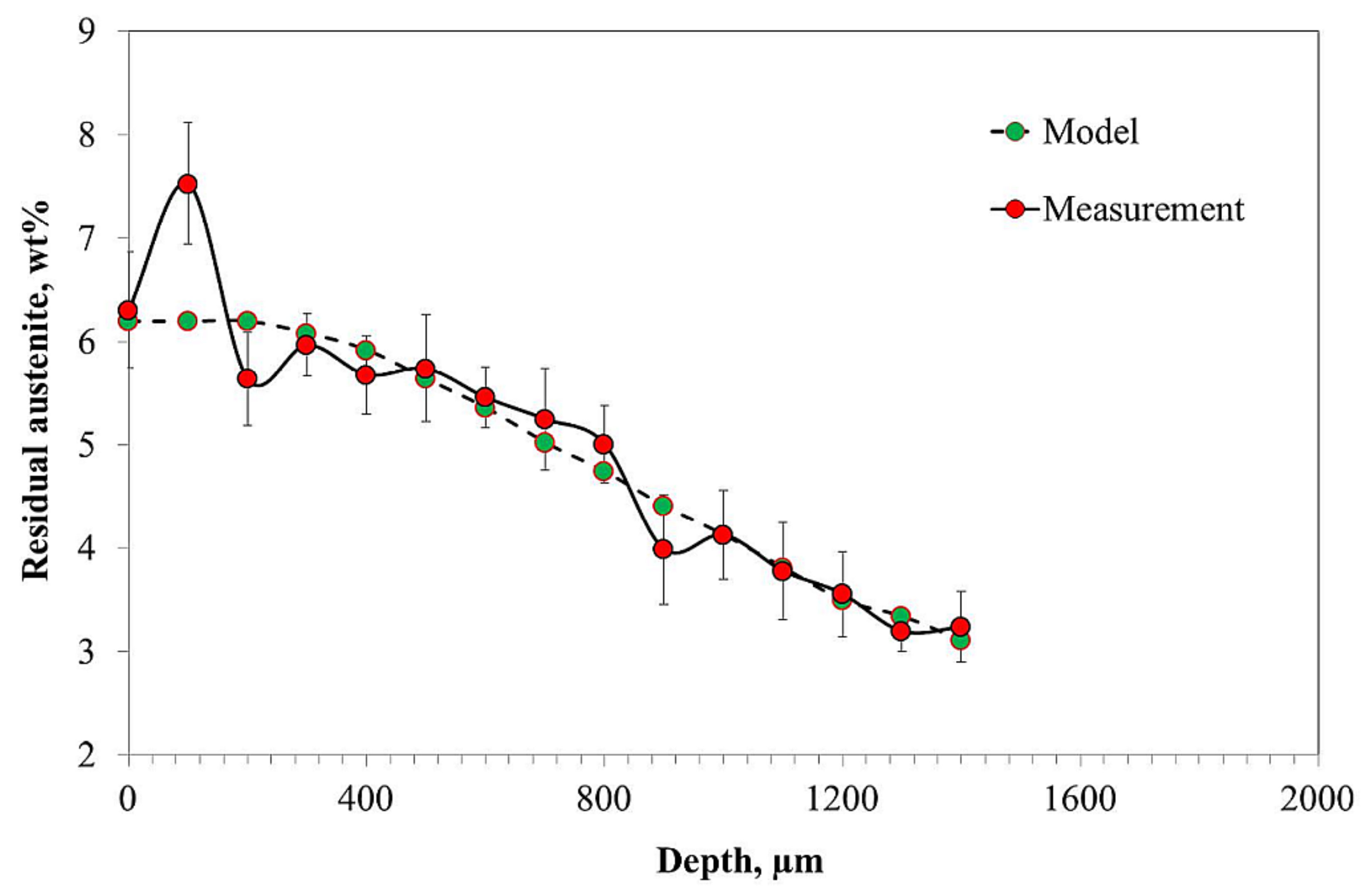
- The hardness profile obtained correlates with the carbon content and residual austenite profiles.
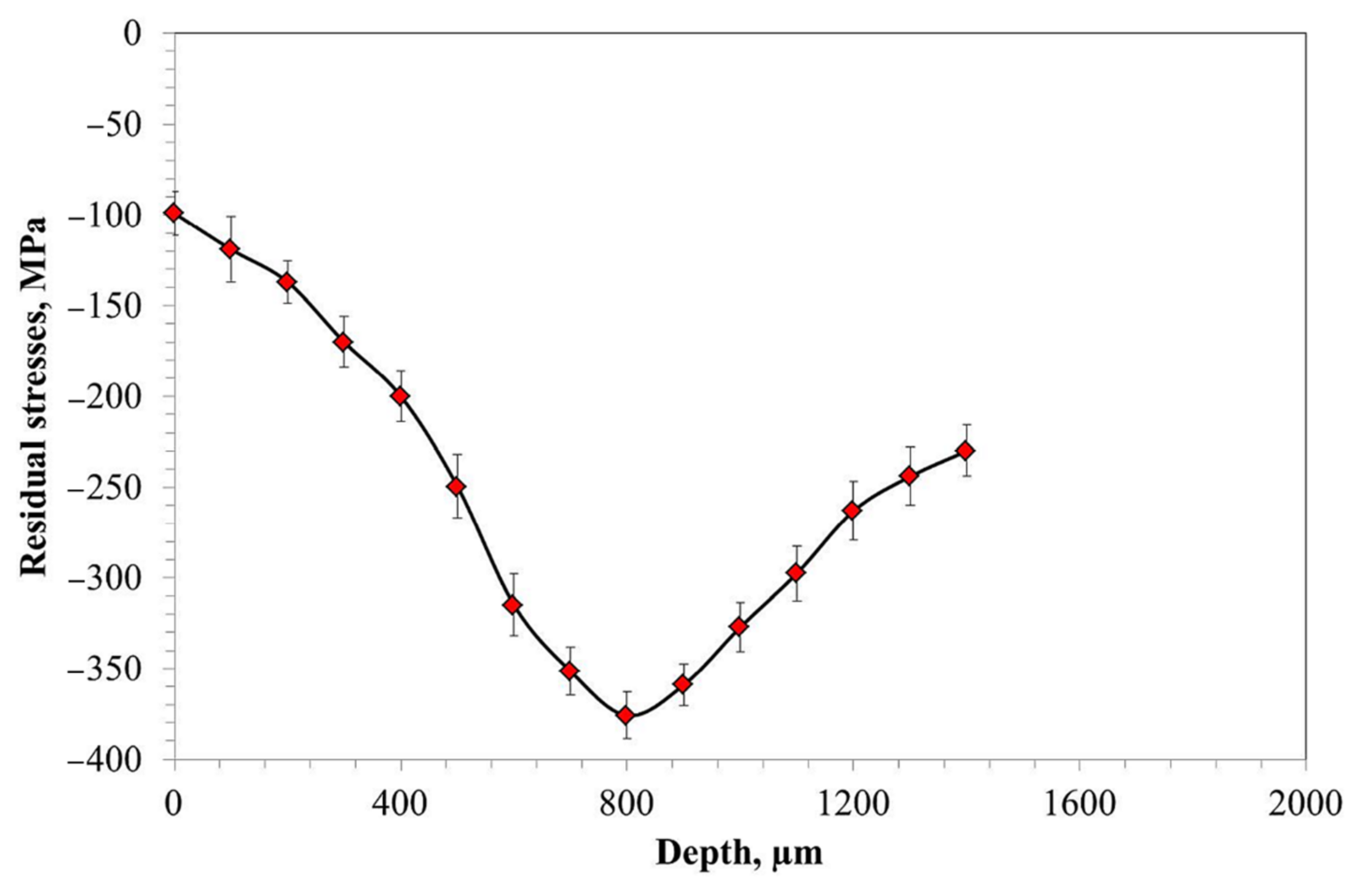
- The results of residual stress measurement revealed the presence of a local extremum at a depth of about 800 µm in the form of a zone showing the highest compressive stresses, despite no differences in hardness, carbon content, or residual austenite content.
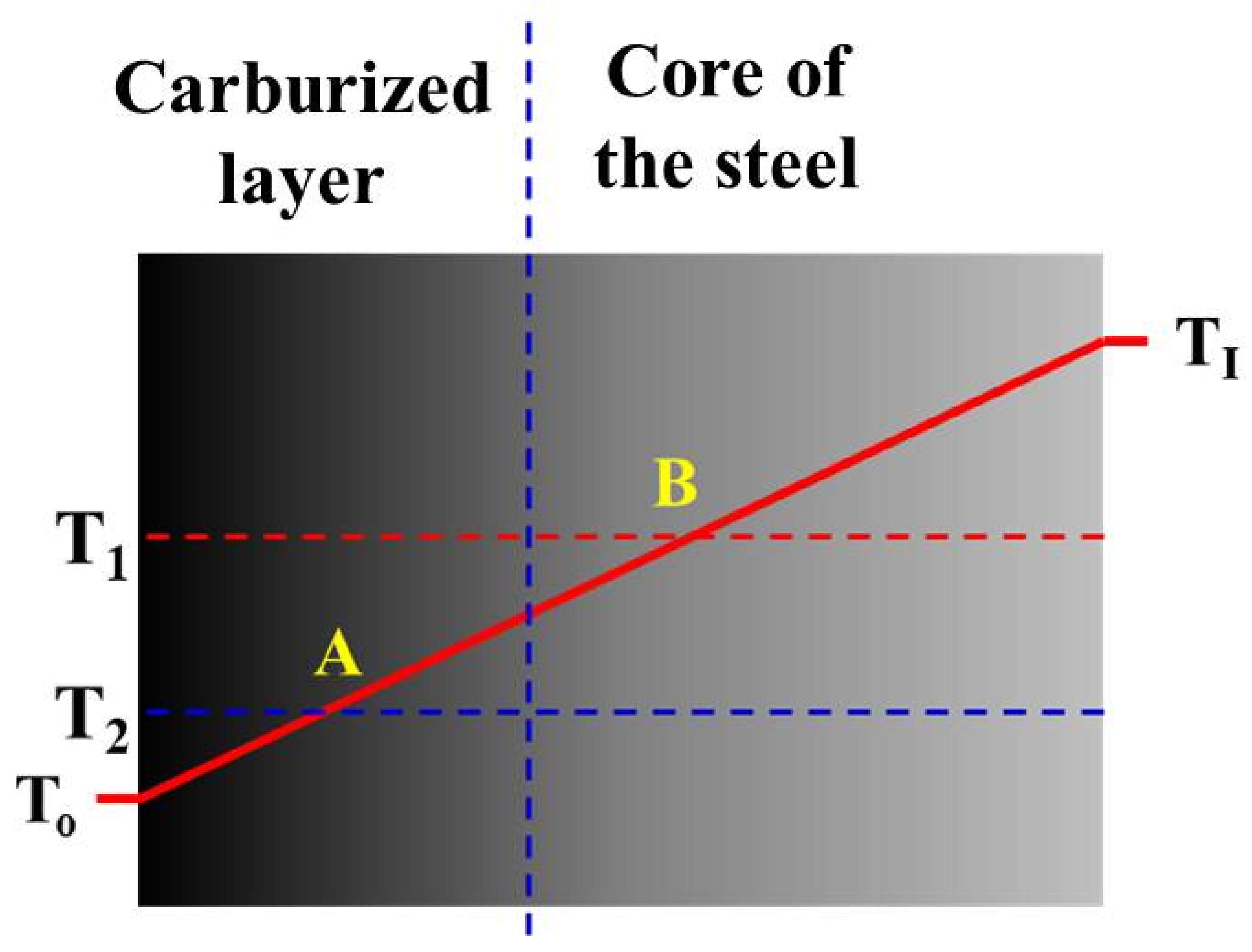
- The occurrence of the extremum is explained by the formation of two fronts of martensite expansion, which result in the formation of a zone with higher compressive stresses at a depth between 600 and 1000 µm.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aramide, F.O.; Ibitoye, S.A.; Oladele, I.O.; Borode, J.O. Effects of carburization time and temperature on the mechanical properties of carburized mild steel, using activated carbon as carburizer. Mater. Res. 2009, 12, 483–487. [Google Scholar] [CrossRef] [Green Version]
- Rajan, T.V.; Sharma, C.P.; Sharma, A. Heat Treatment Principles and Techniques; Prentice Hall: New Delhi, India, 1994. [Google Scholar]
- Denis, S. Coupled temperature stress, phase transformation calculation model numerical illustration of the internal stresses evolution during cooling of a eutectoid carbon steel cylinder. Metall. Trans. A 1987, 18, 1203–1287. [Google Scholar] [CrossRef]
- Leblond, J. Mathematical modelling of transformation plasticity in steels II: Coupling with strain hardening phenomena. Int. J. Plast. 1989, 5, 573–591. [Google Scholar] [CrossRef]
- Wang, K.F.; Chandrasekar, S.; Yang, H.T.Y. Experimental and computational study of the quenching of carbon steel. J. Manuf. Sci. Eng. 1997, 119, 257–265. [Google Scholar] [CrossRef]
- Liu, C.; Xu, X.; Liu, Z. A FEM modeling of quenching and tempering and its application in industrial engineering. Finite Elem. Anal. Des. 2003, 39, 1053–1070. [Google Scholar] [CrossRef]
- Xu, D.H.; Kuang, Z.B. A study on the distribution of residual stress due to surface induction hardening. Int. J. Eng. Mater. Technol. 1996, 118, 571–575. [Google Scholar] [CrossRef]
- Shewmon, G.P. Diffusion in Solids; Mc Graw Hill: Tokyo, Japan, 1963. [Google Scholar]
- Wojtyczka, A.; Iżowski, B. Microstructure and hardness of Pyrowear 53 steel after low-pressure vacuum carburizing at 921 °C. Adv. Manuf. Sci. Technol. 2020, 44, 109–112. [Google Scholar] [CrossRef]
- Aerospace Material Specification AMS6308D. 2007. Available online: https://www.techstreet.com/standards/sae-ams6308d?product_id=1513574 (accessed on 10 May 2021).
- Ferguson, B.; Li, Z.; Freborg, A. Modeling heat treatment of steel parts. Comput. Mater. Sci. 2005, 34, 274–281. [Google Scholar] [CrossRef]
- Dean, S.W.; Banka, A.; Franklin, J.; Li, Z.; Ferguson, B.L.; Freborg, A.; Aronov, M. Improving control of a quenching process by coupling analysis methods. J. ASTM Int. 2009, 6. [Google Scholar] [CrossRef]
- Bongartz, K.; Lupton, D.F.; Schuster, H. A model to predict carburization profiles in high temperature alloys. Metall. Trans. A 1980, 11, 1883–1893. [Google Scholar] [CrossRef]
- Bongartz, K.; Schulten, R.; Quadakkers, W.J.; Nickel, H. A finite difference model describing carburization in high-temperature alloys. Corrosion 1986, 42, 390–397. [Google Scholar] [CrossRef]
- Bongartz, K.; Quadakkers, W.J.; Schulten, R.; Nickel, H. A mathematical model describing carburization in multielement alloy system. Metall. Trans. 1989, 20, 1021–1028. [Google Scholar] [CrossRef]
- Du, H.; Agren, J. Gaseous nitriding iron—Evaluation of diffusion data of nitrogen in of N ın γ′ and ε phases. Int. J. Mater. Res. 1995, 86, 522–529. [Google Scholar] [CrossRef]
- Dimitrov, V.I.; D’Haen, J.; Knuyt, G.; Quaeyhaegens, C.; Stals, L.M. Modeling of nitride layer formation during plasma nitriding of iron. Comp. Mat. Sci. 1999, 15, 22–34. [Google Scholar] [CrossRef]
- Belmonte, T.; Gouné, M.; Michel, H. Numerical modeling of interstitial diffusion in binary systems. Application to iron nitriding. Mater. Sci. Eng. A 2001, 302, 246–257. [Google Scholar] [CrossRef]
- Rozendaal, H.C.F.; Mittemeijer, E.J.; Colijn, P.F.; Schaaf, P.J. The development of nitrogen concentration profiles on nitriding iron. Met. Mater. Trans. A 1983, 14, 395–399. [Google Scholar] [CrossRef]
- Cavaliere, P.; Zavarise, G.; Perillo, M. Modeling of the carburizing and nitriding processes. Comput. Mater. Sci. 2009, 46, 26–35. [Google Scholar] [CrossRef]
- Keddam, M.; Djeghlal, M.; Barrallier, L. A simple diffusion model for the growth kinetics of γ′ iron nitride on the pure iron substrate. Appl. Surf. Sci. 2005, 242, 369–374. [Google Scholar] [CrossRef]
- Wierzba, B.; Romanowska, J.; Kubiak, K.; Sieniawski, J. The cyclic carburization process by bi-velocity method. High Temp. Mater. Process. 2015, 34. [Google Scholar] [CrossRef]
- Bonarski, J.T. Measurement and Use of the Texture Stress Microstructure Characteristics in Materials Diagnostics; Institute of Metallurgy and Materials Science of the Polish Academy of Sciences: Cracow, Poland, 2013. [Google Scholar]
- Skrzypek, S.J. New Possibilities of Measuring Macro Stress of Materials Using X-ray Diffraction in the Geometry of a Constant Angle of Incidence; AGH University of Science and Technology Press: Cracow, Poland, 2002. [Google Scholar]
- Pfeifer, J.P.; Holzbrecher, H.; Quadakkers, W.J.; Breuer, U.; Speier, W. Quantitative analysis of oxide films on ODS-alloys using MCs + -SIMS and e-beam SNMS. Fresenius J. Anal. Chem. 1993, 346, 186–191. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Elschner, A.; Speier, W.; Nickel, H. Composition and growth mechanisms of alumina scales on FeCrAl-based alloys determined by SNMS. Appl. Surf. Sci. 1991, 52, 271–287. [Google Scholar] [CrossRef]
- Nowak, W.J. Characterization of oxidized Ni-based superalloys by GD-OES. J. Anal. At. Spectrom. 2017, 32, 1730–1738. [Google Scholar] [CrossRef]
- Yang, Q.; Ren, J.; Gao, Y.; Li, Y.; Zhao, Y.; Yao, M. Effect of carburization on residual stress field of 20CrMnTi specimen and its numerical simulation. Mater. Sci. Eng. A 2005, 392, 240–247. [Google Scholar] [CrossRef]
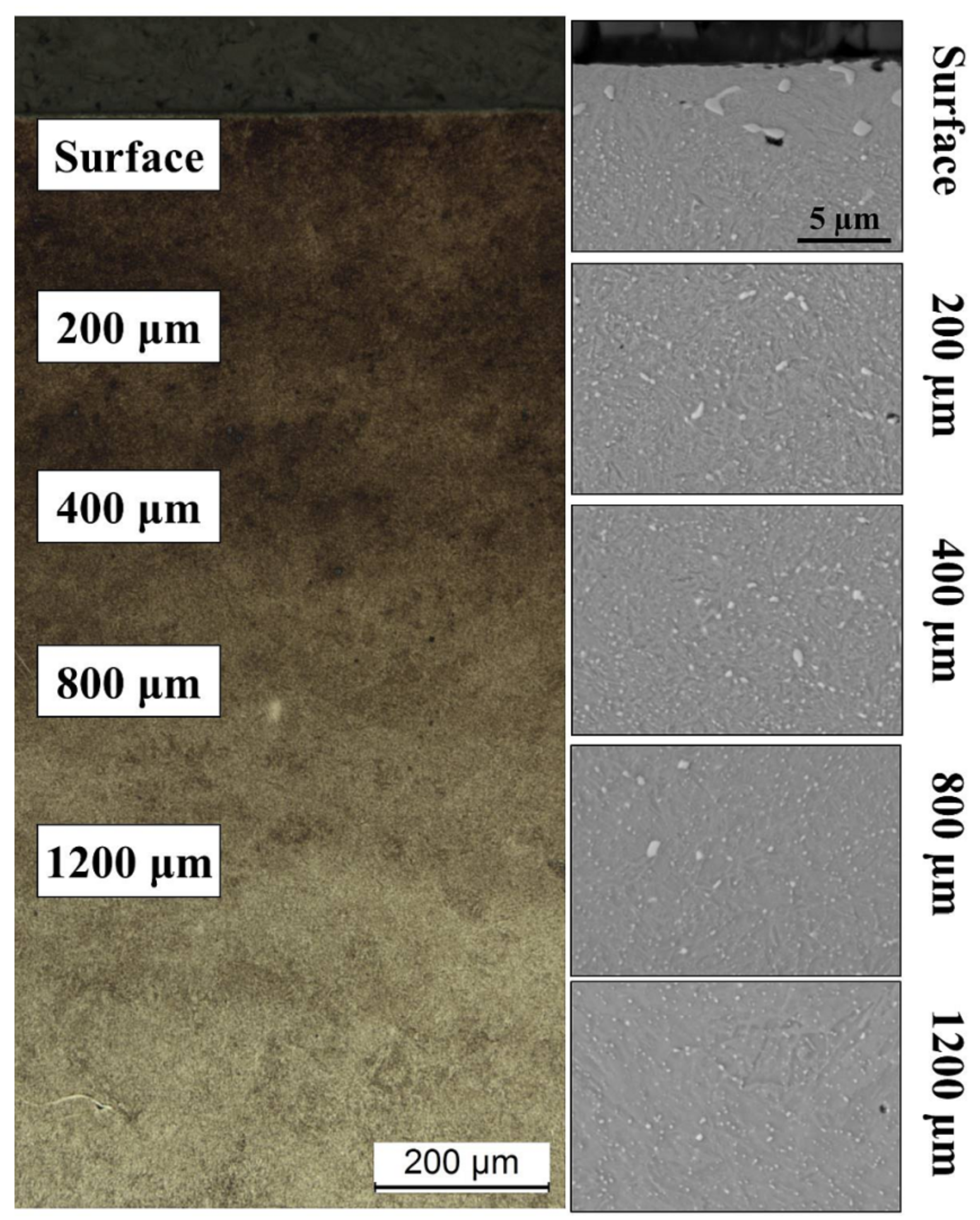
| Element | Fe | C | Mn | Si | P | S | Cr | Ni | Mo | Cu | V |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nominal (wt%) | Bal. * | 0.07–0.13 | 0.25–0.50 | 0.60–1.20 | <0.015 | <0.010 | 0.75–1.25 | 1.60–2.40 | 3.00–3.50 | 1.80–2.30 | 0.05–0.15 |
| Measured (wt%) | Bal. * | 0.13 | 0.41 | 0.93 | 0.011 | 0.003 | 1.04 | 1.97 | 3.23 | 1.92 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filip, R.; Ochał, K.; Gancarczyk, K.; Nowak, W.J.; Kościelniak, B.; Wierzba, B. Characteristics of Impulse Carburization LPC Process. Materials 2021, 14, 4269. https://doi.org/10.3390/ma14154269
Filip R, Ochał K, Gancarczyk K, Nowak WJ, Kościelniak B, Wierzba B. Characteristics of Impulse Carburization LPC Process. Materials. 2021; 14(15):4269. https://doi.org/10.3390/ma14154269
Chicago/Turabian StyleFilip, Ryszard, Kamil Ochał, Kamil Gancarczyk, Wojciech Jerzy Nowak, Barbara Kościelniak, and Bartek Wierzba. 2021. "Characteristics of Impulse Carburization LPC Process" Materials 14, no. 15: 4269. https://doi.org/10.3390/ma14154269
APA StyleFilip, R., Ochał, K., Gancarczyk, K., Nowak, W. J., Kościelniak, B., & Wierzba, B. (2021). Characteristics of Impulse Carburization LPC Process. Materials, 14(15), 4269. https://doi.org/10.3390/ma14154269







