Silver Decorated βTCP-Poly(3hydroxybutyrate) Scaffolds for Bone Tissue Engineering
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tricalcium Phosphate (TCP) Powder Preparation
2.2. Preparation of the Bioceramic Scaffolds
2.3. P(3HB) Synthesis and Purification
2.4. Coating of the Bioceramic Scaffolds with P(3HB)
2.5. Chemical and Phase Composition
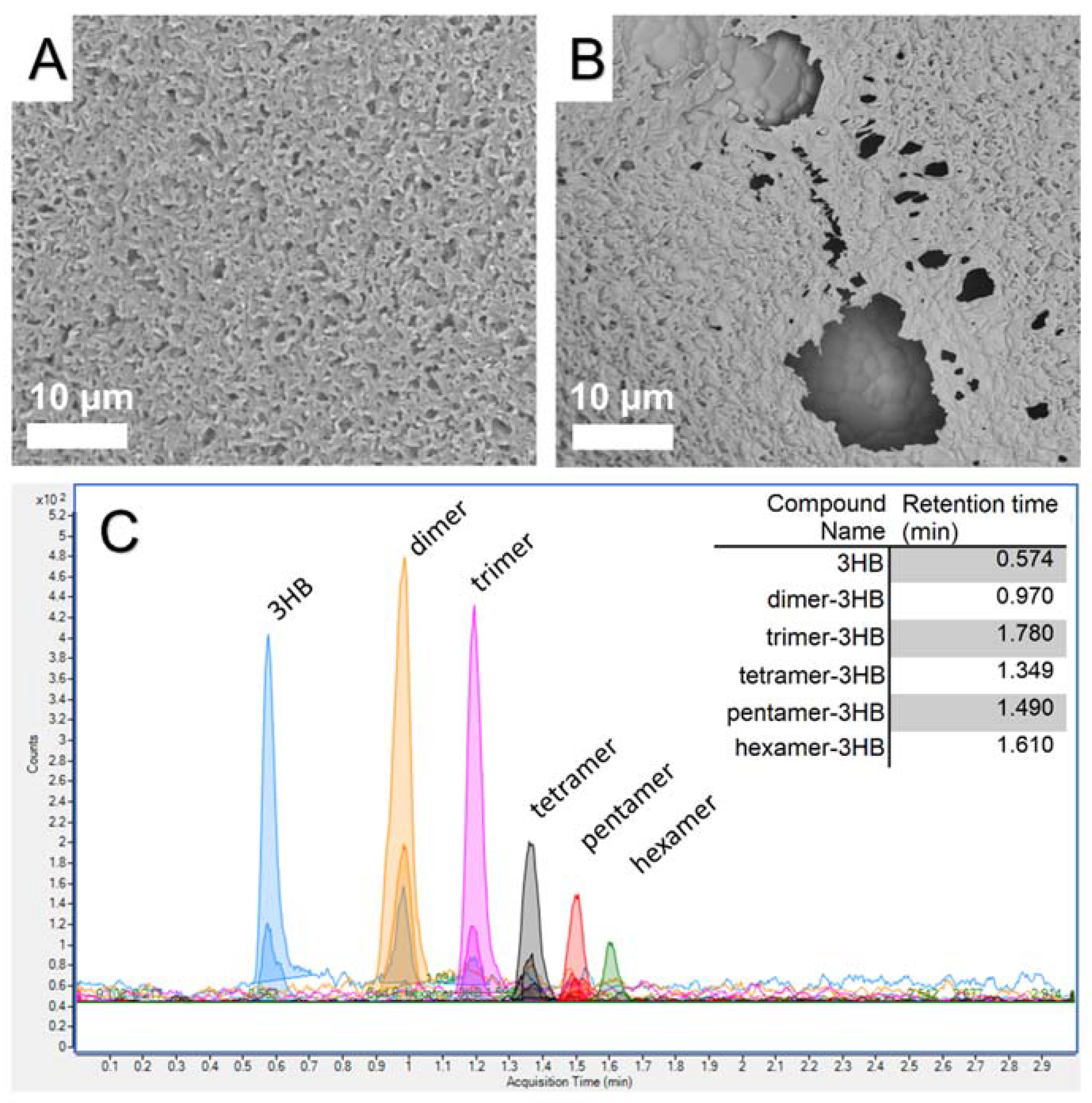
2.6. Microstructure
2.7. Porosity Studies
2.8. Compressive Strength Measurements
2.9. Degradation Studies and UHPLC-MS Measurements
2.10. Statistical Analysis
3. Results and Discussion
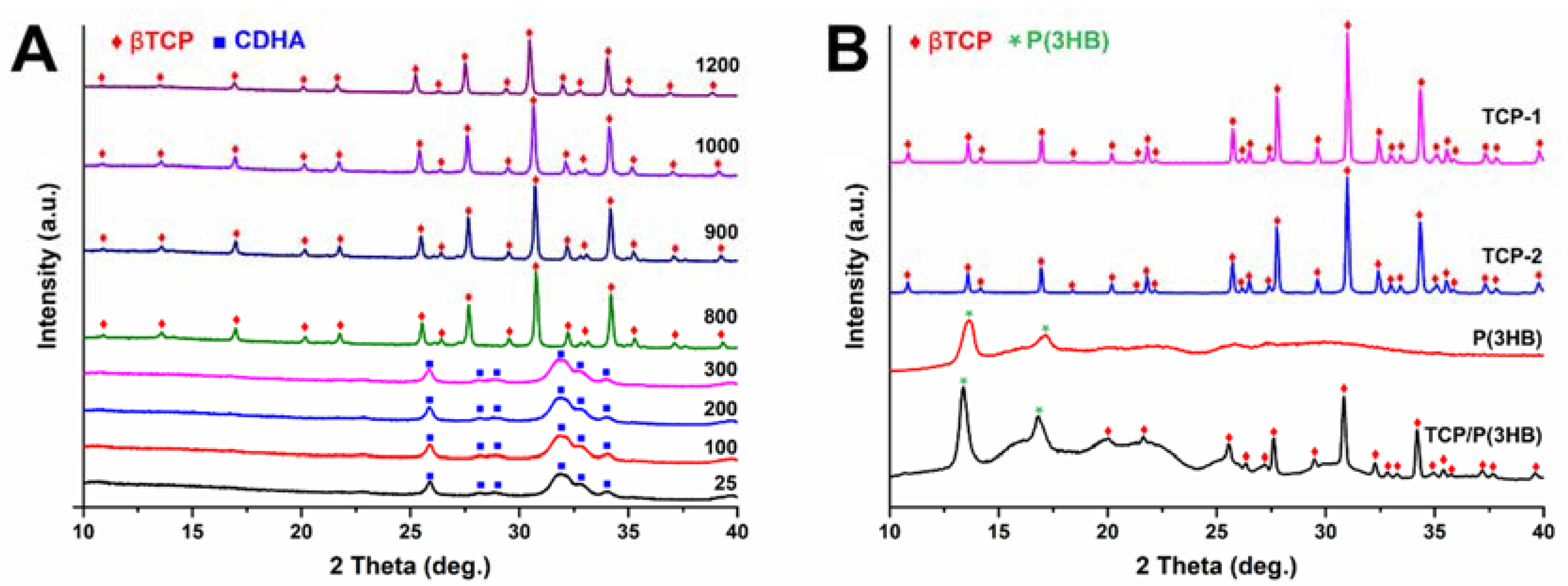
3.1. Chemical and Phase Composition
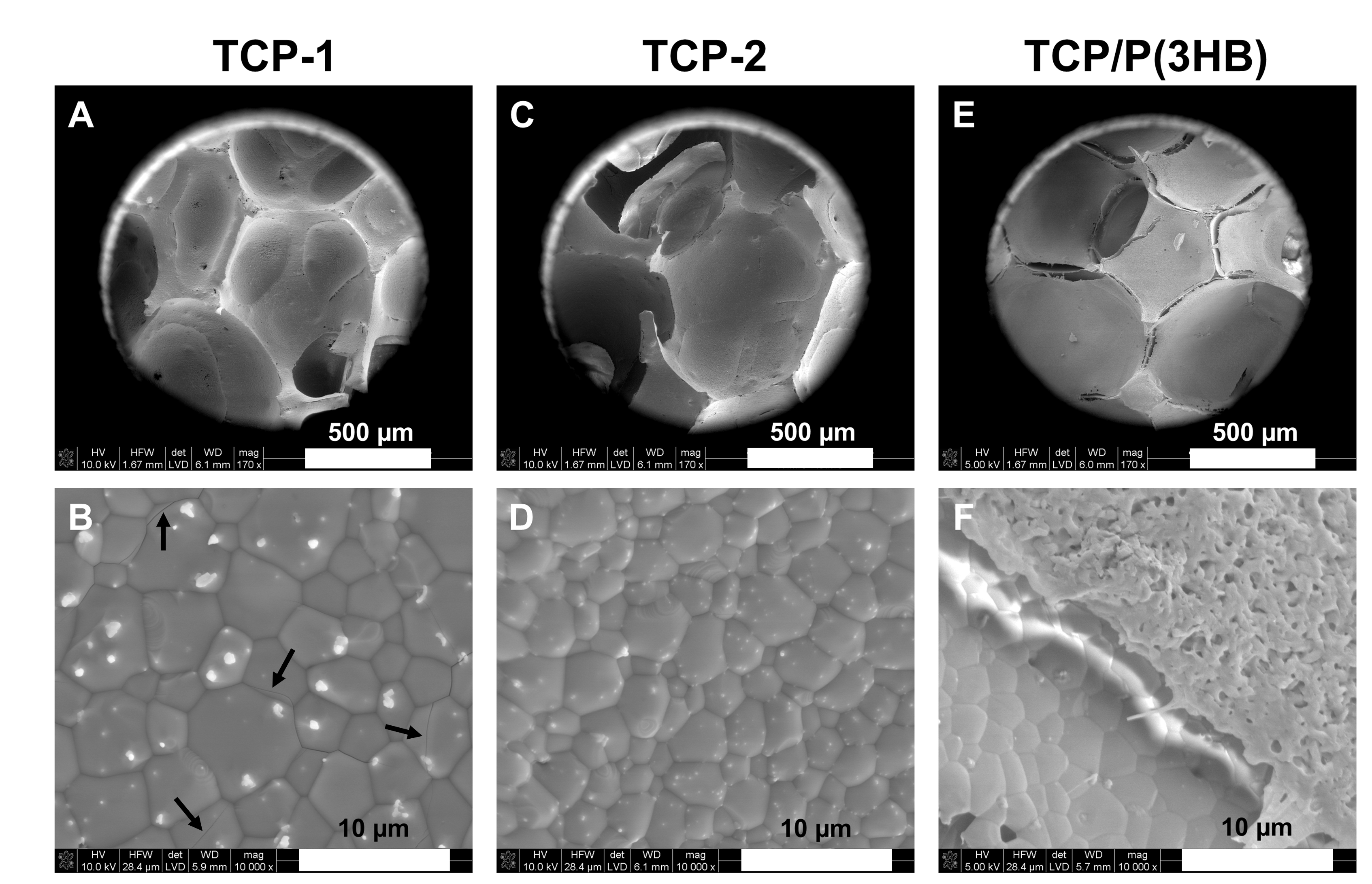
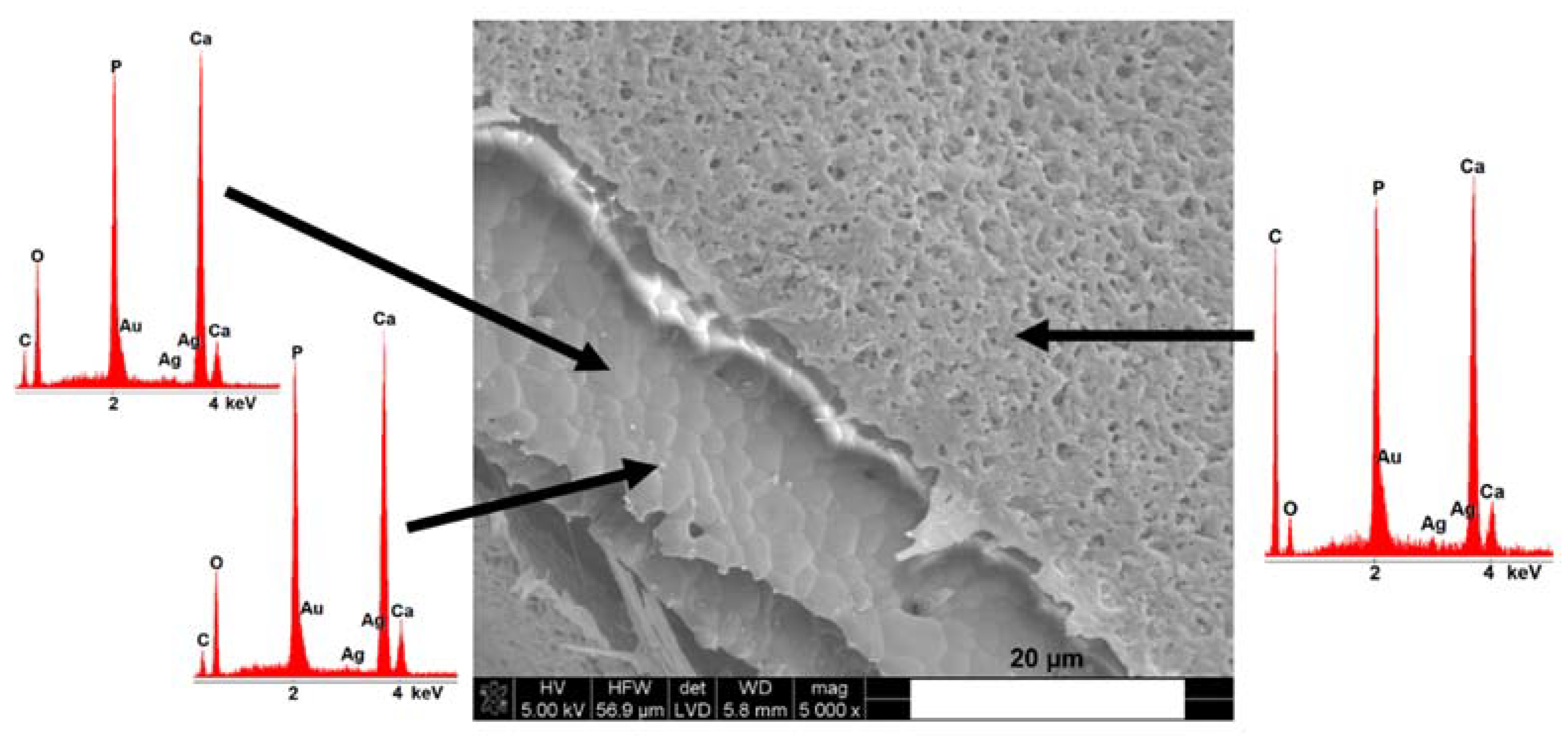
3.2. Microstructure
3.3. Porosity Studies
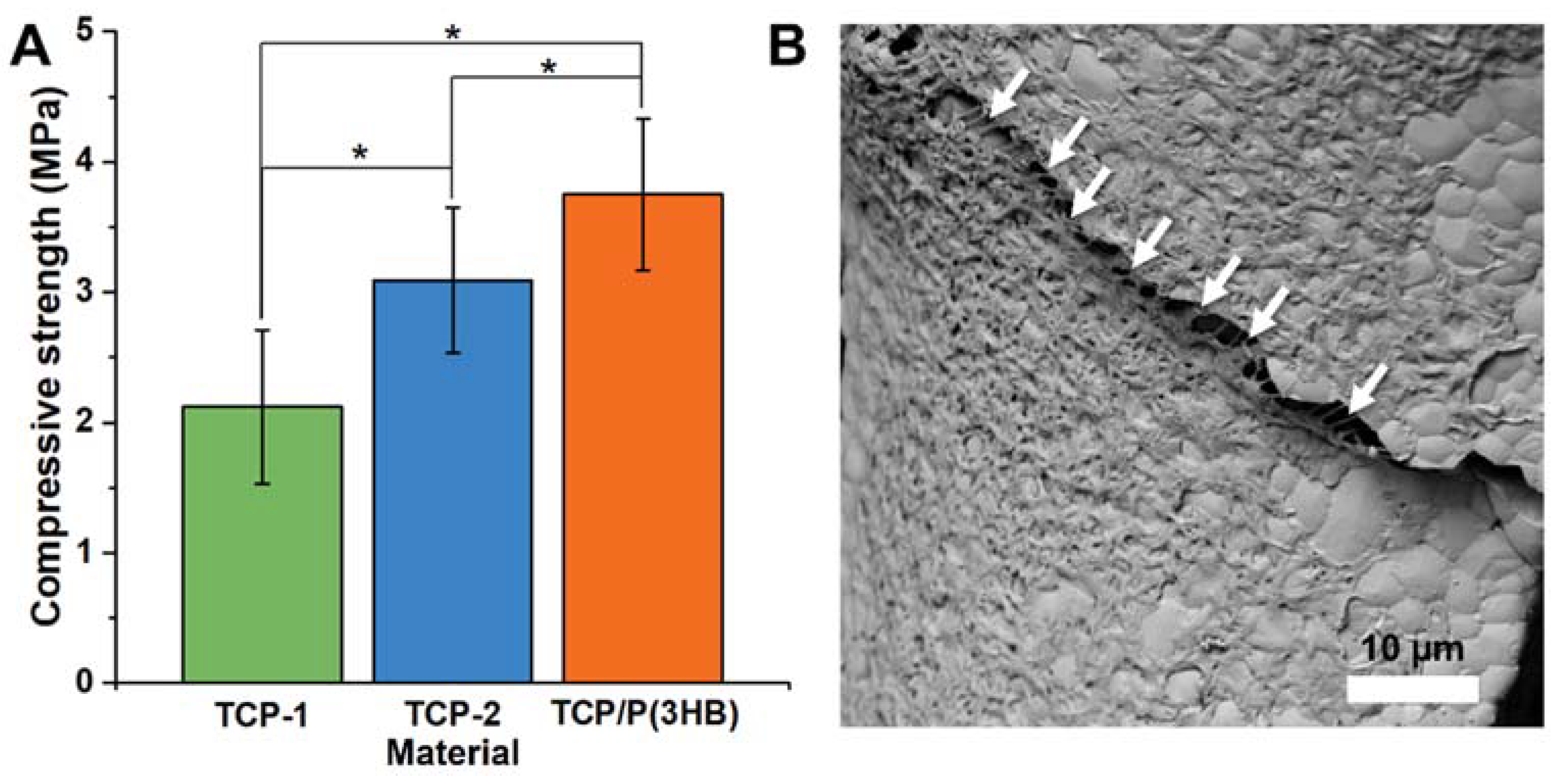
3.4. Compressive Strength Measurements
3.5. Degradation Studies and UHPLC-MS Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoshida, K.; Kondo, N.; Kita, H.; Mitamura, M.; Hashimoto, K.; Toda, Y. Effect of substitutional monovalent and divalent metal ions on mechanical properties of β-tricalcium phosphate. J. Am. Ceram. Soc. 2005, 88, 2315–2318. [Google Scholar] [CrossRef]
- Matsumoto, N.; Sato, K.; Yoshida, K.; Hashimoto, K.; Toda, Y. Preparation and characterization of β-tricalcium phosphate co-doped with monovalent and divalent antibacterial metal ions. Acta Biomater. 2009, 5, 3157–3164. [Google Scholar] [CrossRef]
- Bohner, M.; Santoni, B.L.G.; Döbelin, N. β-tricalcium phosphate for bone substitution: Synthesis and properties. Acta Biomater. 2020, 113, 23–41. [Google Scholar] [CrossRef]
- Honda, M.; Kawanobe, Y.; Nagata, K.; Ishii, K.; Matsumoto, M.; Aizawa, M. Bactericidal and bioresorbable calcium phosphate cements fabricated by silver-containing tricalcium phosphate microspheres. Int. J. Mol. Sci. 2020, 21, 3745. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Champagne, S.; Trenggono, A.; Tolouei, R.; Mantovani, D.; Hermawan, H. Development and characterization of silver containing calcium phosphate coatings on pure iron foam intended for bone scaffold applications. Mater. Des. 2018, 148, 124–134. [Google Scholar] [CrossRef] [Green Version]
- Siek, D.; Ślósarczyk, A.; Przekora, A.; Belcarz, A.; Zima, A.; Ginalska, G.; Czechowska, J. Evaluation of antibacterial activity and cytocompatibility of α-TCP based bone cements with silver-doped hydroxyapatite and CaCO3. Ceram. Int. 2017, 43, 13997–14007. [Google Scholar] [CrossRef]
- Hoover, S.; Tarafder, S.; Bandyopadhyay, A.; Bose, S. Silver doped resorbable tricalcium phosphate scaffolds for bone graft applications. Mater. Sci. Eng. C 2017, 79, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Dastidar, D.G.; Ghosh, D. Silver Nanoparticle Decorated Chitosan Scaffold for Wound Healing and Tissue Regeneration. Macromolecules 2018, 105, 1241–1249. [Google Scholar]
- Hasan, A.; Waibhaw, G.; Saxena, V.; Pandey, L.M. Nano-biocomposite scaffolds of chitosan, carboxymethyl cellulose and silver nanoparticle modified cellulose nanowhiskers for bone tissue engineering applications. Int. J. Biol. Macromol. 2018, 111, 923–934. [Google Scholar] [CrossRef]
- Busuioc, C.; Nicoara, A.I. Bacterial cellulose hydroxyapatite composites decorated with silver nanoparticles for medical applications. Eng. Biomater. 2019, 22, 153. [Google Scholar]
- Torre, E.; Giasafaki, D.; Steriotis, T.; Cassinelli, C.; Morra, M.; Fiorilli, S.; Vitale-Brovarone, C.; Charalambopoulou, G.; Iviglia, G. Silver decorated mesoporous carbons for the treatment of acute and chronic wounds, in a tissue regeneration context. Int. J. Nanomed. 2019, 14, 10147. [Google Scholar] [CrossRef] [Green Version]
- Philippart, A.; Boccaccini, A.R.; Fleck, C.; Schubert, D.W.; Roether, J.A. Toughening and functionalization of bioactive ceramic and glass bone scaffolds by biopolymer coatings and infiltration: A review of the last 5 years. Expert Rev. Med. Devices 2015, 12, 93–111. [Google Scholar] [CrossRef]
- Dziadek, M.; Zima, A.; Cichoń, E.; Czechowska, J.; Ślósarczyk, A. Biomicroconcretes based on the hybrid HAp/CTS granules, α-TCP and pectins as a novel injectable bone substitutes. Mater. Lett. 2020, 265, 127457. [Google Scholar] [CrossRef]
- Cichoń, E.; Haraźna, K.; Skibiński, S.; Witko, T.; Zima, A.; Ślósarczyk, A.; Zimowska, M.; Witko, M.; Leszczynski, B.; Wrobel, A.; et al. Novel bioresorbable tricalcium phosphate/polyhydroxyoctanoate (TCP/PHO) composites as scaffolds for bone tissue engineering applications. J. Mech. Behav. Biomed. Mater. 2019, 98, 235–245. [Google Scholar] [CrossRef]
- Skibiński, S.; Cichoń, E.; Haraźna, K.; Marcello, E.; Roy, I.; Witko, M.; Slosarczyk, A.; Czechowska, J.; Guzik, M.; Zima, A. Functionalized tricalcium phosphate and poly (3-hydroxyoctanoate) derived composite scaffolds as platforms for the controlled release of diclofenac. Ceram. Int. 2021, 47, 3876–3883. [Google Scholar] [CrossRef]
- Ray, S.; Patel, S.K.; Singh, M.; Singh, G.P.; Kalia, V.C. Exploiting polyhydroxyalkanoates for tissue engineering. In Biotechnological Applications of Polyhydroxyalkanoates; Springer: Singapore, 2019; pp. 271–282. [Google Scholar]
- Peptu, C.; Kowalczuk, M. Biomass-derived polyhydroxyalkanoates: Biomedical applications. In Biomass as Renewable Raw Material to Obtain Bioproducts of High-Tech Value; Elsevier: Amsterdam, The Netherlands, 2018; pp. 271–313. [Google Scholar]
- Alves, M.I.; Macagnan, K.L.; Rodrigues, A.A.; de Assis, D.A.; Torres, M.M.; de Oliveira, P.D.; Furlan, L.; Vendruscolo, C.T.; Moreira, A.D.S. Poly (3-hydroxybutyrate)-P (3HB): Review of production process technology. Ind. Biotechnol. 2017, 13, 192–208. [Google Scholar] [CrossRef]
- Koller, M. A review on established and emerging fermentation schemes for microbial production of Polyhydroxyalkanoate (PHA) biopolyesters. Fermentation 2018, 4, 30. [Google Scholar] [CrossRef] [Green Version]
- Steinbüchel, A.; Valentin, H.E. Diversity of bacterial polyhydroxyalkanoic acids. FEMS Microbiol. Lett. 1995, 128, 219–228. [Google Scholar] [CrossRef]
- Zinn, M.; Witholt, B.; Egli, T. Occurrence, synthesis and medical application of bacterial polyhydroxyalkanoate. Adv. Drug Deliv. Rev. 2001, 53, 5–21. [Google Scholar] [CrossRef]
- Singh, A.K.; Srivastava, J.K.; Chandel, A.K.; Sharma, L.; Mallick, N.; Singh, S.P. Biomedical applications of microbially engineered polyhydroxyalkanoates: An insight into recent advances, bottlenecks, and solutions. Appl. Microbiol. Biotechnol. 2019, 103, 2007–2032. [Google Scholar] [CrossRef]
- Ali, I.; Jamil, N. Polyhydroxyalkanoates: Current applications in the medical field. Front. Biol. 2016, 11, 19–27. [Google Scholar] [CrossRef]
- Sanhueza, C.; Acevedo, F.; Rocha, S.; Villegas, P.; Seeger, M.; Navia, R. Polyhydroxyalkanoates as biomaterial for electrospun scaffolds. Int. J. Biol. Macromol. 2019, 124, 102–110. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: Biodegradable polymers with a range of applications. J. Chem. Technol. Biotechnol. Int. Res. Process Environ. Clean Technol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Peng, S.W.; Guo, X.Y.; Shang, G.G.; Li, J.; Xu, X.Y.; You, M.L.; Li, P.; Chen, G.Q. An assessment of the risks of carcinogenicity associated with polyhydroxyalkanoates through an analysis of DNA aneuploid and telomerase activity. Biomaterials 2011, 32, 2546–2555. [Google Scholar] [CrossRef]
- Montazeri, M.; Karbasi, S.; Foroughi, M.R.; Monshi, A.; Ebrahimi-Kahrizsangi, R. Evaluation of mechanical property and bioactivity of nano-bioglass 45S5 scaffold coated with poly-3-hydroxybutyrate. J. Mater. Sci. Mater. Med. 2015, 26, 62. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.H.; Steinbüchel, A. Poly (3-hydroxybutyrate) production from glycerol by Zobellella denitrificans MW1 via high-cell-density fed-batch fermentation and simplified solvent extraction. Appl. Environ. Microbiol. 2009, 75, 6222–6231. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, R.; Sarkar, R. Synthesis and characterization of sintered beta-tricalcium phosphate: A comparative study on the effect of preparation route. Mater. Sci. Eng. C 2016, 67, 345–352. [Google Scholar] [CrossRef]
- Ryu, H.S.; Youn, H.J.; Hong, K.S.; Chang, B.S.; Lee, C.K.; Chung, S.S. An improvement in sintering property of β-tricalcium phosphate by addition of calcium pyrophosphate. Biomaterials 2002, 23, 909–914. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Narushima, T.; Ergun, C. Synthesis and characterization of Ag-containing calcium phosphates with various Ca/P ratios. Mater. Sci. Eng. C 2015, 53, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Gokcekaya, O.; Ueda, K.; Ogasawara, K.; Kanetaka, H.; Narushima, T. In vitro evaluation of Ag-containing calcium phosphates: Effectiveness of Ag-incorporated β-tricalcium phosphate. Mater. Sci. Eng. C 2017, 75, 926–933. [Google Scholar] [CrossRef]
- Yoshida, K.; Hyuga, H.; Kondo, N.; Kita, H.; Sasaki, M.; Mitamura, M.; Hashimoto, K.; Toda, Y. Substitution model of monovalent (Li, Na, and K), divalent (Mg), and trivalent (Al) metal ions for β-tricalcium phosphate. J. Am. Ceram. Soc. 2006, 89, 688–690. [Google Scholar] [CrossRef]
- Gokcekaya, O.; Ueda, K.; Narushima, T.; Nakano, T. Using HAADF-STEM for atomic-scale evaluation of incorporation of antibacterial Ag atoms in a β-tricalcium phosphate structure. Nanoscale 2020, 12, 16596–16604. [Google Scholar] [CrossRef]
- Perez, R.A.; Mestres, G. Role of pore size and morphology in musculo-skeletal tissue regeneration. Mater. Sci. Eng. C 2016, 61, 922–939. [Google Scholar] [CrossRef]
- Lee, D.J.; Kwon, J.; Kim, Y.I.; Wang, X.; Wu, T.J.; Lee, Y.T.; Kim, S.; Miguez, P.; Ko, C.C. Effect of pore size in bone regeneration using polydopamine-laced hydroxyapatite collagen calcium silicate scaffolds fabricated by 3D mould printing technology. Orthod. Craniofacial Res. 2019, 22, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, G.I.; Bowmaker, G.A.; Metson, J.B. The thermal decomposition of silver (I, III) oxide: A combined XRD, FT-IR and Raman spectroscopic study. Phys. Chem. Chem. Phys. 2001, 3, 3838–3845. [Google Scholar] [CrossRef]
- L′vov, B.V. Kinetics and mechanism of thermal decomposition of silver oxide. Thermochim. Acta 1999, 333, 13–19. [Google Scholar] [CrossRef]
- Koga, N.; Yamada, S.; Kimura, T. Thermal decomposition of silver carbonate: Phenomenology and physicogeometrical kinetics. J. Phys. Chem. C 2013, 117, 326–336. [Google Scholar] [CrossRef]
- Peschel, G.; Dahse, H.M.; Konrad, A.; Wieland, G.D.; Mueller, P.J.; Martin, D.P.; Roth, M. Growth of keratinocytes on porous films of poly (3-hydroxybutyrate) and poly (4-hydroxybutyrate) blended with hyaluronic acid and chitosan. J. Biomed. Mater. Res. A 2008, 85, 1072–1081. [Google Scholar] [CrossRef]
- Wang, Y.W.; Yang, F.; Wu, Q.; Cheng, Y.C.; Peter, H.F.; Chen, J.; Chen, G.Q. Effect of composition of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) on growth of fibroblast and osteoblast. Biomaterials 2005, 26, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Miao, X.; Tan, D.M.; Li, J.; Xiao, Y.; Crawford, R. Mechanical and biological properties of hydroxyapatite/tricalcium phosphate scaffolds coated with poly (lactic-co-glycolic acid). Acta Biomater. 2008, 4, 638–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Wu, Q.; Yang, F.; Xu, M.; Leski, M.; Chen, G.Q. Influence of DL-β-hydroxybutyric acid on cell proliferation and calcium influx. Biomacromolecules 2005, 6, 593–597. [Google Scholar] [CrossRef] [PubMed]

| Symbol | Material | Temperature of Heat Treatment (°C) |
|---|---|---|
| Ag-βTCP | Ag-βTCP powder | 900 |
| TCP-1 | Ag-βTCP based scaffold | 1200 |
| TCP-2 | 1150 | |
| TCP/P(3HB) | TCP-2 scaffold coated with P(3HB) | - |
| Chemical Element | Content (wt.%) | ||
|---|---|---|---|
| Ag-βTCP | TCP-1 | TCP-2 | |
| Ca | 39.351 | 39.276 | 41.062 |
| P | 18.608 | 17.330 | 17.436 |
| O | 40.262 | 41.570 | 39.535 |
| Ag | 1.097 | 1.259 | 1.127 |
| Si | 0.197 | 0.063 | 0.068 |
| Al | 0.160 | 0.088 | 0.460 |
| Mg | 0.141 | 0.099 | 0.098 |
| Na | 0.047 | 0.142 | 0.119 |
| Fe | 0.044 | 0.046 | 0.053 |
| S | 0.017 | 0.011 | 0.006 |
| Cl | 0.016 | 0.025 | - |
| Sr | 0.016 | 0.020 | - |
| Material | Ptotal (vol%) | Popen (vol%) | Pclosed (vol%) |
|---|---|---|---|
| TCP-1 | 70.8 ± 1.9 | 66.7 ± 2.7 | 4.0 ± 1.6 |
| TCP-2 | 71.1 ± 2.9 | 68.1 ± 4.6 | 2.9 ± 1.1 |
| TCP/P(3HB) | 71.8 ± 1.8 | 61.8 ± 3.0 | 10.1 ± 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czechowska, J.; Skibiński, S.; Guzik, M.; Zima, A. Silver Decorated βTCP-Poly(3hydroxybutyrate) Scaffolds for Bone Tissue Engineering. Materials 2021, 14, 4227. https://doi.org/10.3390/ma14154227
Czechowska J, Skibiński S, Guzik M, Zima A. Silver Decorated βTCP-Poly(3hydroxybutyrate) Scaffolds for Bone Tissue Engineering. Materials. 2021; 14(15):4227. https://doi.org/10.3390/ma14154227
Chicago/Turabian StyleCzechowska, Joanna, Szymon Skibiński, Maciej Guzik, and Aneta Zima. 2021. "Silver Decorated βTCP-Poly(3hydroxybutyrate) Scaffolds for Bone Tissue Engineering" Materials 14, no. 15: 4227. https://doi.org/10.3390/ma14154227
APA StyleCzechowska, J., Skibiński, S., Guzik, M., & Zima, A. (2021). Silver Decorated βTCP-Poly(3hydroxybutyrate) Scaffolds for Bone Tissue Engineering. Materials, 14(15), 4227. https://doi.org/10.3390/ma14154227










